Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars
Abstract
Aim: To evaluate the efficacy of four final irrigation protocols on the reduction of hard-tissue debris accumulated within the mesial root canal system of mandibular first molars using micro-CT analysis.
Methodology: Forty mesial roots of mandibular molars with a single and continuous isthmus connecting the mesiobuccal and mesiolingual canals (Vertucci’s Type I configuration) were selected and scanned at a resolution of 8.6 μm. Canals were enlarged sequentially using WaveOne Small and Primary instruments activated in reciprocating motion without intracanal irrigation to allow debris to accumulate within the mesial root canal system. Then, specimens were anatomically matched and distributed into four groups (n = 10), according to the final irrigation protocol: apical positive pressure (APP), passive ultrasonic irrigation (PUI), Self-adjusting File (SAF) and XP-endo Finisher (XPF). The final irrigation procedures were performed over 2 min using a total of 5.5 mL of 2.5% NaOCl per canal. Reconstructed data sets were coregistered, and the mean percentage reduction of accumulated hard-tissue debris after the final irrigation procedures was compared statistically between groups using the ANOVA post hoc Tukey test with a significance level set at 5%.
Results: Reduction of accumulated hard-tissue debris was observed in all groups after the final irrigation protocol. Overall, PUI and XPF groups had higher mean percentage reductions of accumulated hard-tissue debris (94.1% and 89.7%, respectively) than APP and SAF groups (45.7% and 41.3%, respectively) (P < 0.05). No significant differences were found when comparing the results of PUI and XPF groups (P > 0.05) or APP and SAF groups (P > 0.05).
Conclusions: The PUI technique and XP-endo Finisher instrument were associated with significantly lower levels of AHTD compared with conventional irrigation and the modified SAF system protocol in mesial root canals of mandibular molars.
Introduction
During biomechanical preparation of the root canal system, the irrigating solution acts as a disinfectant, lubricant and cleaning agent, helping to eliminate tissue debris created by the cutting action of instruments on dentine, and neutralizing microbes and their by-products (Siqueira et al. 2013). Standard irrigation uses a needle adapted to a syringe associated with apical positive pressure. In this approach, the tip of the needle must be positioned 1–2 mm from the working length, and irrigation performed with large volumes and frequent exchange of irrigants to improve disinfection (Gu et al. 2009). Although it allows good irrigant control, conventional syringe irrigation has been reported to be ineffective in flushing out tissue remnants and cleaning the most apical portions of the root canal system (Thomas et al. 2014).
To overcome the limitations of conventional irrigation, several techniques have been proposed. Amongst them, activation of irrigants via sonic, ultrasonic or laser devices has been associated with improvement in the cleaning and disinfection of the root canal system (Gu et al. 2009, Haapasalo et al. 2014, Nusstein 2015). Passive ultrasonic irrigation (PUI) is the activation of an irrigant in the root canal using ultrasonically oscillating small files (Weller et al. 1980) or smooth noncutting wires (van der Sluis et al. 2005a) following the completion of canal preparation. Effectiveness of PUI to remove tissues and debris has been studied extensively (Nusstein 2015). Overall, PUI has been reported to be more effective than conventional needle-delivered irrigation (Paqué et al. 2011, Haapasalo et al. 2014). A new approach for root canal shaping and cleaning has emerged with the development of a hollow, cylinder-like, nickel–titanium motor-driven Self-adjusting File system (SAF; ReDent-Nova, Ra’anana, Israel). Its unique design allows simultaneous and continuous irrigation throughout the mechanical preparation of the canal (Metzgeret al. 2010), and SAF has been shown to be efficient as a potential irrigation adjunct for debris removal after root canal preparation (Dietrich et al. 2012, Paqué et al. 2012a).
Recently, a size 25 universal nontapered nickel–titanium-based instrument (XP-endo Finisher; FKG, La Chaux-de-Fonds, Switzerland) was launched. XP-endo Finisher is made of a proprietary alloy (Martensite-Austenite Electropolish-FleX) that reacts at different temperature levels. When it is cooled, the instrument is straight (M-phase), but when exposed to the body temperature, it changes its shape to the A-phase, which allows the instrument to expand its reach to 6 mm in diameter or 100-fold of an equivalent sized file when in rotation motion (Trope & Debelian 2015). According to the manufacturer, this inherent feature gives the instrument high flexibility that would help to remove packed debris from the complexities of the root canal system, while limiting the impact on dentine (FKG 2015).
Traditionally, debridement of root canals has been evaluated by means of histology, scanning electron microscopy and sectioning methods (Haapasalo et al. 2014). Hard-tissue debris accumulated after different biomechanical protocols has also been quantified using microcomputed tomography (micro-CT) (Paqué et al. 2009, 2011, 2012a,b, Robinson et al. 2012, 2013, De-Deus et al. 2014, 2015, Freire et al. 2015). Even though there is accumulating evidence on the efficacy of several irrigating techniques using conventional methodologies, a comprehensive knowledge regarding the activation of irrigants in different final irrigation protocols, aiming to remove hard-tissue debris from the isthmus area by means of micro-CT technology is still lacking. Thus, the purpose of this ex vivo study was to evaluate the percentage reduction of accumulated hard-tissue debris (AHTD) in isthmus-containing mesial root canals of mandibular molars using different final irrigation protocols. The null hypothesis was that there is no difference in the reduction of AHTD amongst the four irrigation protocols tested.
Material and methods
Sample size estimation
Sample size was calculated after the effect size estimation of the percentage volume of AHTD as reported by Paqué et al. (2012a). In that study, the percentage volume of AHTD after SAF preparation was 1.7%. An a priori ANOVA (fixed effects, omnibus, one-way) was selected from the F-tests family in G*Power 3.1.7 software for Macintosh (Heinrich Heine, Universität Düsseldorf, Dusseldorf, Germany). Nine samples per group were indicated as the ideal size required for observing the same effect of instruments over dentine with an alpha-type error <0.05 and power beta of 99%.
Tooth selection
After ethics committee approval (protocol 0072.0.138.000-09), forty mandibular first molars that had a mesial root with a fully formed apex, mild curvature (15°–20°) in both mesiodistal and buccolingual directions (Schneider 1971), and two mesial canals connected by a single and continuous isthmus that joined together in the apical third to exit in a single foramen (Vertucci’s Type I configuration) were selected. To prevent the introduction of confounding variables, teeth were decoronated ~3 mm above the cemento-enamel junction. Then, mesial roots were mounted on a custom attachment and imaged separately at an isotropic resolution of 8.6 μm using a micro-CT device (SkyScan 1176; Bruker-microCT, Kontich, Belgium). The scanner parameters were set at 90 kV, 278 μA, 180° rotation around the vertical axis, and rotation step of 0.4°, using a 0.1-mm-thick copper filter.
Root canal preparation
Mesial canals were accessed and canal patency con- firmed with a size 10 K-file (Dentsply Maillefer, Ballaigues, Switzerland). When the tip of the instrument was visible through the main foramen, 0.5 mm was subtracted to determine working length (WL). Then, the apical foramen of each root was sealed with fast set epoxy resin to create a closed-end system. Coronal flaring was performed using Gates-Glidden drills 2 and 3 (Dentsply Maillefer), and glide path was achieved to WL using ProGlider instrument (0.16 mm tip diameter; Dentsply Maillefer). Canals were sequentially enlarged using WaveOne Small (size 21, .06 taper over the first 3 mm from apical tip) and Primary (size 25, .08 taper over the first 3 mm from apical tip) instruments (Dentsply Maillefer) activated in reciprocating motion (VDW Silver motor; VDW GmbH, Munich, Germany) until they reached the WL. To allow debris to accumulate into the isthmus area, irrigation and aspiration throughout the preparation procedures were carried out only at the orifice level with a total of 5 mL of distilled water per canal using a 30-gauge NaviTip needle (Ultradent, South Jordan, UT, USA) adapted to a disposable plastic syringe. Then, each canal was slightly dried with one absorbent paper point (WaveOne Small; Dentsply Maillefer) and the roots submitted to a new scan, applying the parameter settings previously mentioned.
The acquired projection images were reconstructed into cross-section slices with NRecon v.1.6.9 software (Bruker-microCT) with a beam hardening correction of 15%, smoothing of five, ring artefact correction of seven and an attenuation coefficient ranging from 0.00007 to 0.025, resulting in the acquisition of 1800–1900 transverse cross-sections per root. The volume of interest was selected extending from the furcation level to the apex of the mesial root, set by integration of all cross-sections. For the purpose of this study, the region of interest in each slice comprised the area of the mesial canals and the isthmus. Pre- and postoperative 3D models of the mesial canals were rendered (CTVol v.2.2.1; Bruker-microCT) and coregistered with their respective preoperative data sets, using the rigid registration module of the 3D Slicer 4.3.1 software (available from http://www.slicer.-org). Then, matched images were examined to calculate volume (in mm3) and surface area (in mm2) of the mesial root canal system, before and after preparation using CTAn v.1.14.4 software (Bruker micro-CT).
For the quantitative analysis of AHTD, the label masks of the registered data sets of each tooth were imported into the Fiji software (Fiji v.1.47n; Madison, WI, USA) and normalized. The sequence of images resulting from this operation was further used to identify the AHTD by means of morphologic operations. Quantification of AHTD was performed by the difference between nonprepared and prepared root canal space using post-processing procedures. The presence of a material with density similar to dentine in regions previously occupied by air in the nonprepared root canal space was considered debris and quantified by intersection between images before and after canal instrumentation (Paqué et al. 2009, Robinson et al. 2012, De-Deus et al. 2014). The total volume of AHTD was calculated in cubic millimetre (mm3) and expressed as the percentage of the total canal system volume after preparation (vol %).
Final irrigation protocols
Aiming to enhance the internal validity of the experiment, the mesial root canals were matched to create 10 groups of four based on the morphological aspects of the root canal system (configuration, length, volume and surface area) and in the vol% of AHTD after preparation. Then, one root from each group was randomly assigned to one of the four experimental groups (n = 10), according to the final irrigation protocol, which was completed within 2 min using a total of 5.5 mL of 2.5% NaOCl per canal by an experienced and previously trained operator:
- Apical positive pressure (APP group): A total of 0.5 mL of 2.5% NaOCl was flushed into the canal using conventional needle/syringe technique and left stand for 1 min. Then, irrigation was performed with 5 mL of 2.5% NaOCl delivered during a 1-min interval using a 30-gauge NaviTip needle (Ultradent, South Jordan, UT, USA) adapted to a disposable plastic syringe placed up to 2 mm short of the WL, with a gentle in-and-out movement.
- Passive ultrasonic irrigation (PUI group): A total of 0.5 mL of 2.5% NaOCl was flushed into the canal and ultrasonically agitated with an E1 Irrisonic tip (0.20 mm in diameter; Helse Dental Technology, São Paulo, Brazil) mounted on a piezoelectric ultrasonic unit (Piezon 150, Electron Medical Systems, Nyon, Switzerland), with the power setting at 10% (30 Hz). The tip was placed 2 mm coronal to the WL, and an up-and-down motion without touching the walls was applied for 20 s with intermittent flux. The canals were then flushed with 1.67 mL of 2.5% NaOCl and activated for another 20 s. This latter procedure was repeated, and a final irrigation was performed with 1.67 mL of 2.5% NaOCl. A total of 5 mL of 2.5% NaOCl was used per canal during a 1-min activation time (three cycles of 20 s). Replenishment of the irrigant was performed using conventional syringe/ needle irrigation, as in the APP group.
- Self-adjusting File (SAF group): A total of 0.5 mL of 2.5% NaOCl was flushed into the canal using a conventional needle/syringe technique and left to stand for 1 min. Then, a 1.5-mm diameter SAF instrument (ReDent-Nova) was inserted into the root canal and operated to WL with an in-and-out motion using a vibrating handpiece (GentlePower Lux 20LP; KaVo, Biberach, Germany) combined with a RDT3 head (ReDent-Nova). In this group, a modified protocol was used with continuous irrigation of 2.5% NaOCl (5 mL) applied for 1 min in each canal using a special irrigation apparatus (VATEA, ReDent-Nova).
- XP-endo Finisher (XPF group): an XP-endo Finisher instrument was placed in a contra-angle hand-piece (VDW GmbH), cooled down (Endo-Frost; Roeko, Langenau, Germany) and removed from the plastic tube in rotation mode by applying a lateral movement. Each canal was filled with 0.5 mL of 2.5% NaOCl, and the XP-endo Finisher was inserted into it without rotation. Then, rotation was turned on (800 rpm; torque of 1 N.cm) and the instrument was activated for 1 min using slow and gentle 7–8 mm lengthwise movements to the full length of each canal. After that, the XP-endo Finisher instrument was removed from the canal and the final irrigation protocol performed with 5 mL of 2.5% NaOCl using syringe/needle irrigation, as in the APP group.
After completion of the final irrigation procedures, the solution was aspirated at the level of coronal orifice and each root canal was slightly dried with one absorbent paper point (WaveOne Small; Dentsply Maillefer). A final micro-CT scanning was performed, data sets were registered with their respective post-preparation counterparts, and the vol% of AHTD in each canal was calculated. Then, percentage reduction (red%) of the AHTD was calculated according to the formula: 100—[(VAF 9 100)/VBF], where VBF and VAF are the volume of AHTD before and after the irrigation protocol, respectively. An examiner blinded to the final irrigation protocol used in each specimen performed all the measurements.
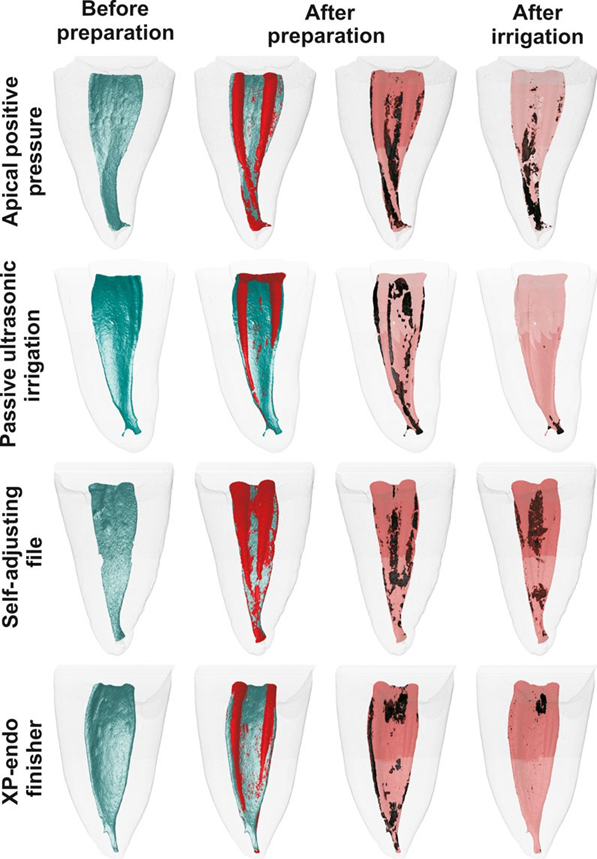
Matched colour-coded root canal models (green and red colours indicating preand postoperative canal surfaces, respectively) and debris (in black colour) enabled qualitative comparison of the distribution of the AHTD in each portion of the root canals, before and after the experimental procedures.
Statistical analysis
Data were normally distributed (Shapiro–Wilk test) and homoscedastic (Levene test). Therefore, results were expressed as means and standard deviations and compared between groups using one-way ANOVA post hoc Tukey test, with a significance level set at 5% (SPSS v17.0; SPSS Inc., Chicago, IL, USA).
Results
Mean volume, surface area and AHTD evaluated before and after root canal preparation and irrigation are detailed in Table 1. Pre- and postoperatively, the degree of homogeneity (baseline) of the groups was confirmed regarding length, volume and surface area of the root canals, as well as, the vol% of AHTD after preparation (P > 0.05).
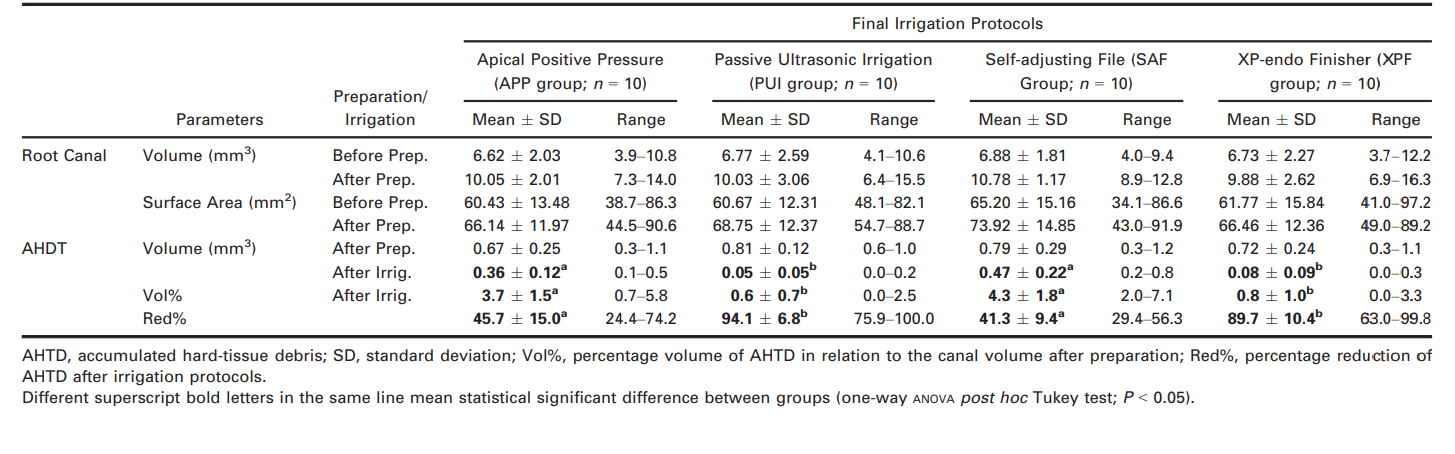
Reduction of AHTD was observed in all groups after the final irrigation protocol. Overall, PUI and XPF groups were associated with greater mean percentage reductions of AHTD (94.1% and 89.7%, respectively) than APP and SAF groups (45.7% and 41.3%, respectively) (P < 0.05). No significant differences were found when comparing the results of PUI and XPF groups (P > 0.05) or APP and SAF groups (P > 0.05). Therefore, the null hypothesis was rejected.
Three-dimensional models of representative mesial root canals show the distribution of the AHTD after preparation and irrigation protocols (Fig. 1). In general, residual debris after final irrigation protocols in the PUI and XPF groups was located at the apical and coronal thirds, respectively, while in the APP and SAF groups debris was commonly observed in both middle and apical thirds.
Discussion
An isthmus has been defined as a narrow, ribbon-shaped communication between two root canals that contains pulp or pulpally derived tissue. In a review of 15 studies, the presence of isthmus communications in the mesial roots of 1615 mandibular first molars averaged 54.8% (de Pablo et al. 2010). In clinical practice, this anatomical variation has been considered one of the most difficult challenges for proper cleaning and disinfection because mechanical instrumentation of this area is unfeasible (Siqueira et al. 2013). Thus, isthmus-containing mesial roots of mandibular molars were selected for this study and specimens were properly matched to reduce the potentially significant anatomical biases that could interfere with the results (Peters et al. 2001, Versiani et al. 2013).
Although nondestructive micro-CT imaging analysis cannot directly evaluate remaining soft tissue or biofilm in the canal, it allows for a three-dimensional quantitative and qualitative analysis of AHTD that is not removed but rather transported into recesses during root canal preparation (Paqué et al. 2009). In infected canals, AHTD may contain bacteria and serve as a nidus for root canal reinfection (Versiani et al. 2015). Besides, it has been claimed that AHTD in the isthmus area may potentially interfere with disinfection by preventing irrigant flow and neutralizing the antibacterial effects of the irrigating solution (Paqué et al. 2012a). Therefore, improving irrigation is conceivable the best way to prevent the formation or remove accumulated debris (Gu et al. 2009, Haapasalo et al. 2014).
The delivery of the irrigant solution has been traditionally achieved using syringe and needle irrigation, and most publications that aimed to evaluate new irrigation techniques use this approach as a control (Gu et al. 2009, Haapasalo et al. 2014). Even though some authors have recommended relatively high flow rates (~0.25 mL s—1) in positive irrigation protocols (Boutsioukis et al. 2007, Khan et al. 2013), in a recent ex vivo study, a flow rate of 4 mL min—1 (or 0.066 mL s—1) using a small-gauge needle positioned 3 mm from the working length, achieved the maximum effectiveness in the irrigant replenishment (Parket al. 2013). In the present study, conventional syringe irrigation was applied with an open-ended needle placed 2 mm short of working length at a flow rate of 0.083 mL s—1 and was able to reduce the percentage volume of AHTD by 45.7%. This result is corroborated by previous studies in which syringe irrigation was unable to remove AHTD or soft tissue remnants from the isthmus area of the mesial root canal system of mandibular molars (Adcock et al. 2011, Endal et al. 2011, Paquéet al. 2011).
Few studies have attempted to use micro-CT technology to investigate the reduction of AHTD in isthmus-containing mesial roots of mandibular molars (Paqué et al. 2011, 2012a,b, Freire et al. 2015, Versiani et al. 2015). Overall, supplementary irrigation procedures after root canal preparation with chelating agents (Paqué et al. 2011, 2012a,b), ultrasonic agitation (Paqué et al. 2011, Freire et al. 2015) and the EndoVac system (Freire et al. 2015, Versiani et al. 2015) have resulted in less AHTD. Clinically, these results can be translated into improved cleanliness in areas within the root canal system generally untouched by instruments during preparation procedures (Nusstein 2015).
In the present study, the highest mean percentage reduction of AHTD was observed after supplementary protocols with PUI (94.1%; range: 75.9–100%) and the novel XP-endo Finisher instrument (89.7%; range: 63.0–99.8%), compared to SAF (41.3%; range: 29.4–56.3%) and conventional irrigation (45.7%; range: 24.4–74.2%) protocols. Previous studies have reported a significant reduction in debris following the use of PUI when compared to needle irrigation (Lee et al. 2004a,b, van der Sluis et al. 2005a,b, 2006, 2007), which is in accordance with the present results. The efficiency of PUI-activated irrigation has been explained because of the production of acoustic microwaves, cavitation and heat generation, favouring the removal of tissue remnants and dentinal debris (Nusstein 2015). However, only two studies using micro-CT technology evaluated the impact of PUI on cleaning noninstrumented recesses of the root canal system. In these studies, Paqué et al. (2011) reported an overall debris reduction after EDTA irrigation and PUI of 50.8 18.7%, while Freire et al. (2015) reported that the use of PUI for irrigant activation led to a 55.55% decrease in the percentage of debris volume. Therefore, half of the debris accumulated during instrumentation could not be removed by the subsequent irrigating steps with PUI, which is disagreement with the present results (94.1%). This higher percentage value compared to those previously reported (Paqué et al. 2011, Freire et al. 2015) might be explained as the consequence of differences in the methodological design including the type of root canal configuration, preparation protocol (apical size and taper), irrigant solution (concentration, volume and flow rate), ultrasonic approach (tip type, activation time and power setting) and the %vol of AHTD after root canal preparation. Additionally, unlike these studies, no chelating agent or intracanal aspiration of irrigant solution was used to avoid the introduction of confounding factors.
To date, only one study attempted to quantify AHTD after using the SAF system in isthmus-containing mesial roots of mandibular molars (Paqu´e et al. 2012a). They reported that only 1.7% of the total canal volume was filled with hard-tissue debris after preparation with SAF. This value is lower than in this study (4.3%) and might be explained by differences in the methodological approach. Paqu´e et al. (2012a) used the recommended protocol for SAF system to prepare Type II mesial canals with a continuous flow of 3% NaOCl delivered at 4 mL min—1 for 4 min, while herein a modified protocol was used (5 mL of 2.5% NaOCl for 1 min) in previously prepared Type I mesial canals. It is worth mentioning that the SAF’s protocol was changed to reflect the clinical conditions under which postoperative procedures are performed, as recommended by the XP-endo Finisher and PUI techniques. It explains the lower performance of the SAF system in comparison with the other agitation protocols, and further studies using its full irrigation protocol with this methodological approach are needed. In the XPF group, the percentage reduction of AHTD (89.7%) was statistically similar to the PUI (94.1%). This may be explained because of the highly flexible proprietary alloy combined with the small core size and zero taper of the XP-endo Finisher instrument, which allowed it to expand its reach when in rotation (FKG 2015, Trope & Debelian 2015). It is plausible to infer that this unique property promoted the agitation of the irrigant solution allowing the disruption of the hard tissue accumulated within the isthmus area and its removal by the final flushing action of the syringe/needle irrigation, similarly to PUI. However, qualitative analysis demonstrated that the XP-endo Finisher instrument was the most effective technique on the removal of AHTD in the apical third.
Although final irrigation protocols with PUI and XP-endo Finisher instruments resulted in significantly less mean percentage volume of AHTD (0.6% and 0.8%, respectively) compared to conventional irrigation and SAF system (3.7% and 4.3%, respectively), this result must be interpreted with caution because this is only one indicator for the assessment of the quality of root canal debridement. Besides, the clinical relevance of AHTD remains unclear and further studies are necessary to evaluate its impact on the success rate of the root canal treatment in isthmus-containing canal system. Considering that none of the irrigation protocols tested so far was able to render root canals free of AHTD (Paqué et al. 2011, 2012a,b, Freire et al. 2015, Versiani et al. 2015) and the high success rate of the root canal treatment (Su et al. 2011), it may be hypothesized that there is a threshold of AHTD within the root canal system below which a favourable host response is expected. Then, maybe the differences amongst the irrigant protocols tested herein regarding AHDT reduction are unlikely to be of clinical significance. Obviously, further studies are necessary to evaluate the reduction of AHTD using complex canal anatomies with different delivery systems, volume, flow and type of irrigation agents, as well as, depth of insertion of different irrigation needles, ultrasonic tips and suction cannulas. Even though it is difficult to draw reliable conclusions from the literature because of the differences in methodological designs, a general agreement exists about the benefits of using irrigant activation at the end of the canal preparation (Nusstein 2015).
Conclusions
PUI and XP-endo Finisher instruments succeeded in rendering the mesial root canal system with significantly lower levels of AHTD compared with conventional irrigation and the modified SAF system protocol.
Authors: G. B. Leoni, M. A. Versiani, Y. T. Silva-Sousa, J. F. B. Bruniera, J. D. Pécora, M. D. Sousa-Neto
References:
- Adcock JM, Sidow SJ, Looney SW et al. (2011) Histologic evaluation of canal and isthmus debridement efficacies of two different irrigant delivery techniques in a closed system. Journal of Endodontics 37, 544–8.
- Boutsioukis C, Lambrianidis T, Kastrinakis E, BekiaroglouP (2007) Measurement of pressure and flow rates during irrigation of a root canal ex vivo with three endodontic needles. International Endodontic Journal 40, 504–13.
- De-Deus G, Roter J, Reis C et al. (2014) Assessing accumulated hard-tissue debris using micro-computed tomography and free software for image processing and analysis. Journal of Endodontics 40, 271–6.
- De-Deus G, Marins J, Silva EJ et al. (2015) Accumulated hard-tissue debris produced during reciprocating and rotary nickel-titanium canal preparation. Journal of Endodontics 41, 676–81.
- Dietrich MA, Kirkpatrick TC, Yaccino JM (2012) In vitro canal and isthmus debris removal of the self-adjusting file, K3, and WaveOne files in the mesial root of human mandibular molars. Journal of Endodontics 38, 1140–4.
- Endal U, Shen Y, Knut A, Gao Y, Haapasalo M (2011) A high-resolution computed tomographic study of changes in root canal isthmus area by instrumentation and root filling. Journal of Endodontics 37, 223–7.
- FKG (2015) XP-Endo Finisher Technical Guide. Available at http://www.fkg.ch/sites/default/files/fkg_xp_endo_brochure_en_vb.pdf. Switzerland: FKG, La Chaux-de-Fonds, pp. 1–16. Accessed July 24, 2015.
- Freire LG, Iglecias EF, Cunha RS, dos Santos M, Gavini G (2015) Micro-computed tomographic evaluation of hard tissue debris removal after different irrigation methods and its influence on the filling of curved canals. Journal of Endodontics 41, 1660–6.
- Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR (2009) Review of contemporary irrigant agitation techniques and devices. Journal of Endodontics 35, 791–804.
- Haapasalo M, Shen Y, Wang Z, Gao Y (2014) Irrigation in endodontics. British Dental Journal 216, 299–303.
- Khan S, Niu LN, Eid AA et al. (2013) Periapical pressures developed by nonbinding irrigation needles at various irrigation delivery rates. Journal of Endodontics 39, 529–33.
- Lee SJ, Wu MK, Wesselink PR (2004a) The effectiveness of syringe irrigation and ultrasonics to remove debris from simulated irregularities within prepared root canal walls. International Endodontic Journal 37, 672–8.
- Lee SJ, Wu MK, Wesselink PR (2004b) The efficacy of ultra-sonic irrigation to remove artificially placed dentine debris from different-sized simulated plastic root canals. International Endodontic Journal 37, 607–12.
- Metzger Z, Teperovich E, Zary R, Cohen R, Hof R (2010) The self-adjusting file (SAF). Part 1: respecting the root canal anatomy - a new concept of endodontic files and its implementation. Journal of Endodontics 36, 679–90.
- Nusstein JM (2015) Sonic and ultrasonic irrigation. In: Bettina B, ed. Endodontic Irrigation: Chemical Disinfection of the Root Canal System. Switzerland: Springer, pp 173–98.
- de Pablo OV, Estevez R, Peix Sanchez M, Heilborn C, Cohenca N (2010) Root anatomy and canal configuration of the permanent mandibular first molar: a systematic review. Journal of Endodontics 36, 1919–31.
- Paqué F, Laib A, Gautschi H, Zehnder M (2009) Hard-tissue debris accumulation analysis by high-resolution computed tomography scans. Journal of Endodontics 35, 1044–7.
- Paqué F, Boessler C, Zehnder M (2011) Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. International Endodontic Journal 44, 148–53.
- Paqué F, Al-Jadaa A, Kfir A (2012a) Hard-tissue debris accumulation created by conventional rotary versus self-adjusting file instrumentation in mesial root canal systems of mandibular molars. International Endodontic Journal 45, 413–8.
- Paqué F, Rechenberg DK, Zehnder M (2012b) Reduction of hard-tissue debris accumulation during rotary root canal instrumentation by etidronic acid in a sodium hypochlorite irrigant. Journal of Endodontics 38, 692–5.
- Park E, Shen Y, Khakpour M, Haapasalo M (2013) Apical pressure and extent of irrigant flow beyond the needle tip during positive-pressure irrigation in an in vitro root canal model. Journal of Endodontics 39, 511–5.
- Peters OA, Laib A, Gohring TN, Barbakow F (2001) Changes in root canal geometry after preparation assessed by high-resolution computed tomography. Journal of Endodontics 27, 1–6.
- Robinson JP, Lumley PJ, Claridge E et al. (2012) An analytical Micro-CT methodology for quantifying inorganic dentine debris following internal tooth preparation. Journal of Dentistry 40, 999–1005.
- Robinson JP, Lumley PJ, Cooper PR, Grover LM, Walmsley AD (2013) Reciprocating root canal technique induces greater debris accumulation than a continuous rotary technique as assessed by 3-dimensional micro-computed tomography. Journal of Endodontics 39, 1067–70.
- Schneider SW (1971) A comparison of canal preparations in straight and curved root canals. Oral Surgery, Oral Medicine, and Oral Pathology 32, 271–5.
- Siqueira JF Jr, Alves FRF, Versiani MA et al. (2013) Correlative bacteriologic and micro–computed tomographic analysis of mandibular molar mesial canals prepared by Self-Adjusting File, Reciproc, and Twisted File systems. Journal of Endodontics 39, 1044–50.
- van der Sluis LW, Wu MK, Wesselink PR (2005a) A comparison between a smooth wire and a K-file in removing artificially placed dentine debris from root canals in resin blocks during ultrasonic irrigation. International Endodontic Journal 38, 593–6.
- van der Sluis LW, Wu MK, Wesselink PR (2005b) The efficacy of ultrasonic irrigation to remove artificially placed dentine debris from human root canals prepared using instruments of varying taper. International Endodontic Journal 38, 764–8.
- van der Sluis LW, Gambarini G, Wu MK, Wesselink PR (2006) The influence of volume, type of irrigant and flushing method on removing artificially placed dentine debris from the apical root canal during passive ultrasonic irrigation. International Endodontic Journal 39, 472–6.
- van der Sluis LW, Versluis M, Wu MK, Wesselink PR (2007) Passive ultrasonic irrigation of the root canal: a review of the literature. International Endodontic Journal 40, 415–26. Su Y, Wang C, Ye L (2011) Healing rate and post-obturation pain of single-versus multiple-visit endodontic treatment for infected root canals: a systematic review. Journal of Endodontics 37, 125–32.
- Thomas AR, Velmurugan N, Smita S, Jothilatha S (2014) Comparative evaluation of canal isthmus debridement efficacy of modified EndoVac technique with different irrigation systems. Journal of Endodontics 40, 1676–80.
- Trope M, Debelian G (2015) XP-3D FinisherTM file — the next step in restorative endodontics. Endodontic Practice US 8, 22–4.
- Versiani MA, P´ecora JD, Sousa-Neto MD (2013) Microcomputed tomography analysis of the root canal morphology of single-rooted mandibular canines. International Endodontic Journal 46, 800–7.
- Versiani MA, Alves FRF, Andrade Junior CV et al. (2015) Micro-CT evaluation of the efficacy of hard-tissue removal from the root canal and isthmus area by positive and negative pressure irrigation systems. International Endodontic Journal doi:10.1111/iej.12559 [Epub ahead of print].
- Weller RN, Brady JM, Bernier WE (1980) Efficacy of ultrasonic cleaning. Journal of Endodontics 6, 740–3.

/public-service/media/default/460/aU9ju_671a20a2e53f3.png)
/public-service/media/default/147/bjsSM_65311952dfadf.jpg)
/public-service/media/default/158/GMj69_65311b2333f75.jpg)
/public-service/media/default/148/ix2WY_6531196adc6ec.jpg)