Supplementary Steps for Removing Hard Tissue Debris from Isthmus-containing Canal Systems
Abstract
Introduction: The purpose of this ex vivo study was to evaluate the percentage reduction of accumulated hard tissue debris (AHTD) in the mesial root canal system of mandibular molars under different final irrigation regimens by means of micro–computed tomographic imaging.
Methods: Sixty curved mesial roots of mandibular molars with 2 independent canals joint apically by an isthmus (Vertucci type II) were selected. Specimens were scanned at a resolution of 12.5 mm, anatomically matched, and distributed into 3 groups (n = 20) according to the preparation protocol: Self-Adjusting File (SAF; ReDent Nova, Ra’anana, Israel), Reciproc (VDW GmbH, Munich, Germany), and Revo-S (Micro-Mega, Besanç on, France) systems. Then, each group was subdivided into 2 subgroups (n = 10) according to the final irrigation protocol with the SAF or EndoVac system (Discus Dental, Culver City, CA). The percentage volume and percentage reduction of AHTD after root canal preparation and final irrigation protocols were statistically compared using 1-way analysis of variance, the paired sample and the in- dependent Student’s t tests. The level of significance was set at 5%.
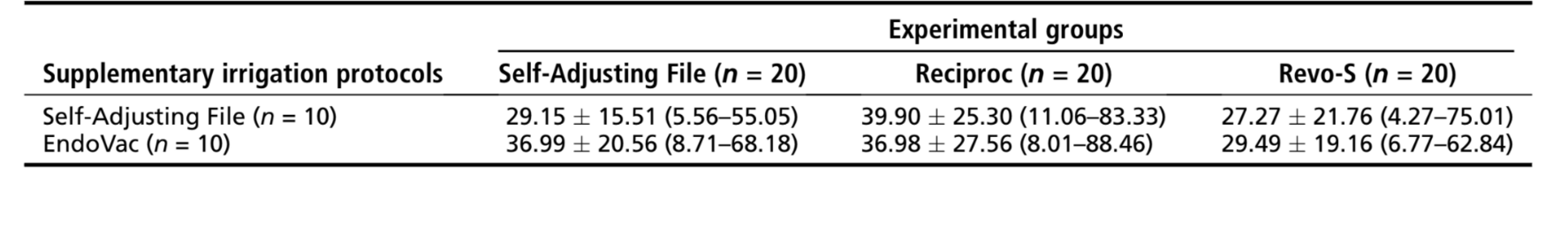
Results: Within groups, the mean percentage volume was significantly reduced after the final irrigation procedures in either the SAF (from 1.52%– 1.78% to 1.01%–1.20%) or EndoVac (from 2.11%–2.23% to 1.31%–1.52%) subgroups (P < .05). In the experimental groups, the mean percentage reduction of AHTD ranged from 29.15%–39.90% after the irrigation protocols, with no statistical difference between groups (P > .05).
Conclusions: None of the irrigation approaches succeeded in rendering the mesial root canal system free of AHTD. A similar percentage reduction of AHTD was achieved after final irrigation protocols using either the SAF or EndoVac system. (J Endod 2016;42:1677–1682)
Endodontic treatment outcome is dependent on successful intracanal infection control by means of using effective chemo-mechanical protocols. With the advent of nickel-titanium rotary and reciprocating instruments, several shortcomings of the conventional preparation procedures that could adversely affect the endodontic therapy were overcome. However, studies using high-resolution micro–computed tomographic (micro-CT) technology have shown that debris created by the cutting action of instruments on dentin during mechanical preparation may be packed into the anatomic complexities of the root canal system preventing the hydrodynamic action of irrigant flow. Consequently, disinfection procedures could be compromised, and persistent microorganisms in these areas may develop or maintain the apical periodontitis.
In the last years, several efforts have been expended toward the development of adjunctive approaches to enhance root canal disinfection. Among the supplementary approaches mostly used to drive irrigants into the anatomic complexities of the root canal system are the sonic devices, ultrasonic techniques, and the EndoVac System (Discus Dental, Culver City, CA). The Self-Adjusting File (SAF; ReDent Nova, Ra’anana, Israel) has also been proven to be effective in the reduction of accumulated hard tissue debris (AHTD) during or after root canal preparation. Despite the fact that recent studies have shown the effectiveness of these approaches as potential irrigation adjuncts for debris removal after root canal preparation, comprehensive knowledge regarding the activation of irrigants in different final irrigation protocols in order to remove hard tissue debris from the isthmus area is still lacking. Therefore, this ex vivo study aimed to evaluate the percentage reduction of AHTD in isthmus-containing mesial root canals of mandibular molars under different final irrigation regimens by means of micro-CT imaging. The null hypothesis was that there is no difference in the reduction of AHTD among the tested irrigation protocols.
Materials and Methods
Sample Size Estimation
The sample size was calculated after the effect size estimation of the percentage volume of AHTD as reported by Paqué et al. In that study, the percentage volume of AHTD after SAF preparation was 1.7%. An a priori analysis of variance (ANOVA) (fixed effects, omnibus, 1-way) was selected from the F test family in G*Power 3.1.7 software for Macintosh (Heinrich Heine, Universität Düsseldorf, Dusseldorf, Germany).
Tooth Specimen Selection
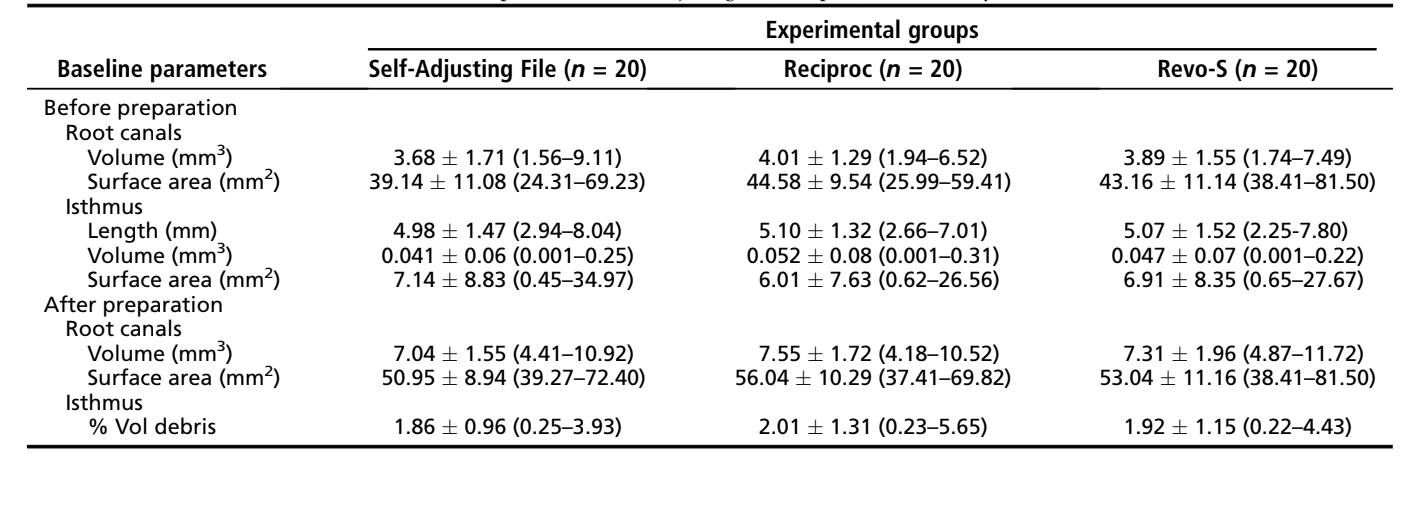
After local research ethics committee approval (protocol no. 2013/145), 250 mesial roots of mandibular molars were obtained from a pool of teeth. Gender and age of the patients were unknown. In order to prevent the introduction of confounding variables, distal roots were sectioned, and teeth were decoronated ~3 mm above the cementoenamel junction. Each mesial root was initially inspected with the aid of a stereomicroscope under 12× magnification and radiographed in both the buccolingual and mesiodistal directions to detect any possible root canal obstruction. The inclusion criteria comprised only teeth presenting mesial roots with moderate curvature (15◦– 20◦) and a fully formed apex. Exclusion criteria were previous canal treatment and the presence of dentinal defects at the external aspect of the roots. As a result, 160 mesial roots were selected and imaged separately at an isotropic resolution of 12.5 mm using a micro-CT device (SkyScan 1172; Bruker-microCT, Kontich, Belgium). The scanner parameters were set at 80 kV, 124 mA, 180◦ rotation around the vertical axis, a rotation step of 0.48◦, and a camera exposure time of 620 milliseconds using a 0.5-mm-thick aluminum filter. Then, 60 moderately curved and fully formed mesial roots of mandibular molars with 2 independent canals joint apically by an isthmus (Vertucci type II configuration) were selected.
The acquired projection images were reconstructed (NRecon v.1.6.9 software, Bruker-microCT), resulting in the acquisition of 1000 to 1200 transverse cross sections per root. The volume of interest was selected extending from the furcation level to the apex, set by integration of all cross sections. For the purpose of this study, the region of interest in each slice comprised the area of the mesial canals and the isthmus. Preoperative 3-dimensional models of the mesial root canal systems were rendered (CTVol v.2.2.1, Bruker micro-CT), and morphologic parameters of the isthmus area (length, volume, and surface area) and the entire root canal system (volume and surface area) were calculated (CTAn v.1.14.4 software, Bruker-microCT). Aiming to enhance the internal validity of the experiment, the mesial canals were matched to create 20 groups of 3 based on the morphologic aspects of the canal. Then, 1 root from each group was randomly assigned to 1 of the 3 experimental groups (n = 20) according to the preparation protocol: SAF, Reciproc (VDW GmbH, Munich, Germany), and Revo-S (Micro-Mega, Besançon, France) systems. After checking the normality assumption (Shapiro-Wilk test) and the equality of variances (Levene test), the degree of homogeneity (baseline) of the groups with respect to each one of the morphologic parameters was confirmed (1-way AN- OVA test, P > .05) (Table 1).
Root Canal Preparation
After the apices of the mesial roots were sealed with fast-set epoxy resin to create a closed-end system, canals were accessed, and the coronal third was sequentially flared with Gates Glidden burs sizes 2 and 3 (Dentsply Maillefer, Baillagues, Switzerland) followed by irrigation with 3 mL 2.5% sodium hypochlorite (NaOCl). Patency was confirmed by inserting a size 10 K-file (Dentsply Maillefer) through the apical foramen before and after completion of canal preparation. For all groups, a glide path was created by scouting a stainless steel size 15 K-file (Dentsply Maillefer) up to the working length (WL), which was established by deducting 1 mm from the canal length. Then, root canals in each group were prepared according to the following protocols (Fig. 1):
- The SAF system (n = 20): A 1.5-mm SAF instrument was operated using an RDT3-NX head (ReDent Nova) adapted to a low-speed handpiece (NSK, Tokyo, Japan) operated at 5000 rpm for 4 minutes. The SAF was gradually inserted into the canal and used to the WL with a pecking motion. Continuous irrigation at a flow rate of 5 mL/min with 2.5% NaOCl was applied throughout the procedure using a special irrigation apparatus (VATEA, ReDent-Nova).
- The Reciproc system (n = 20): The R25 instrument (size 25, 0.08 taper over the first 3 mm) was introduced into the canal until resistance was felt and then activated in reciprocating motion using the VDW Silver motor (VDW GmbH). The instrument was moved slowly in the apical direction using a gentle in-and-out pecking motion of about 3 mm in amplitude. After 3 pecking motions, the instrument was removed from the canal and cleaned. Each time the Reciproc instrument was removed, 2 mL 2.5% NaOCl was applied using a 27-G closed-end tip needle adapted to a disposable plastic syringe and placed 1 mm short of the WL.
- Revo-S system (n = 20): SC1 (size 25, 0.06 taper), SC2 (size 25, 0.04 taper), and SU (size 25, 0.06 taper) Revo-S nickel-titanium rotary instruments were used at 300 rpm in a crown-down manner up to the WL using a gentle in-and-out pecking motion. Irrigation followed the same protocol as in the Reciproc group.
In all groups, preparation was accomplished in 4 minutes using a total of 20 mL 2.5% NaOCl per canal. After preparation, a final rinse with 5 mL 17% EDTA (pH = 7.7) delivered at 1 mL/min rate for 5 minutes followed by a 5-minute 5-mL rinse with bidistilled water were performed in each canal with the tip of the 27-G needle positioned 1 mm short of the WL. Then, canals were slightly dried with absorbent paper points and the roots submitted to a new scan, applying the parameter settings previously mentioned (Fig. 1).
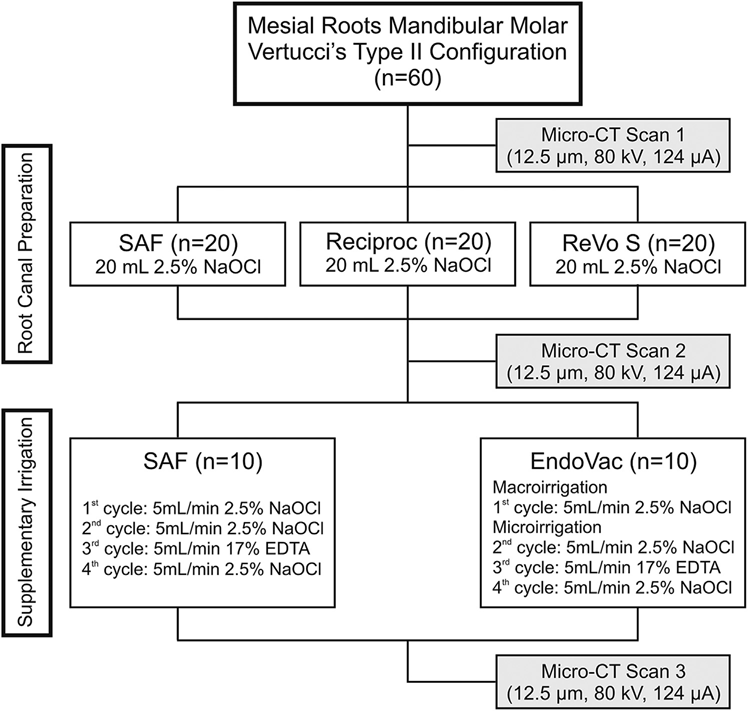
Postoperative scans were coregistered with their respective preoperative data sets using the rigid registration module of the 3D Slicer 4.3.1 software (available from http://www.slicer.org). Quantification of AHTD was performed by the difference between the nonprepared and prepared root canal space using postprocessing procedures with Fiji software (Fiji v.1.47n; Fiji, Madison, WI). The sequence of images resulting from this operation was further used to identify the AHTD by means of morphologic operations (Fig. 2) as described in detail elsewhere. The presence of a material with density similar to dentin in regions previously occupied by air in the nonprepared root canal space was considered debris and quantified by the intersection between images before and after canal instrumentation. The total volume of AHTD was calculated in cubic millimeters (mm3) and expressed as the percentage of the total canal system volume after preparation (%vol) (Table 1).
For the next experimental procedures, a plastic transparent automatrix band was adapted to the coronal part of the roots. Then, the specimens were pair matched in each group with respect to the %vol of AHTD acquired in the postpreparation scan, and 1 root from each pair was randomly assigned to 1 of 2 subgroups (n = 10) according to the following supplementary irrigation protocol (Fig. 1):
- The SAF system (n = 10): The root canals were irrigated in 4 cycles of 60 seconds using a 1.5-mm SAF instrument connected to the VATEA irrigation apparatus. In the first cycle, the SAF was inserted to the WL and moved up and down with a continuous flow of 2.5% NaOCl irrigant at a flow rate of 5 mL/min. After that, the SAF was removed from the canal, and the irrigant was left undisturbed for 60 seconds. This cycle was repeated. Then, in the third and fourth cycles, the SAF was used with continuous irrigation of 5 mL/min 17% EDTA (pH = 7.7) and 5 mL/min 2.5% NaOCl, respectively.
- The EndoVac system (n = 10): The root canals were irrigated in 4 cycles of 60 seconds using the EndoVac System. In the first cycle, macroirrigation with 5 mL 2.5% NaOCl was accomplished using a macrocannula inserted into the canal and moved up and down from a point where it bounded at the canal walls. Then, the irrigant was left undisturbed for 60 seconds. After that, 3 cycles of microirrigation were followed. The microcannula was inserted 1 mm short of the WL and held for 60 seconds, whereas 5 mL of the irrigant was constantly replenished. The microcannula was removed, and the irrigant was left undisturbed for 60 seconds. In the next 2 cycles of microirrigation, 5 mL/min 17% EDTA (pH = 7.7) and 5 mL/min 2.5% NaOCl were used, respectively.
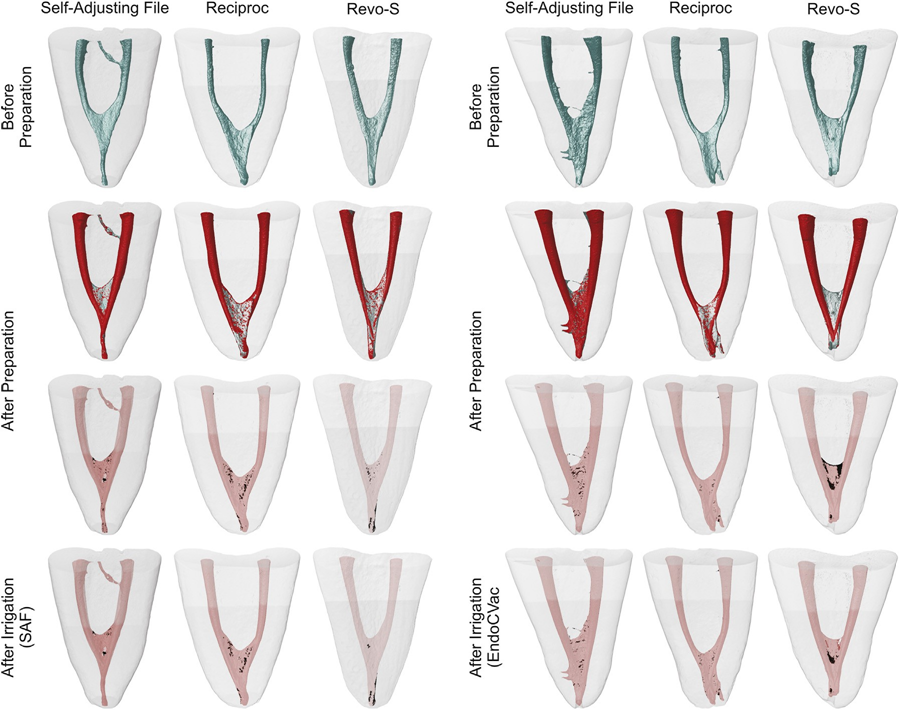
After the supplementary irrigation procedures, the canals were slightly dried with absorbent paper points, and a final scan was performed (Fig. 1). Data sets were registered with their respective counterparts, and the %vol of AHTD in each canal was calculated. Then, the percentage reduction (%red) of the AHTD was obtained according to the following formula: 100 — ([VAF × 100]/VBF), where VBF and VAF are the volume of AHTD before and after irrigation protocols, respectively. Color-coded root canal models (green and red colors indicating pre- and postoperative canal surfaces, respectively) and debris (in black color) enabled qualitative comparison of the matched root canals before and after the experimental procedures.
Statistical Analysis
Data were normally (Shapiro-Wilk test) and homoscedastically (Levene test) distributed. Therefore, results were expressed as the mean and standard deviation and compared between groups using 1-way ANOVA and within group by paired sample and independent Student t tests, with a significance level set at 5% (SPSS v17.0; SPSS Inc, Chicago, IL).
Results
Sample size estimation showed that 9 samples per group were indicated as the ideal sample size (alpha-type error <0.05 and power beta of 99%). Pre- and postoperatively, the degree of homogeneity (baseline) of the groups and subgroups was confirmed regarding the analyzed morphologic parameters of the root canals as well as the % vol of AHTD after preparation (P > .05) (Table 1).
The mean %vol and %red of AHTD evaluated before and after the supplementary irrigation procedures are detailed in Tables 2 and 3, respectively. Within groups, the mean %vol of AHTD was significantly reduced after the final irrigation procedures with either SAF (from 1.52%–1.78% to 1.01%–1.20%) or EndoVac (from 2.11%–2.23% to 1.31%–1.52%) systems (P < .05) (Table 2). Overall, the mean % red of AHTD ranged from 29.15%–39.90% with no statistical difference between the experimental groups (P > .05) (Table 3). Therefore, the null hypothesis was confirmed. Three-dimensional models of representative mesial root canals in each experimental group show the distribution of the AHTD after chemomechanical preparation and supplementary irrigation protocols (Fig. 3). Overall, residual debris after final irrigation protocols were mostly observed at the apical third and in the isthmus area.
Discussion
In general, root canal debridement has been evaluated by means of conventional methods such as root sectioning, scanning electron microscopy, and histology. More recently, nondestructive micro-CT technology was used as a precise tool for 3-dimensional quantitative evaluation of hard tissue debris packed into recesses during canal preparation. Evidence from these studies indicates that dentin particles cut from the canal walls by the endodontic instruments can be actively packed into the anatomic complexities of the canal system, becoming more resistant to removal. Despite the fact that it is difficult to draw reliable conclusions from the literature regarding the most effective irrigation protocol to overcome this problem, a general agreement exists about the benefits of activating the irrigant during and after preparation procedures. Overall, previous studies have shown that the use of supplementary irrigation approaches after canal preparation results in less debris within the complexities of the root canal system, as shown in the present study.
The SAF is a hollow nickel-titanium latticelike instrument that scrubs the canal walls by vertical vibrations and allows simultaneous and continuous irrigation throughout the mechanical preparation of the root canal. To date, only 2 studies attempted to quantify the volume of hard tissue debris accumulated in the isthmus area of the mesial roots of mandibular molars after using the SAF system. In 1 of them, the SAF was used as a finishing instrument after root canal preparation and left 4.3% of the canal volume filled with AHTD. This result was more than twice that reported by Paqué et al and the results found in the present study, but it may be explained because SAF’s protocol was modified and applied for only 1 minute in each canal to equalize the experimental groups in terms of preparation time and amount of irrigant. Therefore, it may be advised that studies using SAF as a supplementary step for root canal cleaning should follow its full protocol, as used herein. However, despite the fact that SAF has been proven efficient as a potential irrigation adjunct for debris removal, it must be highlighted that this instrument is unable to remove all AHTD content from the canal because during its use it continues to scrub the canal walls, producing more dentinal debris. Besides, although SAF conforms to the canal shape, it cannot enter into the isthmus area. Consequently, its efficacy in the present study may be attributed to the continuous flow with a fresh and fully active irrigant replacement.
The EndoVac system comprises a different irrigation regimen that involves apical negative pressure and uses a master tip to deliver and evacuate the irrigant concomitantly at the pulp chamber level, whereas microcannulas are used deeply in the canal. In the literature, the comparison of the cleaning and disinfecting effects of EndoVac and conventional irrigation reached inconclusive results. Although some studies showed superior bacterial elimination and better cleaning using the EndoVac system, other authors found no significant differences between these techniques. Recently, 2 studies evaluated the efficacy of EndoVac to remove hard tissue debris accumulated in isthmus-containing mesial root canals of mandibular molars. It was reported that the median percentage volume of AHTD was reduced to 2.12% and 3.4% after using the EndoVac system as a final irrigation protocol, which is in agreement with the present results. However, it is important to point out that the root canal configuration of the samples selected herein and in those studies (Vertucci type II) may favor the efficacy of the EndoVac system when compared with other irrigation approaches. In this type of configuration in which root canals are connected by an isthmus, the mechanical flushing action of EndoVac produces a current flow of the irrigant toward the apical third in both root canals simultaneously. Consequently, this constant irrigant flow may displace the hard tissue debris from within the isthmus area favoring its removal, which may explain these results.
Although a final irrigation protocol with SAF and EndoVac resulted in a significant reduction in the debris content, which can be translated into improved cleanliness of the root canal system, its clinical relevance remains unclear, and further studies are necessary to evaluate its impact on the success rate of the root canal treatment. It is also worth mentioning that the experimental groups in this study differed not only in the mode of irrigant delivery but also in the delivery protocol; the EndoVac system delivers the irrigant into the pulp chamber and not in the root canal system. However, these differences did not seem to negatively influence the results, probably because of the sample distribution approach used herein, which was based on the morphologic aspects of both root canals and the isthmus area (Table 1). This methodological step is paramount to reduce the potential significant anatomic biases that could interfere with the results, enhancing the internal validity of the experiment.
Despite the findings of this study revealing that the adjunctive irrigation protocols with SAF or EndoVac systems did not succeed in rendering the isthmus-containing mesial root canal system of mandibular molar teeth free of AHTD, their efficacy in reducing the debris created by the instruments during the cleaning and shaping procedures was shown.
Authors: Ali Keleş, Hatice Alçin, Manoel D. Sousa-Neto, Marco A. Versiani
References:
Siqueira JF Jr, Alves FRF, Versiani MA, et al. Correlative bacteriologic and micro– computed tomographic analysis of mandibular molar mesial canals prepared by Self-Adjusting File, Reciproc, and Twisted File systems. J Endod 2013;39:1044–50.
Siqueira JF Jr. Reaction of periradicular tissues to root canal treatment: benefits and drawbacks. Endod Topics 2005;10:123–47.
Hülsmann M, Peters OA, Dummer PMH. Mechanical preparation of root canals: shaping goals, techniques and means. Endod Topics 2005;10: 30–76.
Versiani MA, Pécora JD, Sousa-Neto MD. Flat-oval root canal preparation with self-adjusting file instrument: a micro-computed tomography study. J Endod 2011;37: 1002–7.
De-Deus G, Marins J, Silva EJ, et al. Accumulated hard-tissue debris produced during reciprocating and rotary nickel-titanium canal preparation. J Endod 2015;41: 676–81.
De-Deus G, Roter J, Reis C, et al. Assessing accumulated hard-tissue debris using micro-computed tomography and free software for image processing and analysis. J Endod 2014;40:271–6.
Freire LG, Iglecias EF, Cunha RS, et al. Micro-computed tomographic evaluation of hard tissue debris removal after different irrigation methods and its influence on the filling of curved canals. J Endod 2015;41:1660–6.
Paqué F, Al-Jadaa A, Kfir A. Hard-tissue debris accumulation created by conventional rotary versus self-adjusting file instrumentation in mesial root canal systems of mandibular molars. Int Endod J 2012;45:413–8.
Paqué F, Boessler C, Zehnder M. Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. Int Endod J 2011; 44:148–53.
Paqué F, Laib A, Gautschi H, Zehnder M. Hard-tissue debris accumulation analysis by high-resolution computed tomography scans. J Endod 2009;35: 1044–7.
Paqué F, Peters OA. Micro-computed tomography evaluation of the preparation of long oval root canals in mandibular molars with the self-adjusting file. J Endod 2011;37:517–21.
Paqué F, Rechenberg DK, Zehnder M. Reduction of hard-tissue debris accumulation during rotary root canal instrumentation by etidronic acid in a sodium hypochlorite irrigant. J Endod 2012;38:692–5.
Versiani MA, Alves FRF, Andrade Junior CV, et al. Micro-CT evaluation of the efficacy of hard-tissue removal from the root canal and isthmus area by positive and negative pressure irrigation systems. Int Endod J. 2015 Oct 13. http://dx.doi.org/10.1111/iej.12559. [Epub ahead of print].
Ricucci D, Siqueira JF Jr, Bate AL, Pitt Ford TR. Histologic investigation of root canal-treated teeth with apical periodontitis: a retrospective study from twenty-four patients. J Endod 2009;35:493–502.
Siqueira JF Jr, Rôças IN. Optimising single-visit disinfection with supplementary approaches: a quest for predictability. Aust Endod J 2011;37:92–8.
Adorno CG, Fretes VR, Ortiz CP, et al. Comparison of two negative pressure systems and syringe irrigation for root canal irrigation: an ex vivo study. Int Endod J 2016; 49:174–83.
Leoni GB, Versiani MA, Silva Sousa YT, et al. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int Endod J. 2016 Mar 18. http://dx.doi.org/ 10.1111/iej.12630. [Epub ahead of print].
Susin L, Liu Y, Yoon JC, et al. Canal and isthmus debridement efficacies of two irrigant agitation techniques in a closed system. Int Endod J 2010;43: 1077–90.
Tay FR, Gu LS, Schoeffel GJ, et al. Effect of vapor lock on root canal debridement by using a side-vented needle for positive-pressure irrigant delivery. J Endod 2010;36: 745–50.
Haapasalo M, Shen Y, Wang Z, Gao Y. Irrigation in endodontics. Br Dent J 2014;216: 299–303.
Gu LS, Kim JR, Ling J, et al. Review of contemporary irrigant agitation techniques and devices. J Endod 2009;35:791–804.
Nusstein JM. Sonic and ultrasonic irrigation. In: Bettina B, ed. Endodontic Irrigation: Chemical Disinfection of the Root Canal System. Basel, Switzerland: Springer; 2015:173–98.
Metzger Z, Teperovich E, Zary R, et al. The self-adjusting file (SAF). Part 1: respecting the root canal anatomy—a new concept of endodontic files and its implementation. J Endod 2010;36:679–90.
Versiani MA, Pécora JD, Sousa-Neto MD. Microcomputed tomography analysis of the root canal morphology of single-rooted mandibular canines. Int Endod J 2013;46: 800–7.
