Temporal relationship between sleep-time masseter muscle activity and apnea–hypopnea events: A pilot study
ABSTRACT
Background: Obstructive sleep apnea (OSA) is the most common sleep disorder due mainly to peripheral causes, characterized by repeated episodes of obstruction of the upper airways, associated with arousals and snoring. Sleep bruxism (SB) is a masticatory muscle activity during sleep that is characterized as rhythmic (phasic) or nonrhythmic (tonic) and is not a movement disorder or a sleep disorder in otherwise healthy individuals. Given the potentially severe consequences and complications of apnea, the concurrent high prevalence of SB in daily dental practice, getting deeper into the correlation between these phenomena is worthy of interest.
Study Objectives: The aim of this study was to investigate the correlation between SB-related masseter muscle activity (MMA) and apnea–hypopnea events as well as to assess their temporal sequence.
Methods: Thirty (N = 30) patients with sleep respiratory disorders and clinical sus picion of sleep bruxism (SB) were recruited. Ambulatory polygraphic recording was performed to detect apnea–hypopnea events (AHEs) and sleep bruxism episodes (SBEs). Pearson test was used to assess the correlation between apnea–hypopnea index (AHI) and SB index (SBI). A 5-s time window with respect to the respiratory events was considered to describe the temporal distribution of SBEs. Furthermore, SBI was compared between groups of patients with different AHI severity (i.e., mild, moderate and severe) using ANOVA.
Results: On average, AHI was 27.1 ± 21.8 and SBI 9.1 ± 7.5. No correlation was shown between AHI and SBI. Most of SBEs (66.8%) occurred without a temporal relation ship with respiratory events. Considering OSA, 65.7% of SBEs occurred within 5 s after AHEs, while in the case of central apnea (CA) 83.8% of SBEs occurred before the respiratory event. The participants with severe apnea (N = 9) show a tendency to have higher bruxism indexes when compared to patients with mild (N = 11) and moderate apnea (N = 10).
Conclusions: Findings suggest that: 1. At the study population level, there is no cor relation between AHI and SBI, as well as any temporal relationship between SBEs and respiratory events. 2. Specific patterns of temporal relationship might be identi fied with future studies focusing on the different types of apnea–hypopnea events and bruxism activities.
There are additional details that you can gain in "Online congress on evidence-based temporomandibular disorders and bruxism treatment".
INTRODUCTION
Sleep bruxism (SB) is a masticatory muscle activity during sleep that is characterized as rhythmic (phasic) or non-rhythmic (tonic) and is not a movement disorder or a sleep disorder in otherwise healthy individuals.1,2 Based on sleep architecture studies, the prevalence of SB is high in children3 diminishes to about 8–16% in adults and has a further decrease with age.2,4 Other common sleep disorders, such us obstructive sleep apnea (OSA), show an age-related increase; in fact, its prevalence is 30% and 84% at 50 and 70 years, respectively.5 Apnea and hypopnea events (AHE) are characterized by total (apnea) or partial (hypopnea) airway obstruction related with arousals. In turn, SB has often been associated with arousals,6–8 in response to respiratory efforts.9
Recently, the association between AHE and SB received much attention, based on the suggestion that four different hypothet ical scenarios for a temporal relationship between the two phe nomena might exist: (1) the two phenomena are unrelated; (2) the onset of the OSA event precedes the onset of the SB event within a limited time span; (3) the onset of the SB event precedes the onset of the OSA event within a limited time span; and (4) the onset of the OSA and SB event occurs at the same moment.10 Several studies investigated the possible association between OSA and SB and reported inconsistent results.11–14 For instance, Philipps et al. suggested a positive association between sleep apnea and SB activity,9 in accordance with Saito et al., who se lected 10 patients with OSA and SB and evaluated a 5-min time window to investigate the temporal association between AHE and SB-related rhythmic masticatory muscular activity (RMMA).10 On the contrary, Sjoholm et al. reported that only 3.5% of sleep bruxism episodes (SBEs) are directly associated with the AHE in a group of patients with moderate OSA, and 14.4% in a mild OSA group.11 In a successive investigation, Saito et al. underlined that respiratory events (RE) do not correlate with SBEs, in disagree ment with their previous results.12
As a consequence, some literature reviews concluded that there is no scientific evidence to define a clear relationship be tween SB and respiratory events.15–17 Nonetheless, given the potentially severe consequences and complications of apnea, the concurrent high prevalence of SB in daily dental practice, and the ongoing works of reconceptualization of the bruxism construct, the topic is worthy of further exploration.18,19 Based on that, the aim of this study was to investigate the correlation between sleep bruxism (SB)-related masseter muscle activity (MMA) and apnea– hypopnea events as well as to assess their temporal sequence. The null hypothesis was that there is no correlation between apnea and sleep bruxism severity.
METHODS
Study sample
All patients who were referred for sleep breathing disorders and sus picion of SB during the last 2 months of 2019 were consecutively re cruited for the ongoing study at G.B Morgagni L. Pierantoni Hospital, Forlì, Italy. All participants underwent a two-night in-home record ing with a cardiorespiratory device and additional electrodes on the right masseter muscle.
The research protocol was approved by the Institutional Review Board of the Postgraduate School of Orthodontics, University of Ferrara, Ferrara, Italy. All individuals gave their informed consent in accordance with the Helsinki Declaration and understood that they were free to withdraw from the study at any time.
Participants were excluded if they were positive for current neu ropathic pain, pregnancy, history of neurologic, psychiatric, pulmo nary, cardiac or digestive diseases, history of cognitive disabilities or used medications with an effect on respiratory function and/or muscle activity.
Polygraphic recordings and scoring
First night was used for accommodation, and data gathered during the second night were considered for analysis.
The detection of AHE (apnea, hypopnea event) and SBE was based on cardiorespiratory polygraphy (Nox-T3 device, Nox Medical), which included monitoring of heart rate, nasal air-flow, snoring, chest wall and abdominal excursion, oxygen saturation, body position and audio and bilateral masseter EMG. The recording time was set at 8 h starting at 22.30.
All signals were recorded and fed into a personal computer; the data were analyzed using the Noxturnal software (Nox Medical). Respiratory events were scored as follows: apnea was defined as a cessation (≥ 90%) of airflow for a minimum period of 10 s; hy popnea was identified when the airflow dropped by ≥30% for a pe riod of ≥10 s accompanied by a decline in SpO2 higher than 3%. In the obstructive form, respiratory effort is present with persistence of asynchronous thoracic and abdominal respiratory movements.20 The frequency per hour of sleep was quantified as apnea–hypopnea index (AHI).
Bruxism episodes were classified as phasic, tonic or mixed based on the AASM criteria.21 Bursts with greater amplitude than the 10% maximum voluntary contraction (MVC) value with duration exceed ing 0.25 s were selected. EMG events separated by 3-s intervals were recognized as SBEs if they corresponded to one of the three following patterns: phasic (RMMA, with three or more masseter EMG bursts, each lasting 0.25–2.0 s); tonic (a masseter contraction longer than 2 s); or mixed (a mixture of both contraction types).
The analysis of respiratory and bruxism indexes included apnea– hypopnea index (AHI), supine AHI, non-supine AHI, apnea index, obstructive/mixed and central apnea index, hypopnea index, burst index, sleep bruxism index (SBI), phasic/tonic and mixed SBI.
The correlation between SBEs and AHEs was assessed by taking into account for the respiratory and bruxism indexes. In addition, the temporal relationship between SB episodes and apnea/hypopnea events was assessed using a 5-s time window with respect to the AHE. Based on that, SBEs were classified into:
Antecedent SBE: occurred less than 5 s before the AHE
Contextual SBE: occurred during the AHE
Subsequent SBE: occurred less than 5 s after the AHE
Isolated SBE: occurred outside the 5-s time window.
The data were examined in periods of 30 s according to the AASM manual for scoring of sleep and associated events.20 Exams with an effective duration of less than 4 h were excluded from the study.
An expert in sleep medicine (L.C.) performed a manual analysis of the recordings and scored respiratory events in accordance with the established criteria. The software offered an automatic analysis of phasic, tonic and mixed SBEs, which was developed by the manufacturer according to the standards set out in the Principles and Practices of Sleep Medicine, 4th edition (Nox Medical's Noxturnal Software Version 3.1.1).22 A trained dentist with expertise in brux ism and orofacial pain (A.C.) checked the masseter EMG traces to gether with an expert in sleep medicine to verify the scores. In case of disagreement on the count of AHEs or SBEs, the study supervisors were consulted (C.V., D.M.) and decisions were taken by consensus. Prior to the start of the study, calibration was performed on a pilot sample of 10 recordings.
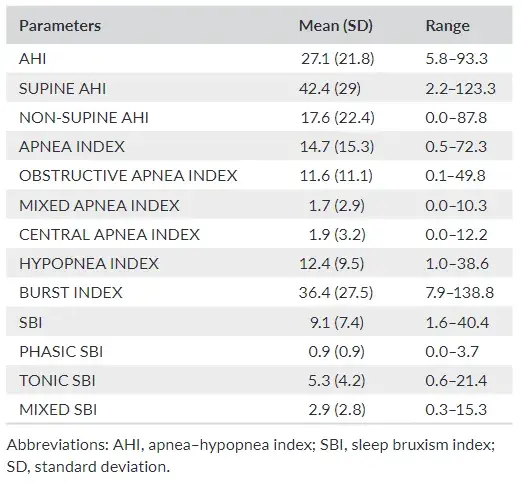
TABLE 1 Descriptive statistics pertaining to the variables
Statistical analysis
Data were stored in a database, and all statistical procedures were performed using IBM Statistical Package for the Social Sciences software for Mac, version 25.0 (IBM SPSS Inc.).
A descriptive analysis of each variable was performed. Pearson correlation test was used to assess the correlation be tween AHI and SBI.
Gender comparison was performed using and Student's t-test. Temporal distribution of the SBEs in relation to AHEs was described as per the above-listed scenarios.
The data were also tested by ANOVA analysis in order to check if there were differences in relation to the AHI severity after dividing the total sample into 3 groups20:
Group 1: AHI <15 (mild OSA).
Group 2: AHI 15–30 (moderate OSA).
Group 3: AHI >30 (severe OSA).
The level of significance was set at p < .05, adjusted for multiple comparisons, when needed.
RESULTS
Within the study sample of 35 participants, five were excluded from data analysis due to technical problems during the recording proce dure or to an insufficient sleep time.
Thus, data are reported on a sample of 30 individuals (23 males, 7 females; mean age 54.1 ± 9.6 years).
In detail:
Group 1: AHI <15 (mild OSA) (11 patients; 6 males, 5 females; mean age 58.3 ± 6.6 years).
Group 2: AHI 15–30 (moderate OSA) (10 patients; 9 males, 1 fe males; mean age 53.8 ± 7.8 years).
Group 3: AHI >30 (severe OSA) (9 patients; 8 males, 1 females; mean age 55.3 ± 17.6 years).
Descriptive data
Descriptive statistics for each respiratory and bruxism indexes, ex pressed as minimum, maximum, mean values and standard deviation, are presented in Table 1.
On average, the participants presented an AHI of 27.1 ± 21.8. Within the apnea group, obstructive, central and mixed apnea events occurred, respectively, with a percentage of 83%, 12% and 5%.
The mean SBI (events/h) was 9.1 ± 7.5. Among the bruxism events, 58% were tonic, 32% mixed and 10% phasic. Gender differences were detected for SUPINE AHI (p = .045), NON SUPINE AHI (p = .007), MIXED APNEA INDEX (p = .003) and PHASIC SBI (p = .003). For all those variables, males showed higher values than females.
Correlation between sleep bruxism and respiratory events
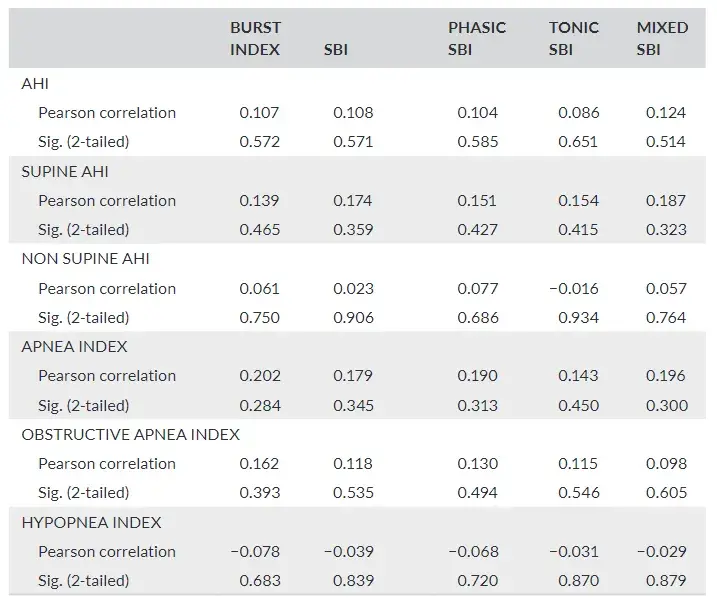
Respiratory indexes did not show a significant correlation with brux ism indexes (Table 2).
TABLE 2 Pearson correlation coefficient test used to evaluate the existence of correlation between respiratory and bruxism indexes
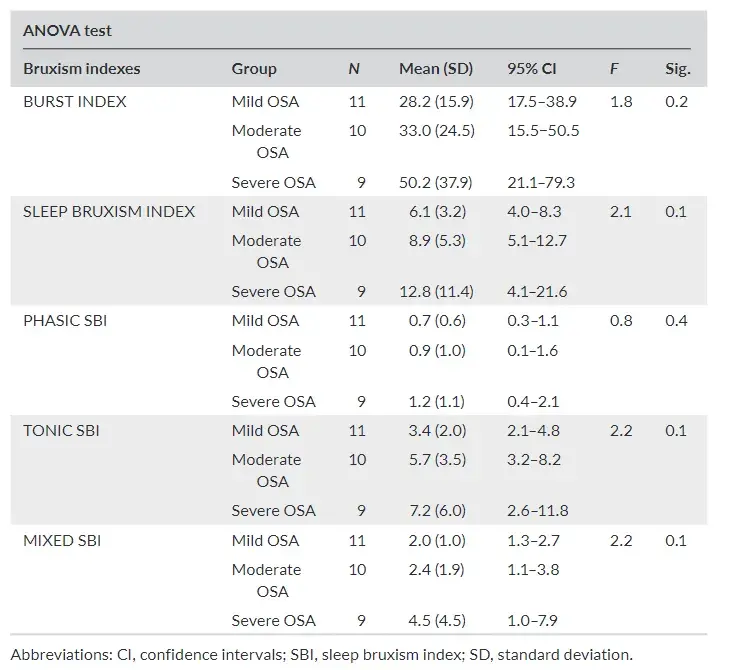
The participants with severe apnea show a tendency to have higher bruxism indexes when compared to patients with mild and moderate apnea. However, there are no statistically significant dif ferences (Table 3) between groups.
TABLE 3 Descriptive analysis of bruxism indexes according to severity of AHI (ANOVA test)
SBEs distribution in relation to AHEs
Most of SBEs (66.8%) occurred without a temporal relationship with AHEs.
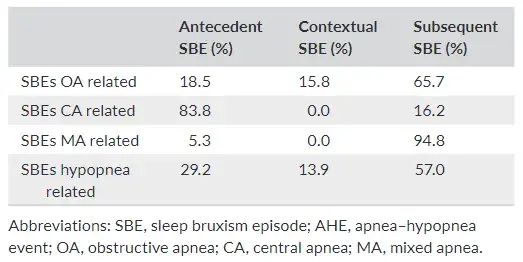
Considering temporally related events, with respect to obstruc tive apnea (OA) events, SBEs occurred as antecedent, contextual and subsequent in 18%, 16% and 66% of the cases, respectively. In the case of central apnea (CA) events, the percentages for antecedent and subsequent SBEs were 84% and 16%, respectively, without any contextual SBEs. Also in the case of mixed apnea (MA) events, no contextual episodes occurred, while 95% of SBEs occurred after, and only 5% before the apnea event. Finally, in the case of hypopnea events, the percentages of antecedent, contextual and subsequent SBEs were 29%, 14% and 57%, respectively (Table 4).
TABLE 4 Descriptive statistics, expressed as a percentage, of SBEs that occurred exclusively in relation to the AHEs
DISCUSSION
The relationship between SB and OSA is gaining increasing attention in several medical fields, but the available evidence is not enough to provide sound conclusions.11–14 Considering this premise, the aim of this study was to get deeper into the topic by investigating the correlation between SB and apnea–hypopnea events in patients with clinical suspicion of sleep breathing disorders as well as trying to identify possible patterns of temporal sequence with respect to the different types of apnea (i.e., central apnea, obstructive apnea, mixed apnea and hypopnea).
The results confirmed the presence of sleep respiratory disorders (i.e., average AHI 27.1 ± 21.8) and showed a mean SBI of 9.1 ± 7.5. Nonetheless, taken as a whole, AHI and SBI were not correlated and not related temporally. Indeed, more than half (66.8%) of the SBEs were «isolated» with respect to the AHEs, viz., not occurring within an arbitrarily set 5-s time frame with respect to the respiratory event. These results are in accordance with several studies11,12 that conclude that SBEs did not correlate with respiratory events (RE) and the SBE are rarely directly associated with the end of AHEs. On the other hand, other authors9,10,23 reported a positive association between OSA and SB activity, thus suggesting to get deeper into the possible reasons for the inconsistency of literature on the topic.
Although most SBEs were «isolated» with respect to the AHEs, the distribution of SBE in relation to a specific respiratory event (i.e., central apnea, obstructive apnea, mixed apnea and hypopnea) is in teresting. With respect to OA events, the majority of SBEs (66%) oc curred subsequently, whilst the opposite happened in CA, with 84% of SBEs occurring antecedently. The latter finding is accordance with Tsujisaka et al., who found that most of the central apnea events occurred after the SB episode.24 As for hypopnea events, most SB episodes (57%) occurred after the respiratory event, as in the case of obstructive apneas. The slightly lower percentage (57% vs 66%) could be related to the fact that no distinction was made between central and obstructive hypopnea events. An explanation for this distribution could be that SBEs are part of the cascade of events that occur during respiratory arousals, rather than hypothesizing a direct of this finding. In contrast, Martynowicz et al. found that SB tends to decrease in relation to the increase in AHI severity26; however, it seems difficult to compare the data due to the different study design.
In general, the results of this study support the common claims that there is not enough scientific evidence yet to define a clear re lationship between SB and respiratory events during sleep.11–17 On one hand, this could be explained by the use of non-standardized data collection strategies, especially as regards the time window in which the events would be considered associated; on the other hand, it may also be hypothesized that the relative predominance of one specific sequence of events may vary at the individual level.10 Within this premise, it must be remarked that the 5 s time window has been chosen arbitrarily based on the observation that patients with severe apnea have such an high frequency of events that lon ger time windows would have overlapped events. Nonetheless, it is likely that adopting other arbitrarily set time windows for evaluating the temporal association between events might lead to different re sults than this investigation. Such issue must be considered for fu ture comparison purposes.
The main limitations of this investigation are the sample size and the use of cardiorespiratory polygraphy instead of polysomnogra phy, which are common to all studies on the topic. While enlarg ing the sample is useful to collect as many data as possible towards the identification of individual phenotypes, including sleep-lab PSG might be helpful for a better assessment of sleep arousals. Given the complexity of sleep architecture and the quick evolution of knowledge on the clinical relevance and relationship between dif ferent phenomena, the creation of multicenter working groups and research task forces might be an interesting option to standardise and improve research methods.
Future studies, possibly taking into account other risk factors for apnea and bruxism, could be designed to build multiple variable models that may predict the presence of sleep bruxism in apnea patients. Based on that, a multidisciplinary approach to the diagnosis of obstruction, involving the assessment of anatomical features, may allow identifying the candidates to present a potentially protective SB. For instance, from an anatomical viewpoint, it might be hypothesized that they are likely those OSA patients with an obstruction of the upper airways, viz., at the oropharyngeal level.10 In these patients, the AHE could lead to respiratory efforts causing the SBE-inducing arousal. Arousal triggers a cascade of events that ends with an increase in the muscle activity and may result in mandibular protrusion and consequent opening of the airways.1,10,27 If this association will be confirmed, SB would become a clinical predictor of OSA in some individuals and, contextually, treat ing OSA would be the causal approach to manage the clinical consequences of SB (e.g., tooth wear, orofacial pain). As part of the multiple variable models, other conditions, factors, and habits that are emerg ing as correlated with both SB and OSA (e.g., gastroesophageal reflux, psychological issues, smoking, pharmacological treatments) must also be take into account. Finally, ongoing works of reconceptualization of the bruxism construct suggest moving on from the adoption of cut-off points to discriminate between bruxers and non-bruxers and embrace an evaluation based on the continuum of jaw muscles activities.1,2,18 Within these premises, the adoption of standardized data collection strategies and an evaluation of the anatomical location of the airway obstruction site to select the study sample seem to be fundamental criteria for future studies on the SB-AHE relationship.
CONCLUSION
Within the limits of the present study, findings suggest that in a pop ulation with sleep apnea more than half of the SBEs were «isolated» with respect to the AHEs. Therefore, from a global point of view, there is no correlation between the two phenomena. However, if the different types of respiratory events are considered separately (i.e., central apnea, obstructive apnea, mixed apnea and hypopnea), SBEs occurred with different temporal sequences. With respect to OA, the majority of SBEs (66%) were subsequent, while an opposite temporal relationship was found with CA, with 84% of SBEs occur ring antecedently. In addition, participants with severe apnea show a tendency to have higher bruxism indexes when compared to pa tients with mild and moderate apnea. As a general remark, these re sults, therefore, suggest the need to investigate for the presence of possible different phenotypes in terms of the temporal relationship between SB and respiratory events.
More details you can discover in our author's course "Airways for dentist" by Roger Price.
REFERENCES
Lobbezoo F, Ahlberg J, Raphael KG, et al. International consensus on the assessment of bruxism: report of a workin progress. J Oral Rehabil. 2018;45(11):837-844.
Manfredini D, Colonna A, Bracci A, Lobbezoo F. Bruxism: a sum mary of current knowledge on aetiology, assessment and manage ment. Oral Surg. 2019;13(7):358-370.
Restrepo C, Lobbezoo F, Castrillon E, et al. Agreement between jaw-muscle activity measurement with portable single-channel electromyography and polysomnography in children. Int J Paediatr Dent. 2018;28(1):33-42.
Manfredini D, Winocur E, Guarda Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults. A systematic review of litera ture. J Orofac Pain. 2013;27(2):99-110.
Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441-446.
American Academy of Sleep Medicine (ed). International Classification of Sleep Disorders, 3rd edn. American Academy of Sleep Medicine; 2014.
Lobbezoo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013;40(1):2-4.
Klasser GD, Rei N, Lavigne GJ. Sleep bruxism etiology: the evolu tion of a changing paradigm. J Can Dent Assoc. 2015;81:f2.
American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: Diagnostic and coding manual. American Academy of Sleep Medicine; 2005.
Manfredini D, Guarda-Nardini L, Marchese-Ragona R, Lobbezoo F. Theories on possible temporal relationships between sleep brux ism and obstructive sleep apnea events. An expert opinion. Sleep Breath. 2015;19(4):1459-1465.
Phillips BA, Okeson J, Paesani D, Gilmore R. Effect of sleep position on sleep apnea and parafunctional activity. Chest. 1986;90(3):424-429.
Saito M, Yamaguchi T, Mikami S, et al. Temporal association be tween sleep apnea-hypopnea and sleep bruxism events. J Sleep Res. 2014;23:196-203.
Sjoholm TT, Lowe AA, Miyamoto K, Fleetham JA, Ryan CF. Sleep bruxism in patients with sleep-disordered breathing. Arch Oral Biol. 2000;45(10):889-896.
Saito M, Yamaguchi T, Mikami S, et al. Weak association between sleep bruxism and obstructive sleep apnea. A sleep laboratory study. Sleep Breath. 2016;20(2):703-709.
De Luca CG, Singh V, Gozal D, Major PW, Flores-Mir C. Sleep brux ism and sleepdisordered breathing: a systematic review. J Oral Facial Pain Headache. 2014;28(4):299-305.
Jokubauskas L, Baltrušaitytė A. Relationship between obstructive sleep apnoea syndrome and sleep bruxism: a systematic review. J Oral Rehabil. 2017;44(2):144-153.
Da Costa Lopes AJ, Cunha TCA, Monteiro MCM, Serra-Negra JM, Cabral LC, Júnior PCS. Is there an association between sleep brux ism and obstructive sleep apnea syndrome? A systematic review. Sleep Breath. 2020;24(3):913-921.
Manfredini D, Ahlberg J, Wetselaar P, Svensson P, Lobbezoo F. The bruxism construct: from cut-off points to a continuum spectrum. J Oral Rehabil. 2019;46(11):991-997.
Manfredini D, Ahlberg J, Aarab G, et al. Towards a Standardized Tool for the Assessment of Bruxism (STAB)-Overview and general remarks of a multidimensional bruxism evaluation system. J Oral Rehabil. 2020;47(5):549-556.
Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respi ratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events—Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597-619.
Iber CA-IS, Chesson A, Quan SF. The AASM manual for the scor ing of sleep and associated events: rules, terminology and technical specifications. Sleep Med. 2012;8:597-619.
Medical N. In: Ehf NM, editor. Sleep bruxism customer support doc ument. Iceland; 2013.
Hosoya H, Kitaura H, Hsshimoto T, et al. Relationship between sleep bruxism and sleep respiratory events in patients with ob structive sleep apnea syndrome. Sleep Breath. 2014;18(4):837-844.
Tsujisaka A, Haraki S, Nonoue S, et al. The occurrence of respira tory events in young subjects with a frequent rhythmic masticatory muscle activity: a pilot study. J Prosthodont Res. 2018;62(3):317-323.
Kato T, Rompre P, Montplaisir JY, Sessle BJ, Lavigne GJ. Sleep brux ism: an oromotor activity secondary to microarousal. J Dent Res. 2001;80(10):1940-1944.
Martynowicz H, Gac P, Brzecka A, et al. The relationship between sleep bruxism and obstructive sleep apnea based on polysomno graphic findings. J Clin Med. 2019;8(10):E1653.
Huynh N, Kato T, Rompre PH, et al. Sleep bruxism is associated to micro-arousals and an increase in cardiac sympathetic activity. J Sleep Res. 2006;15(3):339-346.
