A Systematic Approach to Deep Caries Removal End Points: The Peripheral Seal Concept in Adhesive Dentistry
The objective of this article is to present evidence-based protocols for the diagnosis and treatment of deep caries lesions in vital teeth. These protocols combine caries-detecting dye with anatomical and histologic knowledge to arrive at ideal caries removal end points for adhesive restorations. DIAGNOdent laser fluorescence technology can also be used to confirm these end points. These ideal caries removal end points generate a peripheral seal zone that can support long-term biomimetic restorations. A review of the published literature since 1980 on caries, caries diagnosis, and caries treatments and their relationships to adhesive bonding techniques was carried out. Combinig anatomical measurements and pathologic and histologic knowledge with caries-detecting dye and DIAGNOdent laser fluorescence technologies can produce ideal caries removal end points for adhesive dentistry without exposing vital pulps. (Quintessence Int 2012;43:197–208)
There are additional information about teeth biomimetic restorations you can obtain in our course "The biomimetic concept: composite restoration protocols".
The most common pathology clinicians treat is caries and its resulting decay.1 The treatment of this disease involves the diagnosis and management of the patient’s biofilm and then the remineralization or restoration of the damaged tooth structure. Treating decay without treating the cause of decay is a problem that the CAMBRA (Caries Management By Risk Assessment) program is seeking to resolve. Small lesions can often be treated nonsurgically, according to revised International Caries Detection and Assessment System (ICDAS II). After the systemic disease is treated and incipient lesions are remineralized9 or infiltrated, clinicians are left to determine how much of the caries should be removed before restoration. For small, shallow lesions limited to the enamel and superficial dentin closest to the dentinoenamel junction (DEJ), complete removal of caries by the traditional visual and tactile technique has been successful. The minimally invasive dental treatments for these smaller lesions using air abrasion, sonic diamond tips, glass-ionomer cement, and bonded composite resin have reduced the need for traditional preparations that eliminate important anatomical structures. However, for lesions of medium and large depths, more sophisticated techniques are required for determining ideal caries removal end points.
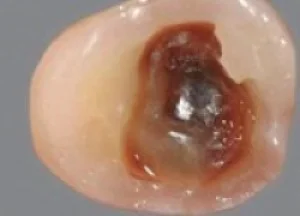
Fig 1. Intermediate and deep caries lesions have many visual and tactile complexities that can be systematically approached with caries removal end point and peripheral seal zone protocols.
Using traditional visual and tactile techniques for these larger lesions is often inconsistent for etermining optimal caries removal end points that consistently preserve tooth structure and remove unfection without exposing the pulp. Such ideal caries removal end points would preserve pulp vitality without limiting the strength and durability of the the adhesive reconstruction. Researchers and clinicians have struggled with the problem of too much vs not enough when it comes to removal of decayed tissue.
This paper outlines a system for determining more predictable caries removal end points for deeper lesions in vital teeth. This approach is based on detailed knowledge of three-dimensional dental anatomy, histology, microbiology, and adhesive dental science. This knowledge is then integrated with visual dye staining. Laser fluorescence technologies can also be added to guide the clinician in deep caries diagnosis and removal. This combination of multiple overlapping techniques can remedy the shortcomings of using only the tactile and visual method.
The general objectives of this systematic approach to caries removal end point determination are the maintenance of pulp vitality after restoration by adhesive methods; the elimination of dentinal infections by removing, deactivating, or sealing in bacteria; and the conservation of intact tooth structure for long-term biomimetic function. The specific objectives of caries removal end point determination are the creation of a peripheral seal zone and the absolute avoidance of pulpal exposure while generating a highly bonded restoration with excellent long-term prognosis. First, by creating a peripheral seal zone 1- to 3-mm wide consisting of normal superficial dentin, DEJ, and enamel (Fig 2), a bond strength of approximately 45–55 MPa can be generated.
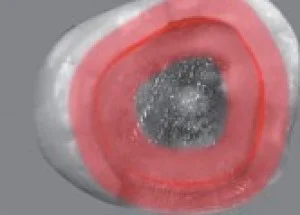 Fig 2. The concept of a peripheral seal zone is that the enamel, DEJ, and superfcial dentin constitute the caries-free area of a highly bonded adhesive restoration.
Fig 2. The concept of a peripheral seal zone is that the enamel, DEJ, and superfcial dentin constitute the caries-free area of a highly bonded adhesive restoration.
This peripheral seal zone will be confirmed by the total absence of caries-detecting dye staining. This cariesfree zone can also be confirmed by a DIAGNOdent (KaVo) reading of approximately 12. Commercial products such as Caries Detector (Kuraray), Caries Finder (Danville), and Seek (Ultradent) are examples of caries-detecting dye. Second, by leaving the slightly infected and partially demineralized but highly bondable affected inner carious dentin inside the peripheral seal zone, a bondability of approximately 30 MPa will be obtained in the deeper areas of the preparation. This will be confirmed by light pink staining from caries-detecting dye. DIAGNOdent can also hepl determine the caries removal end point with readings approximately 20–24 for intermediate dentin and approximately 36 for deep dentin (Fig3). On average, intermediate dentin is 3 to 4 mm from the occlusal surface and deep dentin is 4 to 5 mm from the occlusal surface. Clinicians can prevent pulp exposure by leaving the infected outer caries inside the peripheral seal zone when removal would risk pulp exposure. This would be in small circumpulpal areas deeper than 5 mm from the occlusal surface. These small infected areas will stain red from caries-detecting dye and have DIAGNOdent readings higher than 36. Achieving these objectives should result in highly bondable preparationsthat will support adhesive layers and remain bonded for the long term, an essential requirement for large biomimetic dental reconstructions (Fig 3).
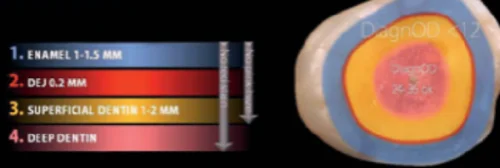
Fig 3. Caries removal end points for the peripheral seal zone can be determined with a combination of caries-detecting dye and DIAGNOdent technologies.
HISTOLOGY OF CARIES LESIONS
In 1980, Takao Fusayama published the research carried out by his team at Tokyo Medical and Dental University on the analysis of caries lesions.34 Using histologic, biochemical, biomechanical, microscopic, and microbiologic techniques, the researchers were able to distinguish two layers in caries lesions that were very diffirent in nature. The first layer was named "outer carious dentin". It was highly infected, acidic, and demineralized. The collagen fibrils in this layer were denatured, having lost most of their intermolecular cross-linkages. This layer was not sensitive to contact and could be removed without anasthesia because it had lost the hydrodynamic system of intact dentinal tubules. This layer also failed to remineralized in natural way because the collagen framework could not return to normal even if the acid environment was neutralized.
The second layer was termed "inner carious dentin". This layer was partially demineralized and slightly infected, but the collagen fibrils retained their natural structure around intact dentinal tubules. Because of this remaining structural integrity, the inner carious dentin was sensitive to removal without anasthesia. The lumens of the dentinal tubules in this layer had no peritubular rings of hydroxyapatite. Instead, the enlarged lumens were now partially or completely filled with large crystals of tribeta calcium phosphate called Whitlockite. Whitlockite is crystallized into the dentinal tubules as hydroxyapatite is dissolved from intertubular dentin by bacterial acids. This inner layer of caries lesion was able to be restored to a normal mineralization with a hydroxyapatite matrix surrounding the collagen fibrils (intertubular dentin) and around the tubules (peritubular dentin) when the pH was neutralized.
Since the late 1960s, the goal of removing only outer caries and saving the inner caries for remineralization has been recognized .37 The problem was that each operator had a different sense of hard and soft. Clinically finding the interphase between the outer and inner carious dentin layers was inconsistent. Adding to the difficulty was the anatomical softening of dentin as it nears the pulp (reparative dentin, laid down during the caries progression, is even softer than deep dentin) and the fact that different instruments (hand, rotary, or ultrasonic) removed more or less of the lesion during excavation. All of this subjectivity and variability made for inconsistent caries removal end points. Fusayama made progress toward a solution to this problem by finding two propylene glycol-based colored solutions (one purple, one r that stained the outer and inner carious dentin layers differently. The outer carious dentin stained dark red, and the inner carious dentin stained lighter (pink for the red dye formula). The interphase between the outer and inner carious dentin was referred to as the turbid layer. This interphase is a mixture of parallel groups of tubules, some of which are outer carious dentin and some of which are inner carious dentin (depending on how long the tubules have been infected and under the influence of bacterial acids). Under the turbid layer, the inner carious dentin becomes the transparent zone. The transparent zone is translucent in histologic examination with a light microscope. The pink staining (often referred to as a pink haze) in the turbid layer becomes lighter as it moves into the transparent zone. In this zone, the large lumens of the dentin tubules are filled to some degree with Whitlockite. These large crystals slow bacterial invasion and reduce dentin permeability. This reduced permeability decreases the outward flow of pulpal fluid, which is referred to as "transudation." It also reduces the movement of pulpal fluid caused by temperature changes. Underneath the transparent zone is an interphase of the transparent zone, as well as normal sensitive dentin called the "subtransparent zone" (Fig 4).
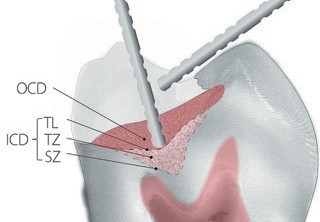
Fig 4. The deep caries lesion has two parts: outer and inner carious dentin. The inner carious dentin has three parts: the turbid layer, transparent zone, subtransparent zone, and normal dentin.
The subtransparent zone stains even more lightly than the transparent zone. Removal of the transparent and subtransparent zones in an attempt to reach hard dentin is the cause of most pulp exposure (Fig 5).
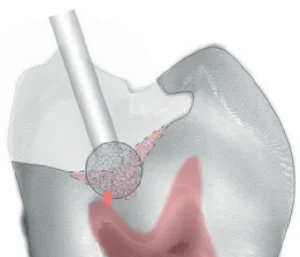
Fig 5. By using only visual and tactile methods for deep caries removal, the pulp is often exposed because the tansparent zone, the subtransparent zone, normal deep dentin, and reparative dentin are all softer than superficial and intermediate dentin.
The pink-haze staining (as differentiated from the red staining) of the inner carious dentin was never discussed by Fusayama in either of his two books or any of his many published articles. He only referred to stained or unstained caries. As a result, many users of caries-detecting dye solutions became confused about exactly how to use it. If all of the lightly stained dentin was removed, under the assumption that it contained a significant number of bacteria, then an increased number of pulp exposures occurred. Other researchers in Japan who helped with Fusayama's original research came to the conclusion that the lightly stained areas were mostly uninfected with intact collagen fibrils surrounded by high levels of hydroxyapatite and Whitlockite and should therefore be preserved for remineralization. Further research in America clarified the relationship between staining and bacterial levels. Histologic and microbiologic analysis showed that the correlation is high in the darkly stained outer caries, but not within the inner caries, which stained lightly.There appeared to be a need for a clinical technology that could assess the amount of bacteria in the lightly stained inner caries.
In the late 1990s, a new laser-fluorescence technology (DIAGNOdent) was introduced as a way to diagnose initial caries lesions (Fig 6). Teams of investigators in Germany and Switzerland found that bacterial metabolic products called porphyrins would fluoresce when irradiated with a 655-nm red laser. This fluorescence could be read and given a numeric value that corresponded approximately to the amount of bacteria present.

Fig 6. DIAGNOdent reads bacterial products called porphyrins and is used to assess the relative amount of bacteria present in a caries lesion.
DIAGNOdent proved its efficiency for the nondestructive diagnosis of pit and fissure caries.49-50 In vivo investigations using DIAGNOdent showed that it might also be used to establish a caries removal end point that correlated with traditional excavation techniques. DIAGNOdent readings for the superficial dentin end point were 8.26 ± 2.69 = ( < 12). The end points for intermediate to deep dentin were 18.75 ± 17.10 = (< 36). These findings were reproduced in a second study at the University of Bern. The different readings in deeper lesions correspond approximately to the proportional differences in pulpal fluid/mm2 at the DEJ vs circumpulpal areas. This is because dentinal tubules are three times more concentrated near the pulp than they are near the DEJ. Depending on the permeability of the inner caries (which is related to the amount of Whitlockite in the dentinal tubules), there will be a greater or lesser diffusion of the porphyrins (hence, the high variance in the DIAGNOdent readings in intermediate and deep inner carious dentin). An increase of demineralized dentin in inner carious dentin and denatured collagen with high demineralization in the outer carious dentin will increase the volume of pulpal fluid in the outer and inner carious dentin. In turn, this will allow the porphyrin diffusion to increase, which will cause higher DIAGNOdent readings in the outer carious dentin and deep inner carious dentin. Boston and Sauble confirmed the German and Swiss experiments and correlated them with the Japanese research using caries-detecting dye. Boston and Liao also investigated the light pink staining of circumpulpal dentin and concluded that it was due to the higher percentage of collagen not completely surrounded by the hydroxyapatite matrix and not from denatured collagen (as in outer carious dentin) or from acidic demineralization (as in inner carious dentin). Staining and remineralization also makes for higher variability and less predictability of any technology. For superficial dentin, the DIAGNOdent readings of 11 or 12 corresponded to a nonstaining and bacteriafree caries-removal end point. A group at Showa University in Tokyo developed a polypropylene glycol-based Caries-Check dye (Nishika) that stained only the outer carious dentin and not the inner carious dentin. This type of caries-detecting dye gave the same results in superficial dentin (DIAGNOdent < 12 with no staining) as Fusayama's propylene glycol-based caries-detecting dye. But because this higher molecular weight cariesdetecting dye formula does not lightly stain the turbid layer, transparent zone, and subtransparent zone, it is not as useful to find the caries removal end point that is ideal for the highest dentin bond strength in the peripheral seal zone. This is because clinicians are not able to detect inner carious dentin that should be removed for the highest bond strength in the peripheral seal zone. However, American and Japanese researchers did not test the deeper lesions like the Europeans did.
Combining caries-detecting dye and DIAGNOdent can give clinicians another way to determine when the excavated lesion is essentially bacteria-free while at the same time not removing affected inner carious dentin inside the peripheral seal zone. The anatomical depth of the lesion needs to be monitored to make the correct determination on whether to proceed with the removal of outer carious dentin inside the peripheral seal zone. Measuring from intact tooth structure with one or two periodontal probes (see Fig 4) is a useful technique to determine when the excavation is into circumpulpal areas (5 to 6 mm from the occlusal surface). If the excavation is into intermediate dentin (3 to 4 mm from the occlusal surface), the caries removal end points with light pink staining can be achieved predictably inside the peripheral seal zone by further excavation of the red outer carious dentin. However, when excavation is near the pulp (> 5 mm from the occlusal surface or > 3 mm from the DEJ) and the caries-detecting dye still stains red, excavation should stop. This protocol will eliminate most pulp exposures (Figs 7 to 9).
Avoiding direct pulp caps has been shown to reduce the need for subsequent endodontic treatment. Conserving more dentin in tooth preparations has also been shown to reduce the incidence of irreversible pulpitis. By eliminating or reducing the surface area and thickness of the nonelastic and deformable outer carious dentin, the performance of a bonded composite under functional loads will also improve.
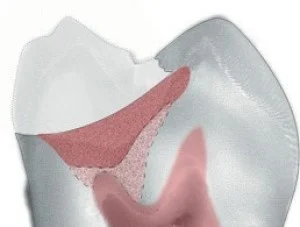
Fig 7. Deep caries lesion showing the outer carious dentin staining red and extending to the circumpulpal dentin ( > 5 mm from the occlusal surface).
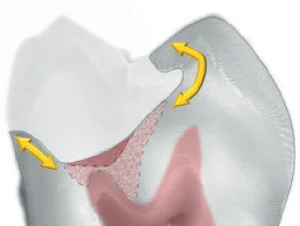
Fig 8. Caries removal end points for a deep lesion. The peripheral seal zone has been created without exposing the pulp. A small amount of outer carious dentin is left on top of the inner carious dentin inside the peripheral seal zone.
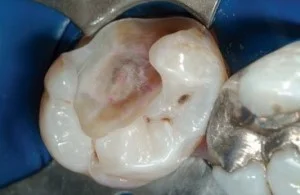
Fig 9. Clinical case illustrating Fig 8. The ideal caries removal end points for highly bonded restorations without pulpal exposure.
The final goal of ideal caries removal end points and peripheral seal zones is to create an adhesive bond that will be preserved for as long as possible. Such a bond to dentin should mimic the strength of a natural tooth. The tensile strength of the DEJ has been measured at 51.5 MPa. Only bonding to sound dentin can achieve and even exceed this tensile bond strength. Using the "gold standards" three-step totaletch or two-step mildly acidic self-etching dentinal bonding systems are the most consistent bonding strategies to obtain these high bond strengths. Adhesive bonding to normal and carious dentin has been studied for the past 15 years at the Medical College of Georgia under the direction of David Pashley. These studies have been continued at many Japanese universities. This research has established the bond strengths of normal and carious dentin. Inner carious dentin loses 25% to 33% of its bondability. Outer carious dentin has a reduction of bondability of over 66%. This reduction in bondability corresponds to the amount of demineralization in the outer and inner carious dentin. The Carisolv chemomechanical technique of caries removal leaves a thin layer of residual outer carious dentin that may reduce the microtensile bond strength (mTBS). This technique can be clinically successful in shallow restorations but is not ideal in larger load-bearing situations.
Simplified two-step total-etch dentinal bonding systems lose 40% to 50% of mTBS when bonded to inner carious dentin. The same decrease in bond strength will occur if acid etching is performed on dentin that is to be bonded with a mild two-step self-etching dentinal bonding system. Dual-cure dentinal bonding systems can have the same negative effect. The acid from caries lesions also activates endogenous collagenase enzymes called matrix metalloproteinases. In the presence of matrix metalloproteinases, a 25% to 30% reduction in bond strength will be observed after (approximately in the first 12 months) restoration placement. A 0.2% to 2.0% chlorhexidine solution will deactivate the matrix metalloproteinases and preserve the maximum bond strength.75-77 Mild selfetching dentinal bonding systems produce an acid/base resistant zone of a 1 to 1.5 micron thickness referred to as "super dentin" because of its ability to withstand high and low pH attacks. SE Protect (Kuraray) with the unique proprietary methacryloyloxydodecylpyridinium bromide monomer containing pyridinium bromide produces this super dentin and also deactivates matrix metalloproteinases. Other mild self-etching dential bonding systems also produce the acid/base resisitant zones but need additional matrix metalloproteinase-deactivating chemicals such as chlorhexidine (Consepsis, Ultradent) or benzalkonium chloride (Micro-Prime B, Danville or Etch-37, Bisco).
The anatomical location of the peripheral seal zone dentin must also be considered to predict potential bond strength. Cervical root dentin loses approximately 20% of its bondability compared with coronal superficial dentin. If the cervical root dentin has inner carious dentin present, the bond strength is only 50% of sound coronal dentin. Deep dentin vs superficial dentin bond strengths are also dependant on the type of dentinal bonding system used. Three-step total-etch and two-step mild self-etching dentinal bonding systems bond equally well to deep dentin, but simplified two-step totaletch and one-step highly acidic self-etching systems can lose up to 50% of their bond strength in deep dentin.
During placement of the restorative material, the ratio of bonded to unbonded surface areas of each layer or increment of composite (the configuration factor or c-factor) will affect the stress of polymerization shrinkage that is applied to the maturing bond to dentin. Higher c-factors always increase stress on the bond to dentin, which decreases its mTBS (unless it is a flowable composite with a low modulus of elasticity compared to dentin). Therefore, high c-factor layering with high modulus composites (thicker than 0.5 mm) should be avoided while the bond to dentin is maturing. This can best be accomplished by using an indirect or semidirect restorative technique. If direct restoration is necessary for socioeconomic reasons, compensatory measures are required to prevent excessive stresses to the bond and remaining hard tissue. This can best be accomplished by multiple thin horizontal layers (which take more time to apply) on a thin layer of flowable composite. A thin (500-micron) microfilled flowable composite or a thick dentinal bonding system adhesive layer (50 to 80 microns) can secure the dentin bond and create a fail-safe layer. Such a resin coating will stay bonded even when overlaying layers fail under high stress. In shallow preparations in superficial dentin, the detrimental effect of resin shrinkage is not as great because the c-factor is reduced. Polyethylene fiber nets used to line high c-factor preparations have also been shown to reduc effects of polymerization stress and cer microleakage. If c-factor stresses are not reduced, the bond strength is decreased by 30% to 50% during the first 24 hours and by another 10% during functional loading in the first years of service. Careful operators who take all of these considerations into account during caries excavation and bonding procedures can decrease the array of differences in regional bond strengths in their restorations.
TREATMENT GOALS FOR DEEP CARIES LESIONS
Create a peripheral seal zone of enamel, DEJ, and normal superficial dentin near the DEJ (this should bond at 55 MPa) (Figs 10 and 11).
Leave the inner carious dentin inside of the peripheral seal zone (this should bond at 30 MPa) (compare Figs 2 and 3 with Figs 10 and 11 ).
Remove highly infected outer carious dentin inside of the peripheral seal zone without exposing the pulp. Small areas of circumpulpal outer carious dentin are left to prevent exposure (see Figs 7 to 9).
Seal in and deactivate any remaining bacteria left inside the peripheral seal zone.
Use adhesive restorative techniques that will maximize the bond strength of the peripheral seal zone and the inner carious affected dentin inside the peripheral seal zone.
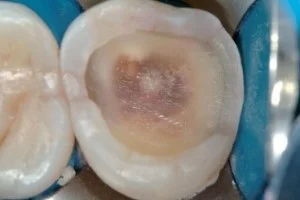
Fig 10. Ideal caries removal end points and peripheral seal zone developed in an intermediate-depth lesion using combined technologies.
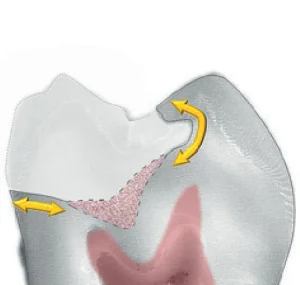
Fig 11. The peripheral seal zone is free of outer and inner carious dentin. Inside the peripheral seal zone, the lightly stained inner carious dentin is retained and will remineralize in vital teeth.
STEP-BY-STEP TECHNIQUE
Test for pulpal vitality with ice or aerosol refrigerant Endo-Ice (ColteneWhaledent). If the test is positive, proceed with caries diagnosis and treatment. If the test is ambiguous or negative, inform the patient of the possible need for endodontic treatment.
Anesthetize the tooth. Isolate it using rubber dam or other isolation techniques.
Access the lesion after removal of any failed restorations. Stain the caries lesion with red caries-detecting dye. Wait 10 seconds and rinse (see Fig 12).
Starting near the DEJ, use a 1-mm round diamond bur of fine to medium grit (30 to 100 microns) to create a peripheral seal zone area free of red-stained outer caries and pink-stained inner caries. This superficial normal dentin will be 1- to 2-mm wide depending on whether it is on the buccal or the occlusal areas of a molar (1 .5 to 2 mm) or on the mesial or distal root dentin (1 mm). Premolars are smaller, and the superficial dentin is narrower in all areas (Figs 10 and 11 ).
Staining and removing outer and inner carious dentin is repeated until the caries removal end point in the peripheral seal zone is stain free. This can be confirmed by DIAGNOdent readings of approximately 12 (see Fig 3) and the total absence of caries-detecting dye. (This indicates virtually bacteria-free superficial dentin.)
Remove the red-stained outer carious dentin from the area inside the peripheral seal zone (being careful to avoid the pulp horn areas). Measure from the occlusal surface to determine if the excavation is in superficial (outer third), intermediate (middle third), or deep (pulpal third) dentin (see Fig 4).
After removing the red and leaving the pink between the pulp horns, the pink inner carious dentin areas in these intermediate dentin areas can be evaluated with DIAGNOdent. The numbers should read approximately 24 (acceptable range, 12 to 36). Those readings indicate a virtually bacteria-free area in the intermediate to deep dentin inside the peripheral seal zone (see Figs 10 and 11).
Move to the deep pulp horn areas last. Carefully remove red-stained outer carious dentin until deep dentin is reached (5 mm from occlusal surface). If the tissue continues to stain red and measurements with the periodontal probe indicate that you are deeper than 5 mm from the occlusal surface(> 3 mm from the DEJ), stop excavation to avoid pulp exposure (compare Figs 4 to 9).
Optional step: Treat the peripheral seal zone, inner carious dentin, and outer carious dentin with 0.2% to 2.0% chlorhexidine for 30 seconds to inactivate both the matrix matalloproteinases and any remaining bacteria; 0.1 % to 1.5% benzalkonium chloride solution in the acid-etch or methacryloyloxydodecylpyridinium bromide monomer in the dentinal bonding system will also achieve these goals.80 If using a three-step total-etch dentinal bonding system, this step is performed after acid etching and rinsing. If using a two-step self-etching dentinal bonding system, after applying chlorhexidine or benzalkonium chloride, dry the preparation for 10 seconds before applying the self-etching primer.
Optional step if using a mild two-step self-etching dentinal bonding system: Use air abrasion on the preparation to maximize the mTBS.
Start dentin bonding with a three-step total-etch or a mild two-step self-etching dentinal bonding system.
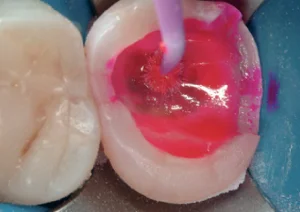 Fig 12. Access the lesion after removal of any failed restorations. Stain the caries lesion with red caries-detecting dye. Wait 10 seconds and rinse
Fig 12. Access the lesion after removal of any failed restorations. Stain the caries lesion with red caries-detecting dye. Wait 10 seconds and rinse
These techniques for caries removal end point determination and peripheral seal zone development are the foundation of conservative dentistry. Such minimally invasive procedures are best performed under magnification. This type of microdentistry is greatly aided by using high-magnification prismatic loupes of 6.5x to 8.0x or with an operatory microscope with similar magnification (Fig 13).

Fig 13. Magnification of 6.5X to 8.0X is ideal for implementing minimally invasive caries removal.
The peripheral seal zone in superficial dentin will allow biomimetic bond strengths of approximately 45-55 MPa to be created.
The intermediate and deeper areas of light pink-stained inner carious dentin will likely generate a dentin bond of 30 MPa. If any outer caries is left in deep circumpulpal areas to prevent pulp from being exposed, the mTBS in those small areas will be approximately 15 MPa. To maximize all of these bond strengths, the dentinal bonding system can be allowed to mature for a certain length of time (3 minutes to 24 hours) before being bonded to another layer of polymerizing resin cement or composite resin. This is why it is important to use the immediate dentin sealing technique whenever possible.
Further information about caries removal and composite restoration you can gain in our course "International schools working with composite restorations".
CONCLUSION
By combining detailed anatomical and pathohistologic knowledge with the technologies of caries-detecting dyes and laser fluorescence, an ideal caries removal end point can be achieved for vital teeth with deep caries lesions. These ideal end points will preserve more vital pulps, conserve more dental hard tissue, and create a highly bondable peripheral seal that will mimic the natural tooth when restored with low stress adhesive techniques.
List of authors:
David Alleman
Pascal Magne
References
1. Fusayama T. A Simple Pain-Free Adhesive Restorative System by Minimal Reduction and Total Etching. Tokyo: lshiyaku EuroAmerica, 1993;1-2.
2. Axelsson P. An Introduction to Risk Prediction and Preventive Dentistry. Chicago: Quintessence, 1999.
3. Axelsson P. Diagnosis and Risk Prediction of Dental Caries. Chicago: Quintessence, 2000.
4. Axelsson P. Preventive Materials, Methods and Programs. Chicago: Quintessence, 2004.
5. Fejerskov 0, Kidd E (eds). Dental Caries: The Disease and its Clinical Management. Oxford, Blackwell Munksgaard, 2003:275-292.
6. Featherstone JD. The caries balance: The basis for caries management by risk assessment. Oral Health Prev Dent 2004;2(suppl):259-264.
7. Young DA, Featherstone JD, Roth JR. Curing the silent epidemic: Caries management in the 21st century and beyond. J Calif Dent Assoc 2007;35:681-685.
8. Ekstrand KR, Martignon S, Ricketts DJ, Qvist V. Detection and activity assessment of primary coronal caries lesions: A methodologic study. Oper Dent 2007;32:225-235.
9. Jenson L, Budenz AW, Featherstone JD, RamosGomez F J, Spolsky VW, Young DA. Clinical protocols for caries management by risk assessment. J Calif Dent Assoc 2007;35:714-723.
10. Paris S, Meyer-Lueckel H, Kielbassa AM. Resin infiltration of natural caries lesions. J Dent Res 2007;86: 662- 666.
11. Milicich G, Rainey JT. Stress distribution in teeth and the significance in operative dentistry. Pract Periodontics Aesthet Dent 2000;12:695-700.
12. Magne P, Oganesyan T. CT scan-based finite elemental analysis of premolar cuspal deflection following operative procedures. Int J Periodontics Restorative Dent 2009;29:361-369.
13. Splieth CH, Ekstrand KR, Alkilzy M, et al. Sealants in dentistry: Outcomes of the ORCA Saturday Afternoon Symposium 2007. Caries Res 2010;44:3-13.
14. Neuhas KW, Ciucchi P, Donnet M, Lussi A. Removal of enamel caries with an air abrasion powder. Oper Dent 2010;35:538-546.
15. Schroeder H. Oral Structural Biology. New York: Thieme Medical Publishers, 1991 :60-72.
16. Neves AA, Coutinho E, Cardoso V, Lambrechts P, Van Meerbeek B. Current concepts and techniques for caries excavation and adhesion to residual dentin. J Ad hes Dent 2011 ;13:7-22.
17. Neves AA, Coutinho E, Cardoso M, de Munck J, VanMeerbeek B. Microtensile bond strength and interfacial characterization of an adhesive bonded to dentin prepared by contemporary caries-excavation techniques. Dent Mater 2011 ;552-562.
18. Neves AA, Coutinho E,de Munck J, Lambrechts P, VanMeerbeek B. Does DIAGNOdent provide a reliable caries removal end point? J Dent 2011 ;39: 351-360.
19. Yazici AR, Baseren M, Gokalp S. The in vitro performance of laser fluorescence and caries-detector dye for detecting residual carious dentin during preparation. Quintessence Int 2005;36:417-422.
20. Shirai K, De Munck J, Yoshida Y, et al. Effect of cavity configuration and aging on the bonding effectiveness of six adhesives to dentin. Dent Mater 2005;21:110-124.
21. Yoshiyama M, Urayama A, Kimochi T, Matsuo T, Pashley D. Comparison of conventional vs selfetching adhesive bonds to caries-affected dentin. Oper Dent 2000;25:163-169.
22. Boston DW, Sauble JE. Evaluation of laser fluorescence for differentiating caries dye-stainable versus caries dye-unstainable dentin in carious lesions. Am J Dent 2005;18:351-354.
23. Krause F, Braun A, Eberhard J, Jepsen S. Laser fluorescence measurements compared to electrical resistance of residual dentin in excavated carvities in vivo. Caries Res 2007;41:135-140.
24. Iwami Y, Shimizu A, Narimatsu M, Hayashi M, Takeshige F, Ebisu S. Relationship between bacterial infection and evaluation using a laser fluorescence device, DIAGNOdent. Eur J Oral Sci 2004;112:419-423.
25. Nakajima M, Ogata M, Okuda M, Tagami J, Snao H, Pashley DH. Bonding to caries-affected dentin using self-etching primers. Am J Dent 1999;12:309-314.
26. Lucci A, Francescut P, Achermann F, Reich E, Hotz P, Megert B. The use of the DIAGNOdent during cavity preparation. Caries Res 2000;34:327-328.
27. Yonemoto K, Eguro T, Maeda T, Tanaka H.Application of DIAGNOdent as a guide for removing carious dentin with Er:YAG laser. J Dent 2006;34:269-276
28. Magne P, Belser U. Bonded Porcelain Restorations in the Anterior Dentition-A Biomimetic Approach. Chicago: Quintessence, 2002:23-55.
29. Magne P. Composite resins and bonded porcelain: The postamalgam era? J Calif Dent Assoc 2006;34: 135-147.
30. Magne P. Esthetic and Biomimetic Restorative Dentistry: Manual for Posterior Esthetic Restorations. Los Angeles: USC School of Dentistry, 2006.
31. Deliperi S, Bardwell D. An alternative method to reduce polymerization shrinkage stress in direct posterior composite restorations. J Am Dent Assoc 2002; 133:1386-1398.
32. Deliperi S, Alleman D. Stress-reducing protocol for direct composite restorations in minimally invasive cavity preparations. Pract Proced Aesthet Dent 2009;21 :e 1-e6.
33. Opdam NJM, Bronkhorst EM, Loomans BAC, Huysmans MCDNJM. 12-year survival of composite vs amalgam restorations. J Dent Res 2010;89:1063-1067.
34. Fusayama T. New Concepts in Operative Dentistry: Differentiating Two Layers of Carious Dentin and Using an Adhesive Resin. Chicago: Quintessence, 1 980:13-59.
35. Ogawa K, Yamashita Y, lchijo T, Fusayama T. The ultrastructure and hardness of the transparent layer of human carious dentin. J Dent Res 1983;62:7-10.
36. Akimoto N, Yokoyama G, Ohmori K, Suzuki S, Koh no A, Cox C. Remineralization across the resin-dentin interface: In vivo evaluation with nanoindentation measurements, EDS, and SEM. Quintessence Int 2001;32:561-570.
37. Massler M. Changing concepts in the treatment of carious lesions. Br Dent J 1 967;123:547-548.
38. Fusayama T. Clinical guide for removing caries using a caries-detecting solution. Quintessence Int 1 988;19:397-401.
39. Anderson MH, Charbeneau GT. A comparison of digital and optical criteria for detecting carious dentin. J Prosthet Dent 1985;53:643-646.
40. McComb D. Caries-detector dyes-How accurate and useful are they? J Can Dent Assoc 2000;66:195-198.
41. Banerjee A, Kidd EAM, Watson TF. In vitro validation of carious dentin removed using different excavation criteria. Am J Dent 2003;16:228-230.
42. Fukushima M. Adhesive resin penetration into carious dentin [in Japanese]. Kokubyo Gakkai Zasshi 1981;48:362-385.
43. Sano H. Relationship between caries detector staining and structural characteristics of carious dentin [in Japanese]. Kukubyo Gakkai Zasshi 1987;54:241-270.
44. Iwami Y, Shimizu, Narimatsu M, Kinomoto Y, Ebisu S. The relationship between the color of carious dentin stained with a caries detector dye and bacterial infection. Oper Dent 2005;30:83-89.
45. Wei S, Sadr A, Shimada Y, Tagami J. Effect of cariesaffected dentin hardness on the shear bond strength of current adhesives. J Ad hes Dent 2008;10:431-440.
46. Boston DW, Graver HT. Histobacteriological analysis of acid red dye-stainable dentin found beneath intact amalgam restorations. Oper Dent 1 994;19: 65-69.
47. Lussi A, Hibst R, Paulus R. DIAGNOdent: An optical method for caries detection. J Dent Res 2004; 83(spec no c):c80-c83.
48. Buchalla W, Attin T, Niedmann Y, Niedman PD, Lennon AM. Porphyrins are the cause of red fluorescence of carious dentine: Verified by gradient reversed-phase HPLC. Caries Res 2008;42:223.
49. Lussi A, lmwinkelried S, Pitts NB, Longbottom C, Reich E. Performance and reproducibility of a laser fluorescence system for detection of occlusal caries in vitro. Caries Res 1999;33:261-266.
50. Lussi A, Megert B, Longbottom C, Reich E, Francescut P. Clinical performance of a laser fluorescence device for detection of occlusal caries lesions. Eur J Oral Sci 2001;109:14-19.
51. Reich E, Berakdar M, Netuschil L, Pitts N, Lussi A. Clinical caries diagnosis compared to DIAGNOdent evaluations. Caries Res 1999;33:299.
52. Brannstrom M. Dentin and Pulp in Restorative Dentistry. London: Wolfe Medical, 1982:93.
53. Boston DW, Liao J. Staining of non-carious human coronal dentin by caries dyes. Oper Dent 2004;29: 280-286.
54. Cortes D, Ellwood R, Ekstrand K. An in vitro comparison of a combined FOTI/visual examination of occlusal caries with other caries diagnostic methods and the effect of stain on their diagnostic performance. Caries Res 2003;37:8-16.
55. ltoh K, Kusunoki M, Oikawa M, Tani C, Hisamitsu H. In vitro comparison of three caries dyes. Am J Dent 2009;22:195-1 99.
56. Boston DW, Jefferies SR, Gaughan JP. The relative location of the dye staining endpoint indicated with polypropylene glycol-based caries dye versus conventional propylene glycol-based caries dye. Eur J Dent 2008;2:29-36.
57. Lennon AM, Attin T, Buchalla W. Quantitiy of remaining bacteria and cavity size after excavation with fluoresence aided caries excavation (FACE), caries detector dye and conventional excavation in vitro. Oper Dent 2007;32:236-241.
58. Thompson V, Craig R, Curro FA, Green WS, Ship JA. Treatment of deep carious lesions by complete excavation or partial removal: A critical review. J Am Dent Assoc 2008;139:705-712.
59. Gruythuysen R, van Strijp G, Wu M. Long-term survival of indirect pulp treatment performed in primary and permanent teeth with clinically diagnosed deep carious lesions. J Endod 2010;36:1490-1493.
60. Casagrande L, Bento LW, Dalpian DM, Garcia-Godoy F, de Araujo FB. Indirect pulp treatment in primary teeth: 4-year results. Am J Dent 2010;23:34-38.
61. Zollner A, Gaengler P. Pulp reactions to different preparation techniques on teeth exhibiting periodontal disease. J Oral Reha bi I 2000;27:93-102.
62. Hevinga MA, Opdam NJ, Frencken JE, Truin GJ, Huysmans MC. Does incomplete caries removal reduce strength of restored teeth? J Dent Res 2010; 89:1270-1275.
63. Urabe I, Nakajima M, Sano H, Tagami J. Physical properties of the dentin-enamel junction region. Am J Dent 2000;13:129-135.
64. De Munck J. An In Vitro and In Vivo Study of the Durability of Biomaterial-Tooth Bonds [thesis]. Belgium: Catholic Universtiy of Leuven, 2004;74-76.
65. Yoshiyama M, Tay F, Torri Y, et al. Resin adhesion to carious dentin. Am J Dent 2003;16:47-52.
66. Yoshiyama M, Tay FR, Doi J, et al. Bonding of selfetch and total-etch adhesives to carious dentin. J Dent Res 2002;81 :556-560.
67. Pugach MK, Strother J, Darling CL, et al. Dentin caries zones: Mineral, structure, and properties. J Dent Res 2009;88:71-76.
68. Albrektsson TO, Bratthall D, Glantz PJ, Lindhe JT (eds). Tissue Preservation in Caries Treatment. London: Quintessence, 2001 :263-274.
69. Yazici A, Atilla P, Ozgunaltay G, Muftuoglu S. In vitro comparison of the efficacy of Carisolv and conventional rotary instruments in caries removal. J Oral Rehab 2003;30:1177-1182.
70. Oikawa M, Kusunoki M, ltoh K, Hisamitsu H. Removal of caries dentin by CarisolvTM system [abstract 3185]. IADR 2009.
71. Zanchi CH, Lund RG, Perrone LR, et al. Microtensile bond strength of two-step etch-and-rinse adhesive systems on sound and artificial caries-affected dentin. Am J Dent 201 O; 23:152-156.
72. Yazici AR, Akca T, Ozgunaltay G, Dayangac B. Bonding strength of a self-etching adhesive system to cariesaffected dentin. Oper Dent 2004;29:176-181.
73. Proenca JP, Polido M, Osorio E, et al. Dentin bond strength of self-etch and total-etch adhesive systems. Dent Mater 2007;23:1542-1548.
74. Say EC, Nakajima M, Senawongse P, Sayman M, Ozer F, Tagami J. Bonding to sound vs caries-affected dentin using photo-and dual-cure adhesives. Oper Dent 2005;30:90-98.
75. Pashley DH, Tay FR, Yiu C, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res 2004;83:216-221 .
76. Hebling J, Pashley DH, Tjaderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res 2005;84: 741-746 [erratum 2006;85:34].
77. Breschi L, Mazzoni A, Nata F, et al. Chlohexidine stabilizes the adhesive interface: A 2-year in vitro study. Dent Mater 2010;26:320-325.
78. Nikaido T, Weerasinghe D, Waidyasekera K, Inoue G, Foxton R, Tagami J. Assessment of the nanostructure of acid-base resistant zone by the application of all-in-one adhesive systems: Super dentin formation. Bio-Med Mater Eng 2009;19:163-171.
79. Donmez N, Belli 5, Pashley DH, Tay FR. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res 2005;84:355-359.
80. Tezvergil-Mutluay A, Murat Mutluay M, Gu L, et al. The anti-MMP activity of benzalkonium chloride. J Dent 2011;38:57-64.
81. Doi J, ltota T, Yoshiyama M, Tay FR, Pashley DH. Bonding to root caries by a self-etching adhesive system containing MDPB. Am J Dent 2004;17:89-93.
82. Nakajima M, Sano H, Zheng L, Tagami J, Pashley DH. Effect of moist vs dry bonding to normal vs cariesaffected dentin with Scotchbond Multi-Purpose Plus. J Dent Res 1999;78:1298-1303.
83. Feilzer AJ, De Gee AJ, Davidson CL. Setting stress in composite resin in relation to configuration of the restoration. J Dent Res 1987;66:1636-1639.
84. Yoshikawa T, Sano H, Burrow MF, Tagami J, Pashley DH. Effects of dentin depth and cavity configuration on bond strength. J Dent Res 1999;78:898-905.
85. Kleverlaan CJ, Feilzer AJ. Polymerization shrinkage and contraction stress of dental resin composites. Dent Mater 2005;21 :1150-1157.
86. Iida K, lnokoshi 5, Kurosaki N. lnterfacial gaps following ceramic inlay cementation vs direct composites. Oper Dent 2003;28:445-452.
87. Nikolaenko SA, Lohybauer U, Roggendorf M '. A, Dasch W, Frankenberger R. Influence o c- ctor and layering technique on micro ten strength to dentin. Dent Mater 2004;20:579-
88. Jayasooriya PR, Pereira PNR, Nikaido T, Tagami J. Efficacy of a resin coating on bond strengths of resin cement to dentin. J Esthet Restor Dent 2003;15: 105-113.
89. Magne P, So W, Cascione D. Immediate dentin sealing supports delayed restoration placement. J Prosthet Dent 2007;98:166-174.
90. He Z, Shimada Y, Sadr A, Ikeda M, Tagami J. The effects of cavity size and filling method on the bonding to Class I cavities. J Ad hes Dent 2008;10:447-453.
91. Schmidlin PR, Huber T, Gohring TN, Attin T, Bindl A. Effects of total and selective bonding on marginal adaptation and microleakage of Class I resin composite restorations in vivo. Oper Dent 2008;33:629-635.
92. Belli S, Donmez N, Eskitascioglu G. The effect of c-factor and flowable resin or fiber use at the interface on microtensile bond strength to dentin. J Adhes Dent 2006;8:247-253.
93. EI-Mowafy 0, EI-Badrawy W, Eltanty A, Abbasi K, Habib N. Gingival microleakage of Class II resin composite restoration with fiber inserts. Oper Dent 2007;32:298-305.
94. Nikaido T, Kunzelmann K-H, Chen H, et al. Evaluation of thermal cycling and mechanical loading on bond strength of a self-etching primer system to dentin. Dent Mater 2002;18:269-275.
95. Shona Y, Ogawa T, Terashita M, Carvalho RM, Pashley EL, Pashley DH. Regional measurement of resin-dentin bonding as an array. J Dent Res 1999; 78:699-705.
96. Toledano M, Osorio R, Moreira MAG, et al. Effect of the hydration status of the smear layer on the wettability and bond strength of a self-etching primer to dentin. Am J Dent 2004;17:310-314.
97. Van Meerbeek B, De Munck J, Mattar D, Van Landuyt K, Lambrechts P. Microtensile bond strengths of an etch and rinse and self-etch adhesive to enamel and dentin as a function of surface treatment. Oper Dent 2003;28:647-660.
98. Dietschi D, Monasevic M, Krejci I, Davidson C. Marginal and internal adaptation of Class II restorations after immediate or delayed composite placement. J Dent 2002;30:259-269.
99. Asaka Y, Miyazaki M, Takamizawa T, Tsubota K, Moore BK. Influence of delayed placement of composites over cured adhesives on dentin bond strength of single-application self-etch systems. Oper Dent 2006;31 :18-24.
100. Magne P, Kim TH, Cascione D, Donovan TE. Immediate dentin sealing improves bond strength of indirect restorations. J Prosthet Dent 2005;94:511-519.
101. Dietschi D. Evaluation of Marginal and Internal Adaptation of Adhesive Class II Restorations: In Vitro Fatigue Tests [thesis]. Amsterdam: Academic Center for Dentistry of the University of Amsterdam and the Vrije University, 2003:35-54, 75-94.
Video
OHI-S
02 April 2024
Video
OHI-S
16 January 2024
Video
OHI-S
21 November 2023


