Efficacy of 3 Supplementary Irrigation Protocols in the Removal of Hard Tissue Debris from the Mesial Root Canal System of Mandibular Molars
Abstract
Introduction: Instrumentation of the mesial root canal system of mandibular molars may hinder disinfection by packing hard tissue debris within the isthmuses. The removal of accumulated hard tissue debris (AHTD) by 3 supplemental irrigation systems, 2 ultrasonically activated and 1 multisonic, was assessed with micro–computed tomographic imaging.
Methods: Twenty-four extracted mandibular molars with 2 mesial canals connected by an isthmus and converging to a single foramen were selected. After preparation of the mesial canals with WaveOne Gold instruments (Dentsply Maillefer, Ballaigues, Switzerland), anatomically matched specimens were assigned to 3 final irrigation protocols (n = 8): intermittent ultrasonic (IU) with an ultrasonically energized 200-mm wire (Irrisafe; Satelec, Bordeaux, France), continuous ultrasonic (CU) with an ultrasonic irrigation needle (ProUltra PiezoFlow, Dentsply Maillefer), and GentleWave (GW) system (Sonendo Inc, Laguna Hills, CA). Specimens were scanned (SkyScan 1176; Bruker-microCT, Kontich, Belgium) at 17.18-mm pixel size before and after preparation and irrigation protocols. Data sets were coregistered, and the percentage reduction of AHTD calculated within the canals and isthmus for each specimen was statistically compared using 1-way analysis of variance and post hoc Tukey tests with a 5% significance level.
Results: The mean percentage reduction of AHTD in canals and isthmuses was significantly higher for GW (96.4% and 97.9%, respectively) than for CU (80.0% and 88.9%, respectively) (P ˂ .05). AHTD reduction for IU (91.2% and 93.5%, respectively) did not differ significantly from GW and CU (P ˃ .05).
Conclusions: GW achieved greater efficacy in the removal of AHTD from the mesial root canal system of mandibular molars compared with CU but not IU. The efficacy of CU and IU was comparable. (J Endod 2019;■:1–7.)
Root canal disinfection, routinely pursued using chemomechanical protocols, may be compromised by the inability of instruments and antibacterial solutions to reach inaccessible canal areas. Apical ramifications, lateral canals, and isthmuses connecting main root canals have all been shown to harbor bacterial cells frequently organized in biofilmlike structures. An isthmus is defined as a narrow communication between 2 canals in the same root that contains pulp-derived tissue. It has also been described as a corridor, a lateral connection, and a transverse anastomosis.
In roots with isthmuses, instrumentation of the main canals inadvertently transports hard tissue debris into the isthmuses where it remains packed despite copious irrigation during and after instrumentation. Accumulated hard tissue debris (AHTD) is an undesirable side effect of the chemomechanical disinfection procedures because it may harbor persistent microorganisms while preventing access for antibacterial irrigation solutions.
Mandibular first molars are the most frequent endodontically treated teeth. These teeth frequently present complex root canal configurations, with isthmus communications present in 55% of the mesial roots and 20% of the distal roots. The isthmus incidence is the greatest at 3–5 mm from the apex, where it was clinically observed during apical surgery in 83% of mesial roots and 36% of distal roots of mandibular first molars.
Considering the high incidence of isthmuses in the root canal system and their inaccessibility to mechanical instrumentation, their disinfection then critically relies on effective delivery of antibacterial solutions.
From traditional syringe needle delivery to machine-assisted agitation systems, irrigation methods have been refined over the years to enhance the delivery of irrigation solutions to mechanically inaccessible areas of the complex root canal system. The application of irrigation solutions and ultrasonic agitation during ultrasonically activated irrigation can either be intermittent or continuous.
While the continuous ultrasonically activated irrigation (CU) method is delivered via an ultrasonically activated irrigation needle, the intermittent ultrasonically activated irrigation (IU) requires a vibrating instrument within the canal and replenishment of the solution with a syringe after each activation cycle.
The GentleWave (GW) system (Sonendo Inc, Laguna Hills, CA) is a novel apical negative-pressure disinfection device requiring minimal root canal instrumentation as per the manufacturer. The system applies advanced fluid dynamics, acoustics, and tissue dissolution chemistry to remove tissue, debris, and biofilms from the entire root canal system simultaneously. The device was recently approved for clinical use in endodontics, and independent evaluation is warranted. To date, only 1 study showed histologically the efficacy of the GW system in debris removal from the isthmuses of maxillary and mandibular molars. The use of nondestructive assessment methods is warranted to investigate the ability of the GW system to enhance cleansing of isthmuses.
Therefore, the aim of the present study was to assess the efficacy of the GW system in comparison with intermittent and continuous ultrasonically activated irrigation in the removal of AHTD from root canals and isthmuses within mesial roots of mandibular molars using micro–computed tomographic (micro-CT) imaging. The null hypothesis tested was that there would be no difference in the reduction of AHTD among these 3 supplementary irrigation protocols.
Materials and methods
Sample Size
The sample size was estimated based on preliminary data obtained from 5 specimens. Following the same instrumentation and final irrigation protocols as described later, 2 specimens were assigned to the GW and CU groups and 1 specimen to the IU group. The effect size of the IU group was established from the one previously reported by Leoni et al (1.25). Using G*Power 3.1.9.2 software
(Heinrich Heine Universität, Düsseldorf, Germany) for 1-way analysis of variance and the data from the pilot study, a minimal total sample of 18 specimens would support analysis with 99% power and a 5% level of significance to statistically substantiate differences between the experimental groups. A total of 24 specimens were included in the final analysis.
Specimen Selection
The study protocol was approved by both institutional ethics boards of the University of Toronto and the University of São Paulo (protocol #35314). Initially, 50 extracted mandibular molars with moderately curved mesial roots (10◦–20◦, Schneider’s method) in both mesiodistal and buccolingual directions were imaged with a micro-CT scanner (SkyScan 1176; Bruker-microCT, Kontich, Belgium) at 17.18 mm (pixel size), 90 kV, 278 mA, 180◦ rotation around the vertical axis, and a rotation step of 0.4◦ using a 0.5-mm-thick aluminum filter. The acquired projection images were reconstructed (NRecon v.1.6.10.4, Bruker-microCT) with a beam-hardening correction of 10%, smoothing of 2, ring artifact correction of 3, and an attenuation coefficient ranging from 0.006–0.04, resulting in the acquisition of approximately 550 slices per root. Then, 24 teeth presenting 2 independent canals in the mesial root connected by an isthmus from the middle to the apical third and exiting in a single foramen (Vertucci type II configuration) were selected. None of the teeth had root fillings, root caries, cracks, fractures, internal or external resorption. To ensure anatomic similarity among the specimens, length (in mm), volume (in mm3), surface area (in mm2), and Structure Model Index (SMI) of the mesial root canals before the experimental procedures were calculated (CTAn v.1.15, Bruker-microCT) (Table 1). The volume of interest was selected extending from the cementoenamel junction level to the apex of the mesial root set by integration of all cross sections.
Root Canal Preparation
The mesial root canals in all specimens were prepared by 1 operator (R.C.) experienced in the use of reciprocating instruments. After access cavity preparation, the mesial canals were negotiated with size 10 K-type files (Dentsply Maillefer, Ballaigues, Switzerland), and emergence of the tip at the apical foramen was verified under 10X magnification (Carl Zeiss, Oberkochen, Germany). The working length (WL) was established 0.5 mm short of the foramen. Next, the foramen was sealed by covering the apical tip of the mesial roots with hot glue to simulate a closed-ended canal system. A glide path was established to the WL with a ProGlider instrument (Dentsply Maillefer), and the root canals were sequentially enlarged with WaveOne Gold Small and Primary instruments (Dentsply Maillefer) to the WL activated in a reciprocating motion (ProMark Endo Motor, Dentsply Maillefer). To facilitate debris accumulation in the isthmus area, irrigation and aspiration throughout the preparation procedures were performed only at the orifice level with a total of 5 mL distilled water per canal using a 30-G ProRinse Endo irrigation needle (Dentsply Maillefer) adapted to a disposable plastic syringe.
Each canal was slightly dried with 1 absorbent paper point (WaveOne Small, Dentsply Maillefer), and the specimens were submitted to a further scan and analysis following the aforementioned parameters. Postoperative scans were coregistered with their respective preoperative data set using the affine registration module of the 3D Slicer 4.10 software (available from http://www.slicer.org), and postoperative 3D parameters (volume, surface area, and SMI) were also acquired (Table 1). Then, spatially registered surface models of the roots were compared regarding the unprepared area of the root canal (Table 1) calculated by the formula (SAu/SAb)*100, where SAu represents the unprepared canal surface area and SAb the root canal surface area before preparation, to ensure the consistency of the instrumentation protocol. A further analysis of the matched images was also performed to calculate hard tissue debris accumulated within the mesial root canal system after instrumentation procedures using CTAn v.1.15 software (Bruker micro-CT).
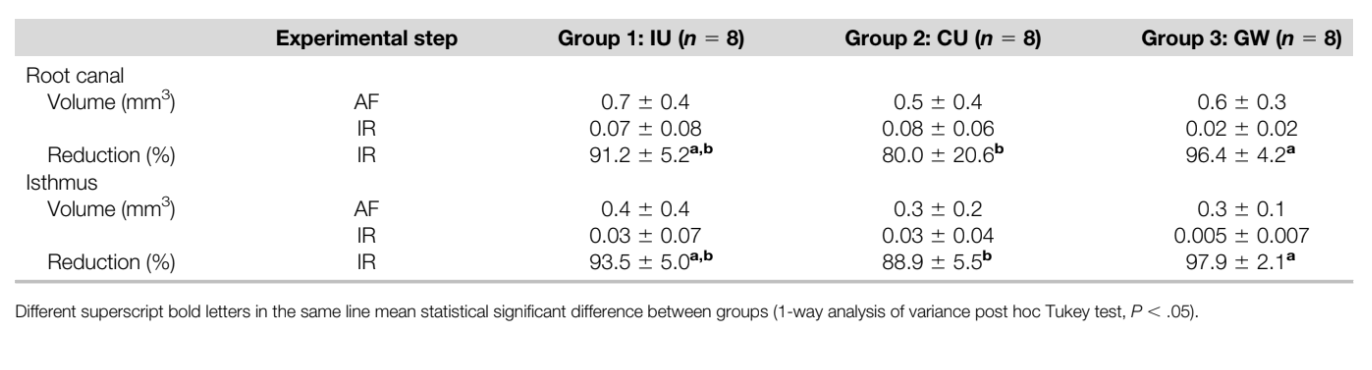
Quantification of AHTD was performed by the difference between non-prepared and prepared root canal space using postprocessing procedures. The presence of a material with density similar to dentin in regions previously occupied by air in the non-prepared root canal space was considered debris and quantified by intersection between images before and after canal instrumentation. The total volume of AHTD was calculated in cubic millimeters (mm3) and expressed as the percentage of the total canal system volume and the isthmus area after preparation (Table 1).
Final Irrigation Protocols
Aiming to enhance the internal validity of the experiment, the mesial root canals were matched to create 8 groups of 3 based on the morphology of the root canal system (length, volume, surface area, and SMI), the unprepared canal surface, and the percentage volume of AHTD after preparation. Then, 1 specimen from each group was randomly assigned to 1 of the following 3 experimental groups (n = 8) according to the final irrigation protocols, which followed the manufacturers’ directions:
- Group 1: IU; a noncutting, 200-mm stainless steel ultrasonic file (Irrisafe; Satelec, Bordeaux, France) driven by the P5 Newtron ultrasonic system (Acteon North America, Mount Laurel, NJ) at a power setting of 9 was placed at 2 mm from the WL and agitated in a 1–2 mm up-and- down motion. The final irrigation protocol began with 6% NaOCl for 3 X 20 seconds followed by 17% EDTA for 3 X 20 seconds and a final rinse with 6% sodium hypochlorite (NaOCl) for 3 X 20 seconds. Irrigation was performed in a flow rate of 15 mL/min per canal.
- Group 2: CU; a ProUltra PiezoFlow ultrasonic irrigation needle (ProUltra, Dentsply Maillefer) was connected to the P5 Newtron ultrasonic system (Acteon North America) at a power setting of 9. The 500-mm ultrasonic irrigation needle was positioned 1 mm short of binding, no deeper than 75% of the working length, and agitated in a 1–2 mm up-and-down motion. The final irrigation protocol began with 6% NaOCl followed by 17% EDTA and a final rinse with 6% NaOCl. Irrigation was performed in a flow rate of 15 mL/min for 1 minute per canal.
- Group 3: GW; before the final irrigation protocol, an occlusal platform was fabricated of a resin material (SoundSeal, Sonendo Inc) and a preformed plastic matrix to ensure an airtight seal between the access cavity and the procedural instrument. The final irrigation protocol began with 3% NaOCl for 5 minutes followed by distilled water for 30 seconds, 8% EDTA for 2 min, and a final rinse with distilled water for 15 seconds in a flow rate of 50 mL/min.
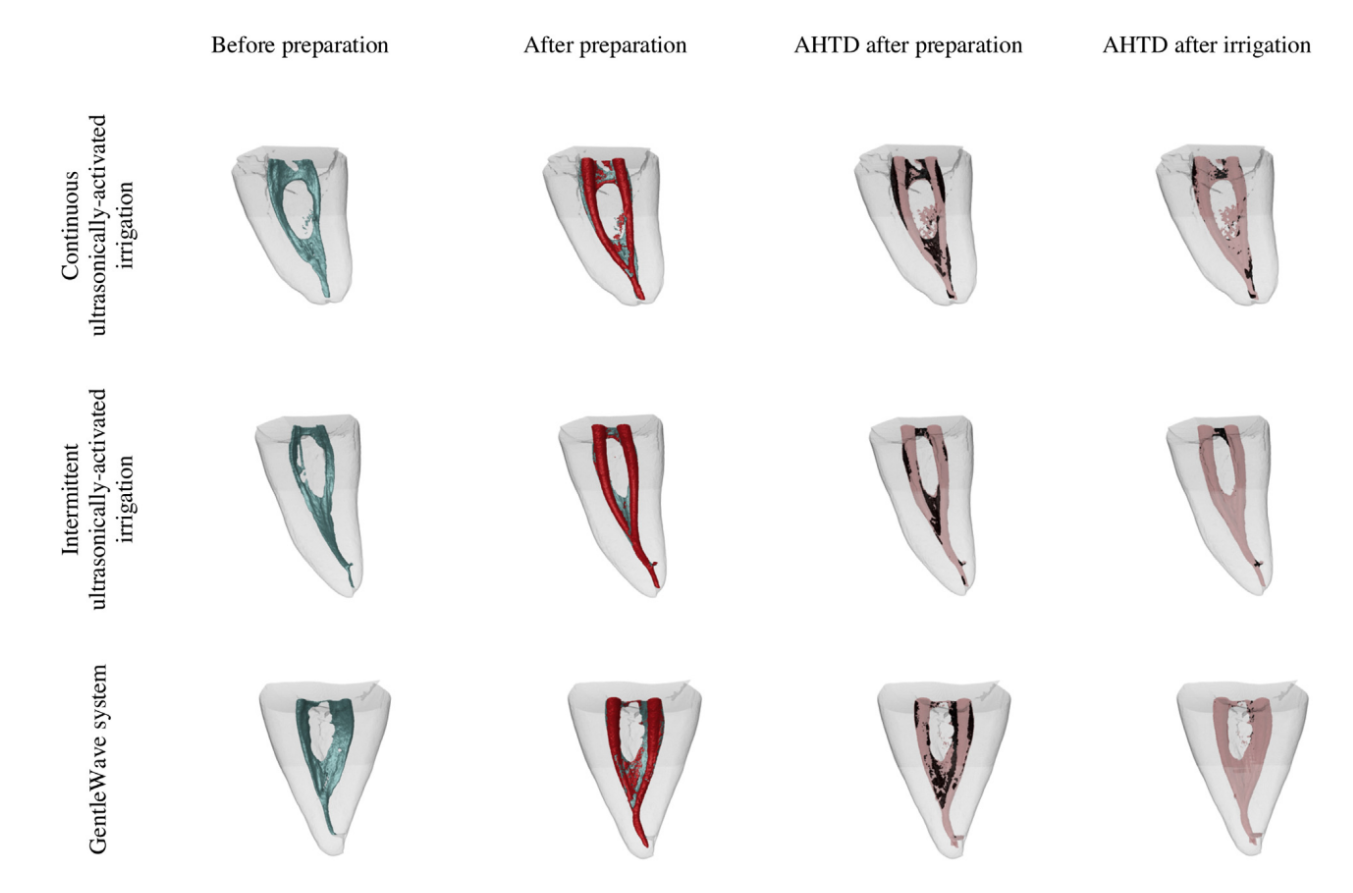
The postirrigation scan was performed after completion of the assigned final irrigation protocols. Data sets were registered with their respective postpreparation counterparts, and the percentage reduction of AHTD was calculated according to the following formula: 100 – ([VAF X 100)/VBF), where VBF and VAF are the volume of AHTD before and after the irrigation protocol, respectively. An examiner blinded to the group assignment of specimens performed all the measurements. Matched color-coded root canal models (green and red colors indicating pre- and postoperative canal surfaces, respectively) and debris (in black color) enabled qualitative comparison of the distribution of the AHTD in each portion of the root canals before and after the experimental procedures.
Scanning Electron Microscopy
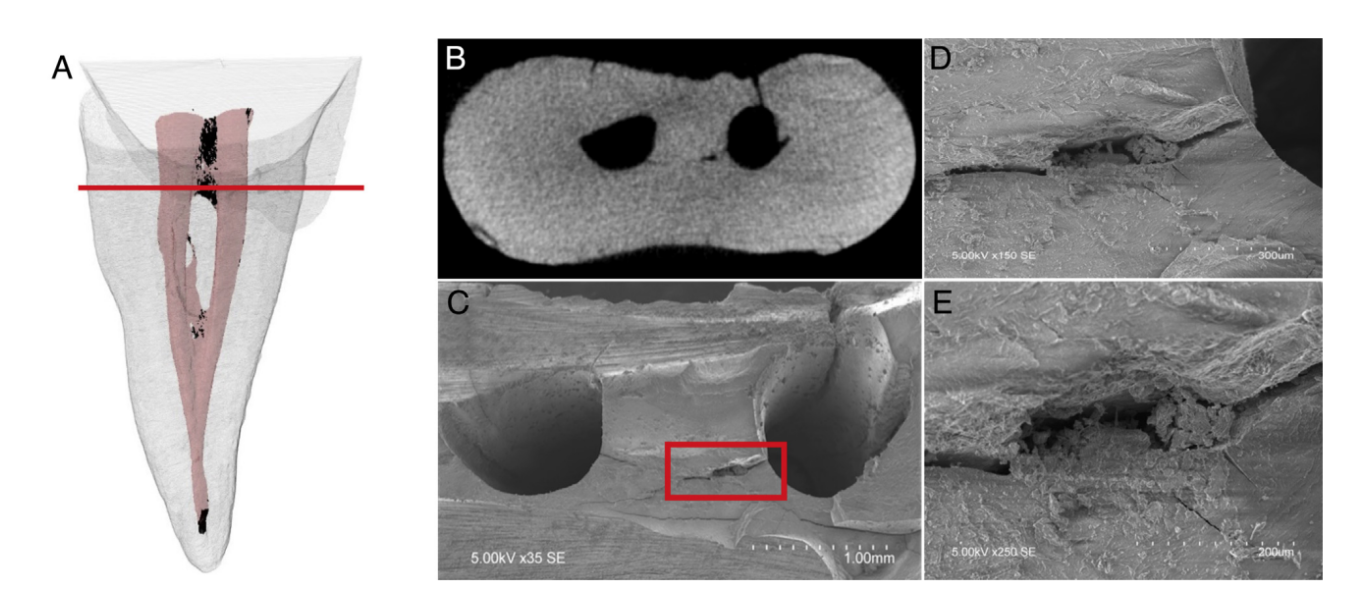
In order to validate the method, after the postirrigation scans, axial cross sections at the coronal, middle, and apical thirds of the root of 2 randomly selected specimens were examined under environmental scanning electron microscopy (S-3400N; Hitachi, Tokyo, Japan) up to 420X magnification to corroborate the presence of debris in the isthmus areas after the final irrigation protocol (Fig. 1A–E). Briefly, after removal of the distal roots at the cementoenamel junction level, grooves were cut on the mesial roots with a diamond-coated disc close to but without exposing the canals and isthmus areas at levels predetermined from the corresponding micro-CT scans where residual AHTD was present. The specimens were then split horizontally with a sharp chisel and mallet. The unprocessed specimens were imaged at an accelerating voltage of 5 kV and at a working distance of 15 mm.
Statistical Analysis
Normal distribution of the data was assessed, and 3-dimensional morphology of the root canals (length, volume, surface area, and SMI), untouched canal surface, and percentage of AHTD after preparation and irrigation protocols were expressed as the mean and standard deviation and compared among groups using 1-way analysis of variance and post hoc Tukey tests with a 5% level of significance.
Results
The degree of homogeneity of the 3 experimental groups was confirmed regarding pre- and postoperative morphologic parameters (canal length, volume, surface area, and SMI), unprepared canal surface, and the volume of AHTD after canal preparation (Table 1, P . .05). Qualitative observation of the scanning electron microscopic images corresponded well to the radiopaque areas within the isthmuses and the AHTD depicted on the micro-CT tridimensional reconstruction of the same specimen (Fig. 1).
Supplementary irrigation protocols considerably reduced AHTD in all groups (Table 2); however, the mean percentage reduction of AHTD was significantly higher for GW in the canals (96.4%) and in the isthmus areas (97.9%) when compared with CU (80.0% and 88.9%, respectively) (P , .05). On the other hand, AHTD reduction for IU in the canals and in the isthmus areas (91.2% and 93.5%, respectively) did not differ significantly from GW and CU (P . .05) (Table 2, Fig. 2). Most residual AHTD after final irrigation was located at the coronal and apical thirds of the root canals in all groups (Fig. 2).
Discussion
Apical periodontitis is a biofilm-mediated disease, and the inability to impact on biofilms within areas of the root canal system that are inaccessible to conventional chemomechanical disinfection protocols may compromise treatment outcomes. The pursuit for improved disinfection efficacy in the anatomically complex root canal system has in recent years focused on the effects within the isthmus areas as a potential target for biofilm eradication. Bacteria with polymicrobial flora organized as biofilms have been identified within the isthmus areas of human mandibular first molars, both immediately after completion of single-visit endodontic treatment and in a tooth associated with posttreatment apical periodontitis. This study assessed the ability of root canal disinfection protocols to remove hard tissue debris from uninstrumented canal irregularities and isthmuses as indication of possible access to biofilms within these rather inaccessible areas.
The experimental design used herein aimed to evaluate the irrigation properties of the tested irrigation devices under standardized conditions, thus disregarding selected clinical application guidelines.
Accordingly, although the manufacturer of GW recommends canal instrumentation up to only size 20/.06, in this study all mesial canals were instrumented to size 25/.07 with minimal irrigation to standardize the volume and distribution pattern of AHTD. In addition, in accordance with a previously established methodology, the Small and Primary WaveOne Gold reciprocating instruments were used in sequence to produce sufficient amounts of AHTD that will allow quantification of its removal efficacy. The volume of produced AHTD, approximately 15% of the total canal volume, was comparable with the 19% reported in a previous study in which the WaveOne reciprocating instruments were used.
In the present investigation, the reduction of AHTD differed significantly among the tested final irrigation protocols; therefore, the null hypothesis was rejected. The GW system removed AHTD by 96.4% within the mesial root canals and by 97.9% within the isthmus areas. These results supported the efficacy of GW in cleansing the complex root canal system in the mesial canals of molars. The IU group reduced AHTD by 91.2% and 93.5% from the canals and isthmus areas, respectively. Its efficacy was statistically comparable with the GW system and appeared superior to that reported in previous micro-CT studies in which similar sequential irrigation steps showed a reduction of 50.8% and 55.6%, respectively. The greater efficacy of IU reported in the present study could be attributed to the higher activation power setting in this study compared with the previous ones. In contrast, the CU group showed the lowest AHTD reduction despite its comparable efficacy to IU, which is in accordance to previous reports. However, it was inferior to that of GW. One limitation of the present study is the use of a different irrigation solution sequence in the ultrasonically activated irrigation experimental groups compared with the GW group.
Although its clinical significance remains to be elucidated, dentin erosion has been observed in vitro when NaOCl is used as a final irrigation solution after demineralization agents. The use of NaOCl as a final rinse in the ultrasonically activated irrigation groups could have potentially created cleaner root canal walls with fewer dentin debris by allowing a deeper penetration of NaOCl into areas previously covered by the smear layer.
Although AHTD removal efficacy was comparable for the GW system and IU, the greater penetration of irrigation delivered by the GW system compared with ultrasonically activated irrigation systems was previously suggested. Ultrasonic irrigation devices rely on the transmission of acoustic energy from an oscillating file in which the file motion is likely to be impeded as the root canal narrows toward the apical portion. In contrast, the GW system uses a broad spectrum of sound waves to distribute fluids throughout the entire root canal system. Compared with ultrasonic energy that is dispersed at a single frequency, multisonic energy emitted by the GW system enables effective delivery of energized irrigation into microsized dentinal tubules at a high flow rate. The interaction between the continuous flow of irrigation solution and the stationary fluid inside the pulp chamber creates a strong shear force that induces a cavitation cloud. The implosion of cavitation bubbles generates multisonic energy produced by a broad spectrum of acoustic waves as well as acoustic streaming with a vortical flow pattern. The hydrodynamic effects are further enhanced by the use of degassed irrigation fluids, which can minimize energy loss and optimize fluid delivery throughout the entire root canal system.
Although the clinical implications of AHTD remain unknown, dentin debris have been shown to significantly alter the biological efficacy of intracanal disinfectants. In addition, dentin debris exhibit inhibitory effects on commonly used irrigation solutions by diminishing free available chlorine and antibacterial properties of NaOCl.
Furthermore, AHTD may protect microorganisms clogged in inaccessible areas by providing a spatial barrier between the bacteria and antimicrobial irrigation.
AHTD may also interfere with the seal provided by the root filling. The aforementioned potential concerns highlight the need for the development of measures to prevent and to disrupt AHTD to enhance access to biofilms inside inaccessible areas of the root canal system in the quest for improving long-term prognosis. Further investigation is warranted to define the relationship between hard tissue debris and biofilms. With further methodologic refinement in micro-CT imaging, future studies should also aim to image and quantify biofilms within the root canal system.
Conclusion
Within the limitations of this in vitro study, none of the tested irrigation protocols was able to render the mesial root canals and isthmus areas of mandibular molars free of dentin debris. The GW system showed better efficacy in the removal of AHTD from the mesial canals and isthmus areas when compared with CU but not IU. The efficacy of IU and CU irrigation systems was comparable.
Authors: Rebecca Chan, Marco A. Versiani, Shimon Friedman, Gevik Malkhassian, Manoel D. Sousa-Neto, Graziela B. Leoni, Yara T.C. Silva-Sousa, Bettina Basrani
References:
- Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol 1983;55:307–12.
- Nair PN, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:231–52.
- Ricucci D, Siqueira Jr JF. Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod 2010;36:1277–88.
- Carr GB, Schwartz RS, Schaudinn C, et al. Ultrastructural examination of failed molar retreatment with secondary apical periodontitis: an examination of endodontic biofilms in an endodontic retreatment failure. J Endod 2009;35:1303–9.
- Weller RN, Niemczyk SP, Kim S. Incidence and position of the canal isthmus. Part 1. Mesiobuccal root of the maxillary first molar. J Endod 1995;21:380–3.
- Green D. Double canals in single roots. Oral Surg Oral Med Oral Pathol 1973;35:689–96.
- Piñeda F, Kuttler Y. Mesiodistal and buccolingual roentgenographic investigation of 7,275 root canals. Oral Surg Oral Med Oral Pathol 1972;33:101–10.
- Vertucci FJ. Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol 1984;58:589–99.
- Paqué F, Laib A, Gautschi H, Zehnder M. Hard-tissue debris accumulation analysis by high- resolution computed tomography scans. J Endod 2009;35:1044–7.
- Paqué F, Boessler C, Zehnder M. Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. Int Endod J 2011;44:148–53.
- Wayman BE, Patten JA, Dazey SE. Relative frequency of teeth needing endodontic treatment in 3350 consecutive endodontic patients. J Endod 1994;20:399–401.
- de Pablo OV, Estevez R, Peix Sanchez M, et al. Root anatomy and canal configuration of the permanent mandibular first molar: a systematic review. J Endod 2010;36:1919–31.
- Teixeira FB, Sano CL, Gomes BP, et al. A preliminary in vitro study of the incidence and position of the root canal isthmus in maxillary and mandibular first molars. Int Endod J 2003;36:276–80.
- von Arx T. Frequency and type of canal isthmuses in first molars detected by endoscopic inspection during periradicular surgery. Int Endod J 2005;38:160–8.
- Cameron JA. The effect of ultrasonic endodontics on the temperature of the root canal wall. J Endod 1988;14:554–9.
- Charara K, Friedman S, Sherman A, et al. Assessment of apical extrusion during root canal irrigation with the novel GentleWave system in a simulated apical environment. J Endod 2016;42:135–9.
- Haapasalo M, Shen Y, Wang Z, et al. Apical pressure created during irrigation with the GentleWave system compared to conventional syringe irrigation. Clin Oral Investig 2016;20:1525–34.
- Haapasalo M, Wang Z, Shen Y, et al. Tissue dissolution by a novel multisonic ultracleaning system and sodium hypochlorite. J Endod 2014;40:1178–81.
- Molina B, Glickman G, Vandrangi P, Khakpour M. Evaluation of root canal debridement of human molars using the GentleWave system. J Endod 2015;41:1701–5.
- Leoni GB, Versiani MA, Silva-Sousa YT, et al. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int Endod J 2016;50:398–406.
- Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol 1971;32:271–5.
- Nair PN. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod 1987;13:29–39.
- Robinson JP, Lumley PJ, Cooper PR, et al. Reciprocating root canal technique induces greater debris accumulation than a continuous rotary technique as assessed by 3- dimensional micro-computed tomography. J Endod 2013;39:1067–70.
- Freire LG, Iglecias EF, Cunha RS, et al. Micro-computed tomographic evaluation of hard tissue debris removal after different irrigation methods and its influence on the filling of curved canals. J Endod 2015;41:1660–6.
- van der Sluis L, Wu MK, Wesselink P. Comparison of 2 flushing methods used during passive ultrasonic irrigation of the root canal. Quintessence Int 2009;40:875–9.
- Tanomaru FM, Torres FF, Chavez-Andrade GM, et al. Intermittent or continuous ultrasonically activated irrigation: micro-computed tomographic evaluation of root canal system cleaning. Clin Oral Investig 2016;20:1541–6.
- Vandrangi P. Evaluating penetration depth of treatment fluids into dentinal tubules using the GentleWave System. Dentistry 2016;6:366.
- van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J 2007;40:415–26.
- Walmsley AD, Williams AR. Effects of constraint on the oscillatory pattern of endosonic files. J Endod 1989;15:189–94.
- Ahmad M, Pitt Ford TJ, Crum LA. Ultrasonic debridement of root canals: acoustic streaming and its possible role. J Endod 1987;13:490–9.
- Haapasalo HK, Siren EK, Waltimo TM, et al. Inactivation of local root canal medicaments by dentine: an in vitro study. Int Endod J 2000;33:126–31.
- Portenier I, Haapasalo H, Rye A, et al. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J 2001;34:184–8.
- Arias-Moliz MT, Morago A, Ordinola-Zapata R, et al. Effects of dentin debris on the antimicrobial properties of sodium hypochlorite and etidronic acid. J Endod 2016;42:771–5.
- Paqué F, Rechenberg DK, Zehnder M. Reduction of hard-tissue debris accumulation during rotary root canal instrumentation by etidronic acid in a sodium hypochlorite irrigant. J Endod 2012;38:692–5.
- Endal U, Shen Y, Knut A, et al. A high-resolution computed tomographic study of changes in root canal isthmus area by instrumentation and root filling. J Endod 2011;37:223–7.
