Adjunctive Steps for Disinfection of the Mandibular Molar Root Canal System: A Correlative Bacteriologic, Micro–Computed Tomography, and Cryopulverization Approach
Abstract
Introduction: This study evaluated the disinfecting ability of chemomechanical preparation with rotary nickel-titanium instruments, followed by 2 distinct adjunctive procedures in the root canals of extracted mandibular molars by means of a correlative analytical approach.
Methods: Twenty-two extracted mandibular molars were selected and anatomically matched between groups on the basis of micro–computed tomographic analysis. In the first phase of the experiment, root canals were contaminated with Enterococcus faecalis and subjected to chemomechanical preparation with BT RaCe instruments and 2.5% NaOCl irrigation. Then either XP-Endo Finisher instrument or passive ultrasonic irrigation was used to supplement disinfection. Micro–computed tomography was used to show whether the percentage of unprepared areas correlated to bacterial counts. In the second phase, the same teeth were contaminated once again, and the adjunctive procedures were used. Samples from the isthmus area of mesial roots and the apical 5-mm fragment of distal roots were obtained by cryopulverization. Samples taken before and after treatment steps in both phases were evaluated by quantitative polymerase chain reaction and statistically analyzed.
Results: In phase 1, preparation in both groups resulted in substantial decrease of bacterial counts (P < .001). The adjunctive approaches led to a further small bacterial reduction, which was significant for XP-Endo Finisher (P < .05). No significant differences were observed between groups for persisting bacterial counts. Correlative analysis revealed no statistically significant relationship between bacterial reduction and the percentage of unprepared areas (P > .05). In phase 2, both methods had significant antibacterial effects in the main canal, but none of them could predictably disinfect the isthmus/recess areas.
Conclusions: Both XP-Endo Finisher and passive ultrasonic irrigation exhibited antibacterial effectiveness, but only the former caused a significant reduction in the bacterial counts after chemomechanical preparation. None of them were effective in predictably disinfecting the isthmus/recess areas. (J Endod 2016;42:1667–1672).
Irrespective of the instrumentation techniques, instruments, and irrigants, a thorough cleaning, disinfection, and shaping of the root canal have not been commonly achieved, especially in teeth with curved canals or unusual anatomies. Studies that used high-resolution micro–computed tomographic imaging technology (micro-CT) have shown that 11%–48% of the main root canal areas remain untouched after instrumentation. These areas may be colonized by biofilms that have the potential to remain unaffected and put the treatment outcome at risk. Moreover, certain anatomic complexities of the root canal system, such as ramifications, recesses, and isthmi, are not commonly reached by instruments and irrigants. Bacteria located in these areas can persist and lead to persistent apical periodontitis. Actually, clinical bacteriological studies have demonstrated that bacteria are still detected in about 30%–60% of the canals after chemomechanical preparation. Bacteria persisting in the canal are the most important risk factor for post-treatment apical periodontitis. Efforts have been expended toward developing adjunctive approaches to enhance root canal disinfection. This involves approaches that drive irrigants to difficult-to-reach areas or allow for the instrument to reach and mechanically debride unaffected regions. One of these adjunctive approaches is passive ultrasonic irrigation (PUI), which comprises the ultrasonic activation of an irrigant. Data from in vitro and in vivo studies evaluating the benefits in terms of antibacterial effects of the adjunctive PUI approach with NaOCl have been inconclusive.
The XP-Endo Finisher (FKG Dentaire, La Chaux-de-Fonds, Switzerland) instrument was recently introduced with the promise of enhancing root canal cleaning and disinfection. This is a size 25 non-tapered instrument made of nickel-titanium (NiTi) MaxWire alloy (Martensite-Austenite Electropolish FleX). At room temperature the instrument is straight in its martensite phase, but at body temperature it changes to the austenite phase and develops a spoon shape; when rotated and moved up and down in the canal, this shape makes the instrument expand and contract to touch the canal walls and shake the irrigant solution. A recent study showed that XP-Endo Finisher and PUI succeeded in rendering the mesial root canal system with significantly lower levels of hard-tissue debris compared with conventional irrigation and a modified Self-Adjusting File system protocol. So far, only 1 study has investigated the antibacterial benefits of using XP-Endo Finisher and reported better results than conventional irrigation. No study has evaluated the antibacterial effects of this instrument after chemomechanical procedures.
The present study was intended to evaluate the disinfecting and shaping ability of chemomechanical preparation with rotary NiTi instruments, followed by 2 distinct adjunctive approaches in the root canals of extracted mandibular molars by means of a correlative bacteriologic and micro-CT analysis. A cryopulverization approach was used to evaluate the bacteriologic conditions of the isthmus and recess areas after the use of either XP-Endo Finisher instrument or PUI adjunctive procedures.
Materials and Methods
Tooth Selection and Preparation
The study protocol was approved by the Ethics Committee of the Estácio de Sá University, Rio de Janeiro, RJ, Brazil. Twenty-two extracted mandibular molars with 2 independent canals joined apically by an isthmus in the mesial root (Vertucci type II) and a single distal canal (Vertucci type I) were selected from a collection of 185 mandibular molars on the basis of radiographs taken in both buccolingual and mesiodistal directions, exploration with small files after access preparation, and micro-CT imaging by using a SkyScan 1174v2 scanner (Bruker-microCT, Kontich, Belgium) operated at 50 Kv, 800 mA, isotropic resolution of 19.86 mm, and 180◦ rotation around the vertical axis with a rotation step of 1.0 by using a 0.5-mm-thick aluminum filter.
Images of each specimen were reconstructed with a ring artifact correction of 5, a beam hardening correction of 15%, and smoothing of 5 (NRecon v.1.6.9.16; Bruker-microCT). CTAn v.1.14.4 software (Bruker-microCT) was used for 3-dimensional (3D) evaluation of the root canal regarding volume and surface area, and CTVol v.2.2.1 software (Bruker-microCT) was used for visualization and qualitative evaluation of the root canal system configuration. The specimens were pair-matched on the basis of the morphologic and anatomic aspects of the mesial and distal root canal systems, assessed by micro-CT, and 1 specimen from each pair was randomly assigned to 1 of the 2 experimental groups.
The root canals were explored with #15 hand K-files until the instrument tip reached the apical foramen, as visualized by a stereomicroscope. This measure was recorded as the patency length, and the canals were initially enlarged up to this point by using the BioRaCe BR2 (25/04) instrument (FKG Dentaire) operated in the VDW Gold motor (VDW, Munich, Germany) at 300 rpm, 1.5 N • cm, to standardize the initial canal diameter and create room for bacterial contamination. Smear layer was removed by using 17% EDTA and 2.5% NaOCl irrigation. NaOCl was inactivated with 5% sodium thiosulfate. The teeth were scanned again in micro-CT by using the previously mentioned parameters, and the obtained data sets were used as baseline for comparison with post-preparation images.
Phase 1
For contamination, the root canals were filled with trypticase soy broth (Difco, Detroit, MI) by using Navitip (Ultradent Products Inc, South Jordan, UT) needles until the broth flowed through the apical foramen. The teeth were placed in a flask containing 50 mL trypticase soy broth and ultrasonicated for 1 minute to release entrapped air and allow for penetration of the culture medium into root canal irregularities. Next, the teeth were sterilized in an autoclave. A fresh culture of Enterococcus faecalis ATCC 29212 grown for 24 hours at 37◦C was used as inoculum for root canal contamination. The teeth were incubated for 30 days at 37◦C under gentle shaking, and the culture medium was replenished every week. Later, all contaminated teeth had the excess culture medium dripped off, and their external root surfaces were wiped with sterile gauze. Two teeth were fixed in 10% buffered formalin and processed for scanning electron microscopy (SEM) to confirm bacterial colonization as described elsewhere.
The apical foramina of each root were sealed with Topdam (FGM, Joinville, SC, Brazil) to prevent apical bacterial leakage and create a closed-end system. Before root canal preparation, the outer root surfaces were cleaned with 3% hydrogen peroxide and disinfected with 2.5% NaOCl, followed by inactivation of the latter with 5% sodium thiosulfate. Teeth were mounted vertically up to the cervical region in blocks made of silicone impression material (President Jet; Coltène AG, Cuyahoga Falls, OH). The tooth crown, including the pulp chamber walls, and the silicone surface were disinfected with 2.5% NaOCl, followed by inactivation of this substance with 5% sodium thiosulfate. Samples were taken from the root canal by using paper points before (P1S1) and after chemomechanical preparation (P1S2) and after the adjunctive approach (P1S3) (Fig. 1A). The root canal was rinsed with 1 mL sterile 0.85% saline solution to remove unattached cells, and 3–5 sterile paper points were used sequentially at the working length (WL), which was established at 0.5 mm of the patency length. Each paper point remained in the canal for 1 minute. Paper points were transferred to tubes containing 1 mL Tris-EDTA buffer (10 mmol/L Tris-HCl, 1 mmol/L EDTA, pH 7.6) and frozen at –20◦C. In the mesial root, samples were taken from each canal, but they were pooled for further bacteriologic analyses because the 2 canals merged into 1 at the apical portion. Canals were prepared at the WL by using the BT RaCe system (FKG Dentaire), operated in the VDW Gold motor at 600 rpm, 1.5 N • cm, up to the BT3 instrument. Irrigation was carried out by using 2.5% NaOCl delivered by Navitip needles taken up to 2 mm short of the WL (Fig. 1A). During instrumentation of the mesial canals, the orifice of the distal canal was sealed with Topdam (and vice-versa) to avoid leakage of irrigants into it. After apical preparation, the canal was irrigated with NaOCl, EDTA (for smear layer removal), and then NaOCl again (Fig. 1A). After inactivation of NaOCl with 5% sodium thiosulfate, P1S2 sample was taken as described above, and teeth from each group were subjected to either PUI or XP-Endo Finisher adjunctive procedures as follows.
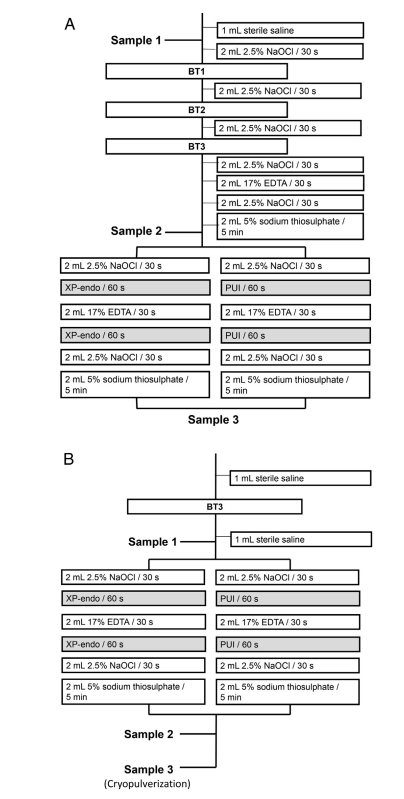
PUI.
Root canals were irrigated with 2 mL 2.5% NaOCl, which was ultrasonically activated in the canal for 1 minute by using the EndoUltra device (Vista Dental Products, Racine, WI), with the probe tip placed 1 mm short of the WL. The canals were irrigated with 2 mL EDTA, which was ultrasonically activated the same way as above, followed by a final flush with 2.5% NaOCl. Finally, NaOCl was inactivated with 2 mL 5% sodium thiosulfate, and P1S3 sample was taken.
XP-Endo Finisher Instrument.
XP-Endo Finisher was operated up to the WL for 1 minute after irrigation with 2 mL 2.5% NaOCl. The instrument was coupled to the VDW Gold motor at 800 rpm, 1 N • cm, with slow up-and-down 7- to 8-mm-long movements. The root canals were then irrigated with 17% EDTA, and XP-Endo was used again. After final irrigations with 2.5% NaOCl and sodium thiosulfate, P1S3 sample was taken.
The same final volume of irrigants was used per group, with 14 mL NaOCl per canal. In both groups, irrigants were preheated at 37◦C for the intracanal procedures, which were carried out at 37◦C inside a cabinet containing a heater (800-Heater; PlasLabs, Lansing, MI).
The specimens were autoclaved and subjected to a new micro-CT scan using the aforementioned parameter settings. Preoperative and postoperative color-coded 3D models of the mesial canals were rendered (CTVol v.2.2.1; Bruker-microCT) and co-registered with their respective preoperative data sets (green and red indicate preoperative and postoperative canal surfaces, respectively) by using the rigid registration module of the 3D Slicer 4.3.1 software (available from http://www.slicer.org), with accuracy greater than 1 voxel. Then, matched images were examined to calculate volume (mm3) and surface area (mm2) of the mesial root canal system before and after preparation by using CTAn v.1.14.4 software (Bruker micro-CT). The area of untouched canal surface was determined by calculating the number of static voxels and expressed as a percentage of the total number of voxels present on the canal surface.
Phase 2
The same tooth specimens from phase 1 were used in the second phase of the experiment. Eighteen teeth were still available. This second phase was carried out to increase the number of teeth with canals positive for bacteria before application of the adjunctive approaches, allowing for a more robust statistic comparison. In addition, it was possible to test both approaches against more controlled initial bacterial loads. Finally, evaluation of the effects of the isolated adjunctive approach on the isthmus areas was included in this second experiment.
Teeth were sterilized in autoclave, contaminated, and mounted as described in phase 1. Next, the root canals were rinsed with 1 mL saline and instrumented once again with a BT3 instrument to slightly reduce the initial bacterial load. P2S1 was taken as in phase 1. Each canal was irrigated with 2 mL 2.5% NaOCl, and either XP-Endo Finisher instrument or PUI was used as previously described (Fig. 1B). After irrigation with EDTA, the adjunctive approaches were performed once again, and then the canal was irrigated with NaOCl and sodium thiosulfate. P2S2 sample was collected.
On the basis of the root canal cross-section micro-CT images, the position of the isthmus in the mesial root was established, and the specimens were cut off by using sterile double-faced diamond disks to generate isthmus-containing root fragments for analysis. The distal roots had a single flattened canal containing recesses and were sectioned 5 mm from the apex. The root fragments corresponding to the isthmus area of the mesial root and the apical part of the distal root had their outer surfaces cleaned with 3% hydrogen peroxide and disinfected with 2.5% NaOCl, which was further inactivated with sodium thiosulfate. In sequence, the external root surfaces were sampled by using a #80 sterile paper point dampened with Tris-EDTA buffer. This sample served as sterility control and was assessed by means of a quantitative real-time polymerase chain reaction (qPCR) assay. These disinfecting and control sampling procedures were conducted under an operating microscope. A 6750 freezer mill (Spex, Metuchen, NJ) operated at the liquid nitrogen temperature was used to cryogenically grind each root fragment as described elsewhere. After grinding, apical root powder samples (P2S3) were suspended in Tris-EDTA buffer and stored at –20◦C.
DNA Extraction and qPCR Assay
DNA was extracted from the samples from both experimental phases and used as template for quantification of E. faecalis cells by using a 16S rRNA gene-targeted qPCR assay. DNA extraction and qPCR steps, controls, and conditions were as described previously. All measurements were done in triplicate.
Statistical Analysis
The Wilcoxon matched pairs test was used to compare the intragroup reduction in bacterial counts from P1S1/P2S1 to P1S2/P2S2, P1S1/P2S1 to P1S3/P2S3, and P1S2/P2S2 to P1S3/P2S3. Initial samples (P1S1/P2S1) were compared between groups by using the non-parametric Mann-Whitney U test, which revealed no significant difference between them (P > .05). Therefore, the same test was used to compare counts in P1S2/P2S2 and P1S3/P2S3 samples between groups. Initially, analyses were performed for mesial and distal roots separately. Because there were no significant differences between mesial and distal canals, data were also gathered to increase robustness of the statistical analysis. Pearson correlation analysis was used to verify the relationships between bacterial reduction and percentage of unprepared areas. Statistical analyses were performed with STATISTICA version 8 (StatSoft, Tulsa, OK) with a significance level set at 5%.
Results
SEM analysis revealed that E. faecalis colonized the root canal walls, generally forming biofilm-like structures (data not shown). Root canal colonization was further confirmed by qPCR positive results in P1S1/P2S1 samples from all teeth.
Phase 1
Table 1 displays the mean, median, and range of E. faecalis counts observed for the test groups. In the XP-Endo Finisher group, E. faecalis counts were substantially reduced from P1S1 to P1S2 (P < .001). After using the XP-Endo instrument (P1S3), there was an additional significant reduction (P < .05). All 20 samples were positive for E. faecalis in P1S1, 6 in P1S2, and 6 in P1S3. In the PUI group, the initial bacterial counts (P1S1) were also significantly decreased after preparation (P1S2) (P < .001). Although the bacterial counts were further reduced in P1S3, they were not significantly different from P1S2 (P > .05). All 20 samples were positive in P1S1, 10 in P1S2, and 7 in P1S3. No significant differences were observed when comparing P1S3 from XP-Endo and PUI groups (P > .05).
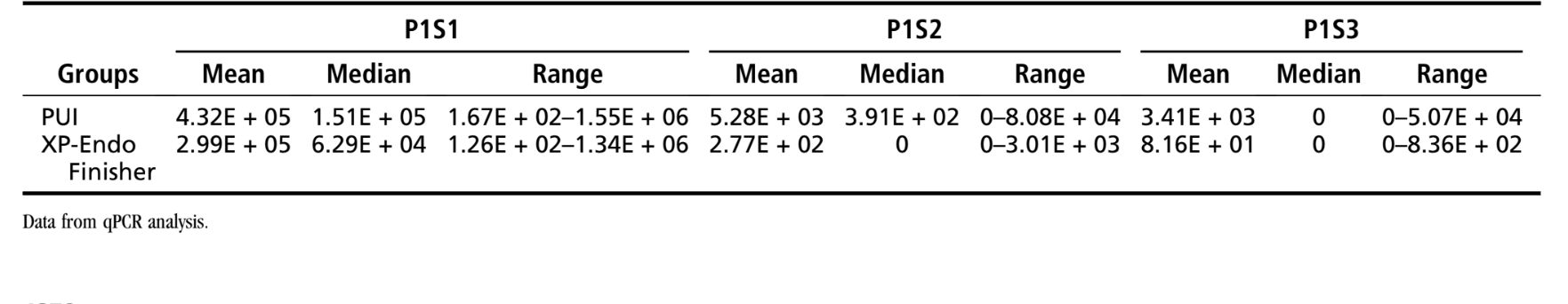
Statistical analysis of the mesial and distal data separately showed absence of significance for all comparisons between P1S2 and P1S3 (P > .05) except for the mesial canals in the XP-Endo Finisher group (P < .05). Data from mesial and distal roots separately are shown in Supplemental Table S1.
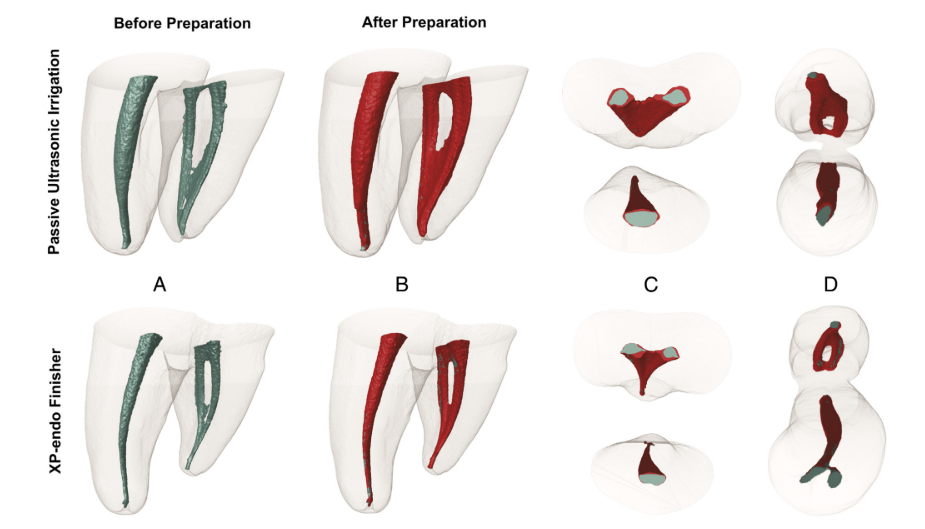
Micro-CT analysis showed no significant differences in the initial canal volumes between groups (P > .05). As for unprepared areas, a mean percentage value of static voxels of 4.5% (median, 4.3%) was observed for the XP-Endo Finisher group and 4.3% (median, 5.4%) for the PUI group (P > .05) (Fig. 2). Correlative analysis revealed no statistically significant relationship between bacterial reduction and the percentage of unprepared areas (P > .05).
Phase 2
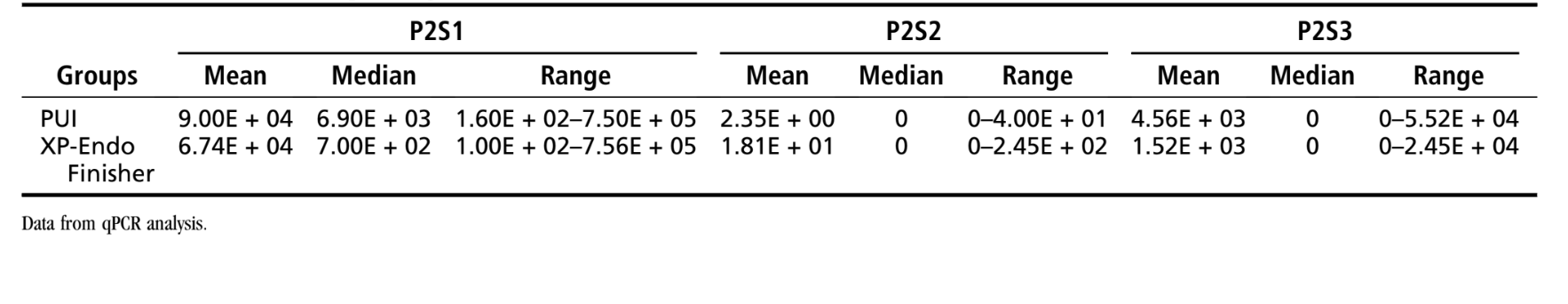
Table 2 depicts the quantitative data from phase 2. In the XP-Endo Finisher group, the E. faecalis counts in P2S1 were significantly reduced after using this instrument (P < .001). All 18 samples were positive for bacteria in P2S1, 2 in P2S2, and 4 in P2S3 (pulverized samples). The PUI procedure was also highly effective in reducing the bacterial counts in the main canal (P < .001). All 18 samples were positive for bacteria in P2S1, 1 in P2S2, and 6 in P2S3 (pulverized samples). No significant differences were observed when comparing XP-Endo and PUI effects in P2S2 (main canals) or P2S3 (isthmi/recesses) (P > .05). Statistical analysis of the mesial and distal data separately showed no significant differences between groups either (P > .05) (Supplemental Table S2).
Discussion
This study correlated different analytical tools to evaluate, in 2 experimental phases, the antibacterial effects of adjunctive approaches in the mandibular molar root canal system. In the first phase, the effects of the 2 procedures used immediately after chemomechanical preparation with rotary NiTi instrumentation and NaOCl irrigation were evaluated. This analysis was restricted to the antibacterial effects in the main root canal as determined by paper point sampling. Because 50% (PUI group) and 70% (XP-Endo Finisher group) of the cases showed negative results for bacteria in P1S2, reducing the sample size for intergroup analysis of P1S3, a second phase was devised to increase the number of canals subjected to both approaches and include analysis of their effects not only in the main canals but also in the isthmus and recess areas by cryogenically grinding the root for sample taking.
In the first phase, chemomechanical instrumentation with BT RaCe and NaOCl irrigation promoted substantial bacterial elimination in both groups, which agrees with previous studies. Whereas the additional procedure step with PUI did not succeed in significantly further reducing the bacterial counts, the intracanal E. faecalis counts after using XP-Endo Finisher were significantly lower than those obtained immediately after preparation. The significantly better results for XP-Endo occurred in the mesial roots. In the second phase, the initial samples consisted of an initial lower bacterial load to permit comparison of both approaches against bacterial numbers compatible with what the adjunctive procedures are expected to face in the clinical situation. In this phase, PUI and XP-Endo Finisher approaches were similar and highly effective in reducing the bacterial counts in the main canal.
As for the isthmus/recess areas, the destructive nature of the cryopulverization approach did not permit a longitudinal analysis to be made. Therefore, it is not possible to infer whether negative results were because of the effects of the adjunctive approaches or unsuccessful bacterial contamination in those areas. However, there were no significant differences in bacterial counts between groups. Counts in the isthmus area (P2S3) were indeed higher than in the main canal (P2S2), suggesting that the effects of PUI and XP-Endo Finisher in that region are not predictable. This along with the findings of several teeth with canal-negative and isthmus-positive samples highlights the limitation of the paper point approach in sampling the root canal system.
The overall results for the XP-Endo Finisher instrument were encouraging because it generally performed in a comparable way with PUI, a widely recommended adjunctive approach. In a recent study, XP-Endo Finisher instrument was more efficient than other techniques in disinfecting the main canal space. The design and helical movement of the instrument may have allowed it to reach previously untouched areas and displace bacterial biofilms. The increase of touched areas was not evaluated by micro-CT because of the minimal, if any, cutting ability of the XP-Endo Finisher instrument.
Our findings with PUI are in agreement with several studies that reported no significant improvement in disinfection after preparation. The antibacterial effects of PUI are suggested to be related to cavitation, acoustic streaming, and heating of the irrigant, but if these phenomena really happen in the root canal, they do not seem to suffice to significantly enhance bacterial elimination.
There was no correlation between the percentage of unprepared areas and the bacterial levels in P1S3, which is in consonance with a previous study. The possibility exists that in some specimens the untouched areas of the main canal may not have been colonized by bacteria. Moreover, it is possible that uninstrumented areas were indeed disinfected by NaOCl irrigation. Limitations of the paper point sampling procedure may also have accounted for this lack of correlation.
The present experimental design has some noteworthy aspects. Micro-CT scans were used to select and match the teeth according to anatomic similarities before distribution between groups, minimizing variables inherent to anatomy. In addition, heated irrigants were used throughout the experiments. This had to be done because XP-Endo Finisher instrument undergoes phase transformation at body temperature. Then we decided to include warmed solutions in all phases of the experiment, which was also performed inside a cabinet with temperature kept at 37◦C. Most previous studies in extracted teeth were con- ducted at room temperature. Because temperature may affect the antibacterial activity of NaOCl, it is advisable to perform antibacterial tests under a temperature similar to body temperature. Another advantage of this study was the use of qPCR for bacterial quantification. This approach is very sensitive and could be reliably used in cryopulverized samples (pilot studies that used culture showed loss of bacterial counts because of the grinding approach). The use of cryopulverization in turn was essential to permit the analysis of the antibacterial effects of the tested approaches in areas such as isthmi and recesses, which may not be properly sampled by paper points.
Although this study is innovative in combining different analytical approaches, it also has limitations. Sample taking with paper points is usually restricted to the main canal, and some areas such as irregularities, isthmi, and recesses may not be sampled. Limitation of the paper point technique was evident in phase 2, when more canals were positive for bacteria in P2S3 than in P2S2; higher mean counts were also shown for P2S3. In addition, because paper point sampling does not distinguish the segment of the main canal, it remains unknown in which part bacteria remained. Cryopulverization can sidestep these limitations, but it is a destructive method and can only be used for cross-sectional analysis. Another limitation may refer to the use of qPCR for bacterial detection. There is a concern that DNA from cells that recently died as a consequence of antibacterial treatment can also be detected by this method. Nevertheless, a previous study that used a similar in vitro protocol showed no significant difference for bacterial counts between culture and qPCR in post-treatment samples. This, along with the high occurrence of negative qPCR results in post-treatment samples, strongly suggests that DNA from dead cells may not have been a significant problem in the present study. Free DNA can be degraded by NaOCl or may have been washed away during irrigation.
In conclusion, this study demonstrated that both adjunctive approaches caused a small reduction in bacterial counts after chemomechanical preparation, which was significant only for XP-Endo Finisher. Neither XP-Endo nor PUI was effective in predictably disinfecting the isthmus/recess areas of mandibular molars.
Authors: Flavio R.F. Alves, Carlos V. Andrade-Junior, Marılia F. Marceliano-Alves, Alejandro R. Perez, Isabela N. Rôças, Marco A. Versiani, Manoel D. Sousa-Neto, Jose C. Provenzano, Jose F. Siqueira
References:
- Siqueira JF Jr, Araujo MC, Garcia PF, et al. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod 1997;23:499–502.
- Peters OA, Schönenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J 2001;34: 221–30.
- Vera J, Siqueira JF Jr, Ricucci D, et al. One- versus two-visit endodontic treatment of teeth with apical periodontitis: a histobacteriologic study. J Endod 2012;38: 1040–52.
- Paqué F, Zehnder M, De-Deus G. Microtomography-based comparison of reciprocating single-file F2 ProTaper technique versus rotary full sequence. J Endod 2011; 37:1394–7.
- Markvart M, Darvann TA, Larsen P, et al. Micro-CT analyses of apical enlargement and molar root canal complexity. Int Endod J 2012;45:273–81.
- Siqueira JF Jr, Alves FR, Versiani MA, et al. Correlative bacteriologic and micro-computed tomographic analysis of mandibular molar mesial canals prepared by self-adjusting file, Reciproc, and Twisted File systems. J Endod 2013;39:1044–50.
- Peters OA, Arias A, Paque F. A micro-computed tomographic assessment of root canal preparation with a novel instrument, TRUShape, in mesial roots of mandibular molars. J Endod 2015;41:1545–50.
- Ricucci D, Siqueira JF Jr. Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod 2010;36:1277–88.
- Ricucci D, Siqueira JF Jr, Bate AL, Pitt Ford TR. Histologic investigation of root canal-treated teeth with apical periodontitis: a retrospective study from twenty-four patients. J Endod 2009;35:493–502.
- Paiva SS, Siqueira JF Jr, Rôças IN, et al. Supplementing the antimicrobial effects of chemomechanical debridement with either passive ultrasonic irrigation or a final rinse with chlorhexidine: a clinical study. J Endod 2012;38:1202–6.
- Byström A. Evaluation of endodontic treatment of teeth with apical periodontitis [odontological dissertation no. 27]. Umea, Sweden: University of Umea; 1986.
- Vianna ME, Horz HP, Gomes BP, Conrads G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J 2006;39:484–92.
- Sjögren U. Success and failure in endodontics [odontological dissertation no. 60]. Umea, Sweden: University of Umea; 1996.
- Neves MA, Provenzano JC, Rôças IN, Siqueira JF Jr. Clinical antibacterial effectiveness of root canal preparation with reciprocating single-instrument or continuously rotating multi-instrument systems. J Endod 2016;42:25–9.
- Siqueira JF Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod 2008;34:1291–13013.
- Siqueira JF Jr, Rôças IN. Optimising single-visit disinfection with supplementary approaches: a quest for predictability. Aust Endod J 2011;37:92–8.
- Alves FR, Almeida BM, Neves MA, et al. Disinfecting oval-shaped root canals: effectiveness of different supplementary approaches. J Endod 2011;37:496–501.
- Tardivo D, Pommel L, La Scola B, et al. Antibacterial efficiency of passive ultrasonic versus sonic irrigation: ultrasonic root canal irrigation. Odontostomatol Trop 2010;33:29–35.
- Carver K, Nusstein J, Reader A, Beck M. In vivo antibacterial efficacy of ultrasound after hand and rotary instrumentation in human mandibular molars. J Endod 2007; 33:1038–43.
- Paiva SS, Siqueira JF Jr, Rôças IN, et al. Molecular microbiological evaluation of passive ultrasonic activation as a supplementary disinfecting step: a clinical study. J Endod 2013;39:190–4.
- Debelian G, Trope M. Cleaning the third dimension. Endodontic Practice 2015;8: 22–4.
- Leoni GB, Versiani MA, Silva-Sousa YT, et al. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int Endod J 2016 Mar 18. http://dx.doi.org/ 10.1111/iej.12630. [Epub ahead of print].
- Azim AA, Aksel H, Zhuang T, et al. Efficacy of 4 irrigation protocols in killing bacteria colonized in dentinal tubules examined by a novel confocal laser scanning microscope analysis. J Endod 2016;42:928–34.
- Siqueira JF Jr, Alves FR, Almeida BM, et al. Ability of chemomechanical preparation with either rotary instruments or self-adjusting file to disinfect oval-shaped root canals. J Endod 2010;36:1860–5.
- Alves FR, Siqueira JF Jr, Carmo FL, et al. Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. J Endod 2009;35:486–92.
- Antunes HS, Rôças IN, Alves FR, Siqueira JF Jr. Total and specific bacterial levels in the apical root canal system of teeth with post-treatment apical periodontitis. J Endod 2015;41:1037–42.
- Alves FR, Rôças IN, Almeida BM, et al. Quantitative molecular and culture analyses of bacterial elimination in oval-shaped root canals by a single-file instrumentation technique. Int Endod J 2012;45:871–7.
- van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J 2007;40:415–26.
- Martin H. Ultrasonic disinfection of the root canal. Oral Surg Oral Med Oral Pathol 1976;42:92–9.
- Ahmad M, Pitt Ford TR, Crum LA. Ultrasonic debridement of root canals: an insight into the mechanisms involved. J Endod 1987;13:93–101.
- Sirtes G, Waltimo T, Schaetzle M, Zehnder M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J Endod 2005;31:669–71.
- Siqueira JF Jr, Rôças IN. Exploiting molecular methods to explore endodontic infections: part 1—current molecular technologies for microbiological diagnosis. J Endod 2005;31:411–23.
- McCarty SC, Atlas RM. Effect of amplicon size on PCR detection of bacteria exposed to chlorine. PCR Methods Appl 1993;3:181–5.
- Fouad AF, Barry J. The effect of antibiotics and endodontic antimicrobials on the polymerase chain reaction. J Endod 2005;31:510–3.

/public-service/media/default/147/bjsSM_65311952dfadf.jpg)
/public-service/media/default/158/GMj69_65311b2333f75.jpg)
/public-service/media/default/460/aU9ju_671a20a2e53f3.png)
/public-service/media/default/145/GbhGY_65311921a3b65.jpg)