Contrast-enhanced micro-CT to assess dental pulp tissue debridement in root canals of extracted teeth: a series of cascading experiments towards method validation
Abstract
Aim: To validate a new method for the evaluation of pulp tissue debridement in the root canals of extracted teeth using an impregnation protocol involving potassium triiodide, a radiocontrast solution known as Lugol’s, combined with micro-computed tomographic (micro-CT) imaging.
Methodology: The impact of NaOCl on the radiopacity of Lugol’s solution was assessed using a two-fold dilution series of Lugol in distilled water and 5.25% NaOCl, which were then pipetted into transparent dishes and radiographed. To verify the influence of Lugol on the proteolytic effect of NaOCl, a dissolution test was performed using fresh bovine meat. Ten slices did not undergo any tissue processing, whilst twenty slices were fixed in formaldehyde for 24 h. After that, 10 of them were immersed in Lugol for another 24 h. Then, all specimens were placed in NaOCl and the time required for a complete tissue dissolution was recorded. For the last experiments (histological validation and micro-CT assessment), 8 extracted mandibular premolars with formerly vital pulps were immersed in buffered formalin, scanned in a micro-CT device, accessed, immersed in Lugol for 7 days and scanned again. Then, the root canals of 5 teeth were prepared and scanned, and the volume of remaining pulp tissue identified and quantified, whilst 3 teeth were histologically processed. The same specimens were subjected to histological assessment, and the images of the histologic sections were registered with the corresponding micro-CT images to verify whether the pulp tissue in the histological sections matched its counterpart in the Lugol-impregnated tissues identified in the micro-CT slices.
Results: There was no discernible effect on radiopacity when NaOCl was mixed with Lugol’s solution. Tissue processing did not affect the time required for the complete dissolution of fresh bovine meat. Histological evaluation revealed a correlation between micro-CT and histological images confirming the identification of Lugol-impregnated pulp tissue in micro-CT images. Conclusions The radiocontrast Lugol’s solution was unaffected by NaOCl and did not interfere with its soft tissue dissolution capability. The impregnation protocol using Lugol’s solution allowed the visualization of pulp tissue on the micro-CT images and the identification of pulp remnants after chemical–mechanical canal procedures.
Introduction
Conventional analysis of histological sections and use of X-ray micro-computed tomography (micro-CT) are considered the gold standard methods to evaluate cleaning and shaping procedures during root canal preparation. Whilst micro-CT allows the mineralized tissues of the root canal removed by mechanical preparation to be identified and quantified, the amount of remaining (unmineralized) pulp tissue is usually assessed on histological sections. Thus, despite the usefulness of micro-CT technology, this method has been limited to the evaluation of changes along the canal walls, including transportation and the creation of aberrations, such as zips and perforations. Because of the penetrating power of X-rays, micro-CT techniques provide a 3D density map of specimens and tissues that strongly absorb this radiation (Alfaro et al. 2015, Cunha et al. 2015). However, it is unsuitable for imaging soft tissues, such as the dental pulp, as these tissues absorb X-rays to a very limited degree (Gignac & Kley 2014).
Recently, a correlative approach using histology as a complementary method for micro-CT assessment was employed in two studies to evaluate various chemomechanical protocols in root canals (Lacerda et al. 2017, Siqueira et al. 2018). Both studies demonstrated histologically the presence of pulp tissue remnants attached to untouched canal walls previously identified by micro-CT analysis. Although this correlative approach using different methods may allow the assignment of causation mechanisms, histologic analysis usually allows only a few sections per root to be assessed, which provides very limited data and is inconsistent with the amount of information in hundreds of cross-sectional images usually produced by the micro-CT scanning of a typical root canal. Moreover, histological sectioning is a time-consuming and expensive procedure that destroys the specimen.
It is obviously desirable to develop a reliable nondestructive experimental method able to simultaneously assess the quality and quantity of soft and hard tissues in a heterogenous specimen such as the human tooth. In other research areas, this limitation has been overcome by using various contrast agents such as osmium, gold, barium sulphate and iodine-based dyes (Metscher 2009a, b, Faulwetter et al. 2013, Pauwels et al. 2013). Overall, it has been demonstrated that an aqueous solution of Lugol’s iodine, also called iodine–potassium iodide (I2KI), is amongst the most effective means for rapidly differentiating a diversity of soft tissue types. Lugol’s solution is a simple, cost-effective, nontoxic and rapid staining option for contrast enhancement of soft tissues. Yet, its use has been limited to anatomical studies of a wide variety of biological specimens using a broad range of different concentrations of iodine and staining durations, depending on the type of the tissue (Heimel et al. 2019). Currently, despite the fact that Lugol impregnation is the most widely used contrast agent in the anatomical study of soft tissues, it is still not clear whether this solution would be suitable in the micro-CT assessment of pulp tissue after root canal debridement ex vivo. One of the fundamental issues when using Lugol concerns sodium hypochlorite (NaOCl), the main endodontic irrigant used to dissolve necrotic pulp tissue (Zehnder 2006), which reacts with iodine (Vogel 1978) and could thus interfere with its impregnation and visibility on radiographic images.
The present communication reports on a series of cascading experiments seeking to introduce and explore the potential to visualize dental pulp tissue on micro-CT images using Lugol as a contrast-enhancing solution. The cascade of experiments aimed to validate the impregnation protocol and the contrast-enhanced micro-CT (CE-CT) method whilst identifying the advantages and potential limitations of this novel methodology. The cascade experiments were design to:
- Evaluate the potential of NaOCl to reduce the degree of radiographic contrast associated with Lugol (radiopacity test);
- Verify the possibility that Lugol’s solution affects the proteolytic effect of NaOCl (soft tissue dissolution test);
- Assess the ability of Lugol’s solution to impregnate pulp tissue properly by correlative imaging of Lugol-enhanced micro-CT and conventional histology (histological validation);
- Measure the remaining volume of Lugol-impregnated pulp tissue (volumetric micro-CT assessment).
Materials and methods
Impact of NaOCl on the radiopacity of Lugol’s solution
Lugol’s solution (I2KI) used for all the experiments had a concentration of 5% I2 and 10% KI. To assess the impact of NaOCl on its radiopacity, a 1:1 dilution series was performed in distilled water and 5.25% NaOCl (1.5 mL total volume). The pure solution and its dilutions were pipetted into round transparent polystyrene dishes (Semadeni, Ostermundigen, Switzerland) with an inner diameter of 23.4 mm to a depth of 3.5 mm. Radiopacity was determined using a standard set-up as described previously (Hertig et al. 2017). In brief, electronic data sets were generated using a fixed unit (Trophy, Paris, France) at 65 kV, 8 mA and 0.22 s with a focus-film distance of 25 cm and electronic sensors (Digora; Soredex, Tuusula, Finland). Images were analysed using ImageJ (Bethesda, MD, USA). Grey values were normalized in each image against an aluminium step wedge, with an individual standard curve for each image. Experiments were done in triplicates. The relative radiopacity of the Lugol’s solution and its dilutions is expressed as the aluminium equivalent (in mm) per mm solution.
Soft tissue dissolution test
Thirty slices of fresh bovine meat were adjusted to a similar weight (2 mg) and dimensions (4 x 4 mm) using a no. 15 surgical blade. Ten slices did not undergo any tissue processing, whilst the other 20 were fixed in formaldehyde for 24 h. After that, 10 of the fixed slices were further immersed in Lugol’s solution for another 24 h. Then, all specimens were placed individually into flasks containing 40 mL of 5.25% NaOCl and the total time required for complete pulp tissue dissolution (in min) was recorded. All testing procedures were performed at room temperature. This investigation was not classified as an animal study because it had no influence on the premortal fate or the slaughtering process of the animals. Preliminary analysis of the raw data indicated the adherence to a Gaussian distribution (Shapiro–Wilk test, P < 0.05). Data were compared between groups using one-way ANOVA followed by Tukey’s HSD test. Alpha-error was considered at 5%.
Micro-CT assessment of pulp tissue remnants
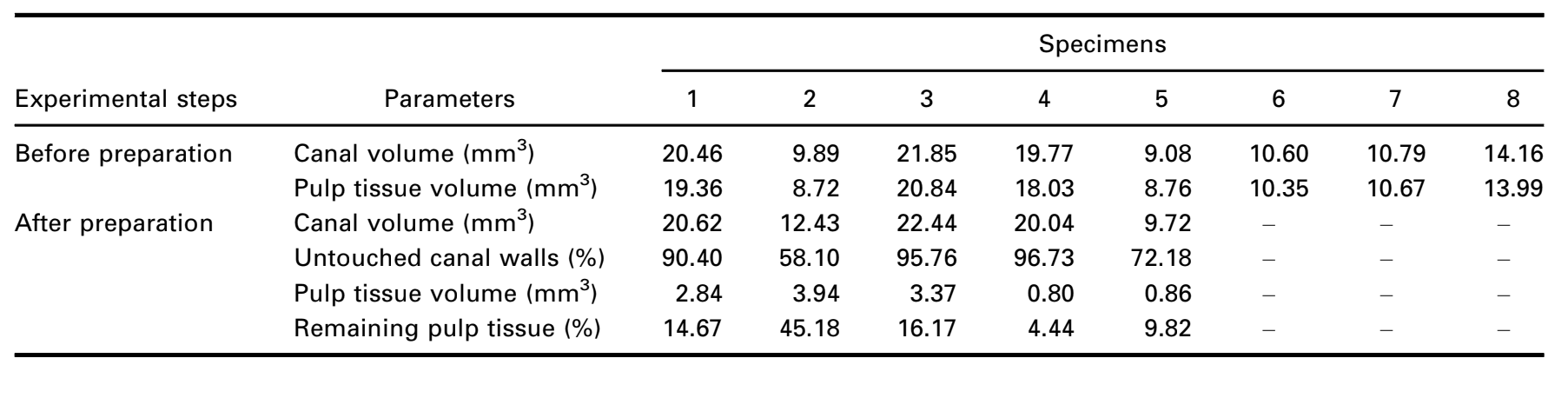
After the approval by the local ethics committee (protocol no. 12127319.3.0000.5243), eight noncarious single-rooted mandibular premolars and one mandibular molar with vital pulps, extracted for orthodontic reasons, were immersed in 10% buffered formalin and stored for up to 30 days at 15 °C. Afterwards, the teeth were scanned at a pixel size of 14.37 lm using a micro-CT device (SkyScan 1173; Bruker microCT, Kontich, Belgium) set at 70 kV, 114 mA, 360° rotation around the vertical axis with a rotation step of 0.5°, frame average of 5, using a 1.0-mm-thick aluminium filter. NRecon v.1.7.16 software (Bruker micro-CT) was used to reconstruct the micro-CT projections into axial cross sections using a ring artefact correction of 4, contrast limits ranging from 0.006 to 0.05 and beam hardening correction of 40%, resulting in 800–900 grayscale images per tooth from the cementum-enamel junction to the apex. To verify the canal morphology, the cross-sectional images were segmented using an automatic routine (De-Deus et al. 2020) in the FIJI/ImageJ soft- ware (Fiji v.1.51n; Madison, WI, USA), and the aspect ratio of the root canal, defined as the ratio of the major to the minor diameters, was measured in each slice from the orifice to the apical foramen. Then, canal volume (in mm3) was calculated as the volume of binarized root canal within the volume of interest.
After conventional access cavity preparation, each tooth was immersed in Lugol’ solution for 7 days and submitted to a new scan and reconstruction procedures using the previously mentioned parameters in order to assess the impregnation of the entire pulp tissue by the contrast solution. This set of images was coregistered with the unstained data set using the affine algorithm implemented on the 3D Slicer 4.6.0 software (http://www.slicer.org) (Fedorov et al. 2012), and the root dentine of the tooth following immersion was removed through a Boolean operation to reduce the noise generated by the pulp tissue segmentation. Thus, the pulp tissue impregnated with Lugol’s solution was observed and quantified (in mm3) by the segmentation process with a specific threshold value, using the Object Counter tool available in the FIJI/ ImageJ software. After that, the root canals of 5 out of the 8 selected premolars were chemomechanically prepared, whilst the other 4 sound teeth (3 premolars and 1 molar) were prepared for histological sectioning to confirm the presence of the pulp tissue remnants (control group).
Root canal preparation
The root canals were prepared up to the working length with Reciproc R25 instrument (VDW GmbH, Munich, Germany) driven by a VDW Silver motor (VDW GmbH) in the ‘RECIPROC ALL’ preset programme using light apical pressure with a slow in- and-out pecking motion of 3 mm amplitude. After completing three pecking movements, the instrument was removed from the canal and its flutes cleaned by insertion into a sponge moistened with alcohol. The working length was achieved after 3 waves of instrumentation. Apical patency was performed with a size 15 K-file (Dentsply Sirona Endodontics, Ballaigues, Switzerland) throughout the preparation procedures. Irrigation was performed with a total of 12 mL of 5.25% NaOCl dispensed into the root canal with a 31-G NaviTip double side port needle (Ultradent Inc., South Jordan, UT, USA) taken up to 1 mm short of the working length throughout the preparation procedures. After root canal preparation, the specimens were rescanned, reconstructed and coregistered using the aforementioned parameters. Then, the volume of pulp tissue remnants impregnated with Lugol’s solution was calculated (in mm3) and quantified as a percentage value based on the initial volume of the pulp tissue.
Histological assessment
After the experimental procedures described above, the specimens were demineralized in 22.5% (vol/vol) formic acid plus 10% (wt/vol) sodium citrate solution for a period of 2–3 weeks. The end-point was monitored radiographically. The specimens were then rinsed for 24 h in tap water, dehydrated and processed for routine histological examination. Teeth were embedded in paraffin blocks, and serial 0.6 µm thick cross sections were obtained every 1 mm from the cemento-enamel junction to the apex, resulting in 8 slices per tooth. The acquired sections were mounted on glass slabs and stained with haematoxylin–eosin. Histological images were visualized using an Axioplan 2 Imaging fully motorized light microscope (Carl Zeiss Vision, Hallbergmoos, Germany).
Matching the Lugol-impregnated micro-CT images and histological slices
The acquired micro-CT slices containing the Lugol- impregnated pulp tissue were inspected along the z-axis using a reference coordinate system based on a landmark-based registration algorithm (Analyze software; Biomedical Imaging Resource, Mayo Clinic, Rochester, MN, USA) to align them with the micro-radiograph images of the histologic sections. After selecting the corresponding images, a dimensional standardization adjustment was performed including automatic magnification, resizing and cropping, by means of a computer-assisted procedure. This procedural step allowed the examiners to reliably inspect the roots at the same levels and thus, qualitatively verify if the pulp tissue in the histological section matched its counterpart in the Lugol-impregnated micro-CT slice, confirming the efficacy of the impregnation protocol and scanning parameters. Two precalibrated examiners used a proforma with predefined criteria to analyse the degree of matching between Lugol-impregnated and histological images. The image analysis procedure was performed in a 34’ high-quality computer monitor with the possibility of escalating images (up to 10x) and reversing the colour mode. To validate the analytical process, analyses were repeated twice at 10-day intervals to appraise the reproducibility.
Results
Impact of NaOCl on the radiopacity of Lugol’s solution
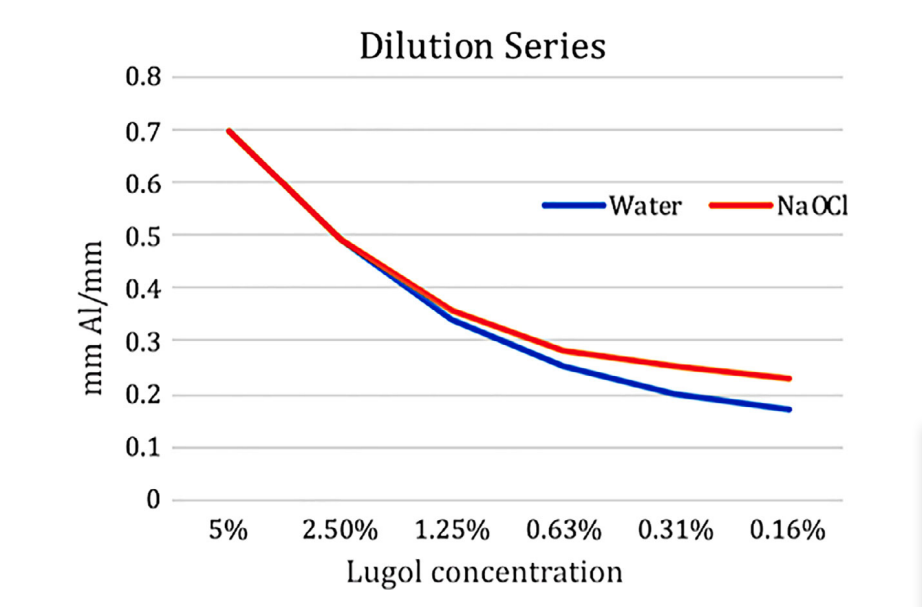
Pure Lugol’s solution had a radiopacity of 0.70 ± 0.09 mm Al mm-1. Dilution in 5.25% NaOCl had a similar effect on radiopacity as the control pro- cedure in water, with the higher dilutions in NaOCl showing slightly higher radiopacity (Fig. 1), which is based on the difference in radiopacity between the pure 5.25% NaOCl solution and water of 0.05 mm Al mm-1. There was no discernible effect on radiopacity caused by the chemical interaction between the NaOCl and Lugol’s solution, which was visible by clearing of the brown colour in the presence of NaOCl.
Soft tissue dissolution test
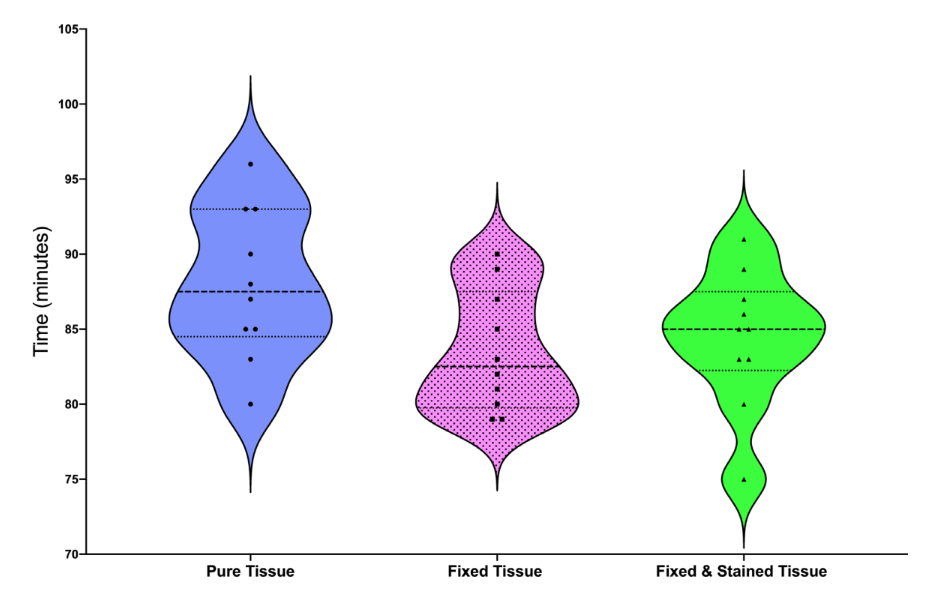
Violin plots illustrate the mean, minimum and maximum values, as well as the data distribution of tissue dissolution amongst the specimens (Fig. 2). Tissue processing in formaldehyde and Lugol’s solution did not affect the time required for the dissolution of fresh bovine meat (P > 0.05).
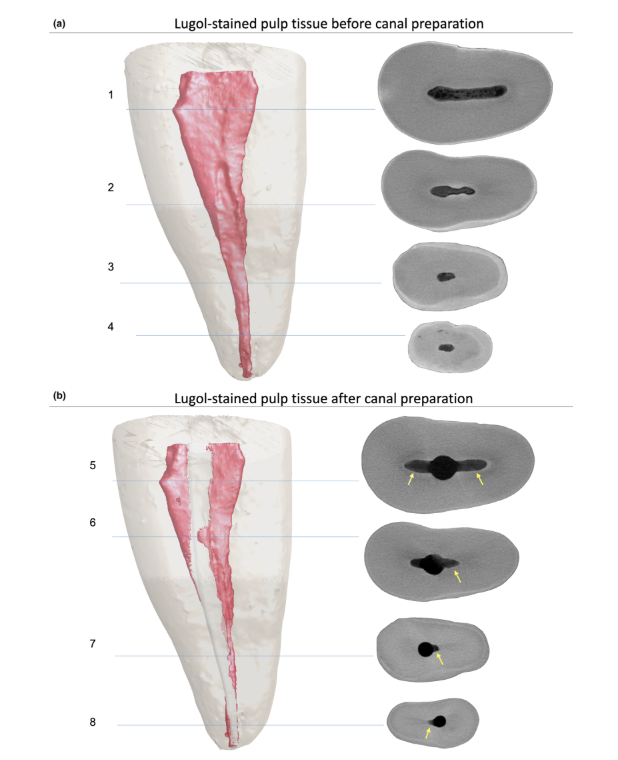
Histological validation
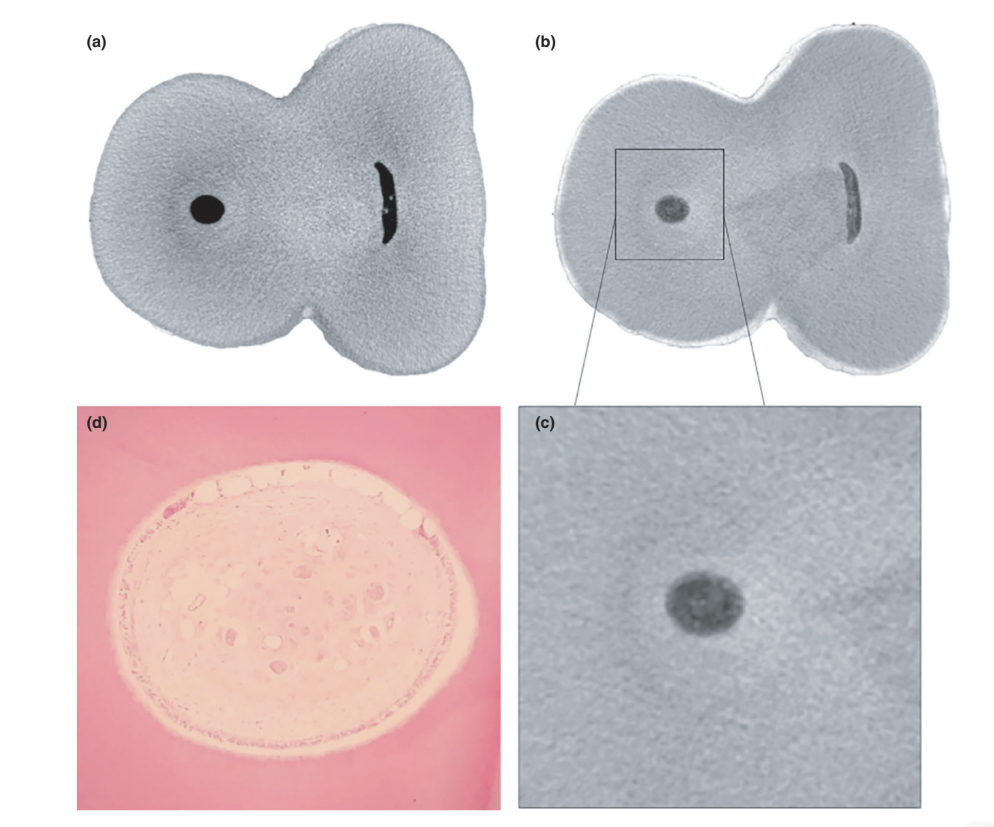
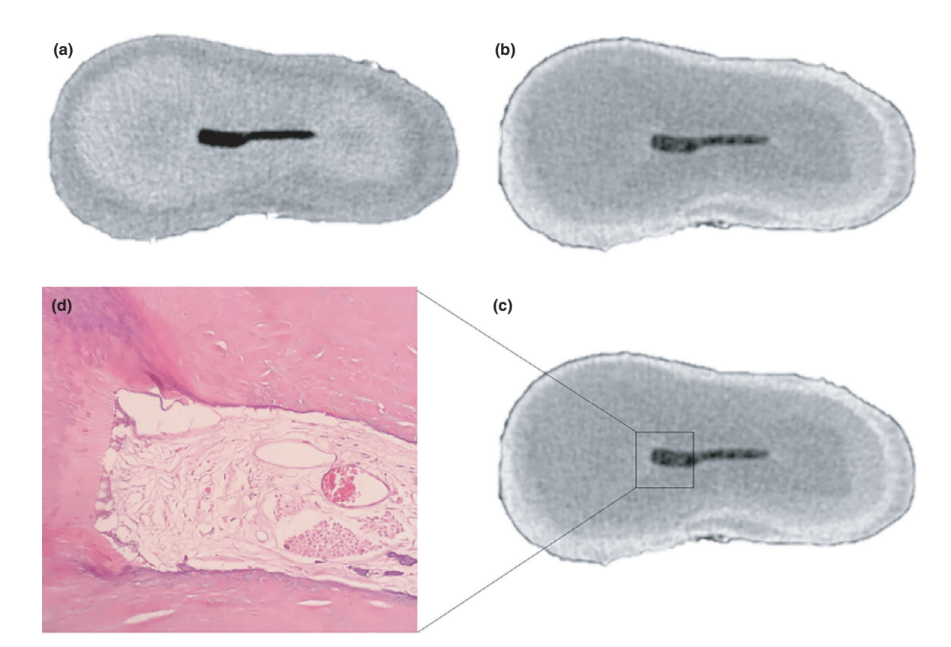
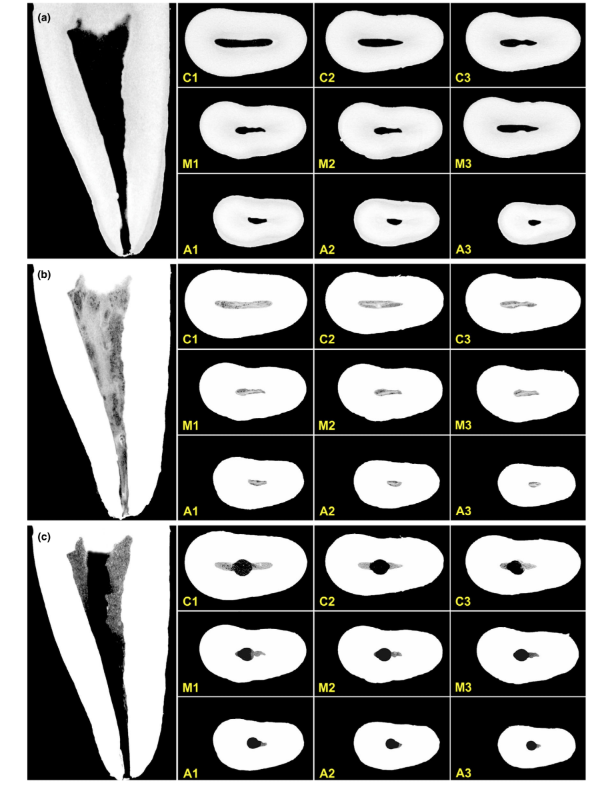
The overall quality of the staining protocol used is illustrated in Figure 3. Correlative analysis between micro-CT and histological images confirmed the identity of the Lugol-impregnated pulp tissue in the micro-CT images. The results of the matched micro-CT and histological images are shown in Figures 4 and 5, confirming the quality of the staining protocol.

Volumetric micro-CT assessment
Additionally, segmented pulp remnants after root canal preparation were quantified and expressed as a percentage value. Instrumented root canal volume and noninstrumented canal areas acquired by the micro-CT method were also quantified (Table 1) and illustrated in Figures 6 and 7 and Supplementary Video 1. The volume of the root canal and aspect ratio directly influenced the removal of pulp tissue during instrumentation. Teeth with aspect ratio values less than 3.5, expressed by the graphic curve and high volumes, were associated with smaller volumes of pulp tissue remnants.
Discussion
This communication introduces a novel staining method for dental pulp tissue in the context of the micro-CT assessment of root canal debridement with obvious beneficial uses for future research in this field. The proposed nondestructive method is able to provide high-resolution images and 3D information on pulp tissue and dentine simultaneously thus allowing the longitudinal and quantitative volumetric assessment of root canal cleaning and shaping procedures. Since Gysi & Röse (1894) published the first high-quality photomicrographs depicting details of the vascular, lymphatic and nervous elements of the pulp–dentine complex of a mandibular molar, and Kölliker (1852) provided the first description of the dental pulp, named by him as Pulpa dentis, in his classic book on the minute structures of the tissues and organs of the body, many studies have investigated teeth using histological methods. Using this technique, Hatton et al. (1928) were the first to demonstrate that the canal was only superficially cleaned and much of the pulp tissue was not removed after preparation with stainless steel instruments. However, it was only after Walton (1976) published a seminal study assessing the amount of the remaining pulp tissue after the cleaning and shaping procedures that paraffin-based histological sectioning became the standard method to determine the efficacy of debridement procedures within the root canal space.
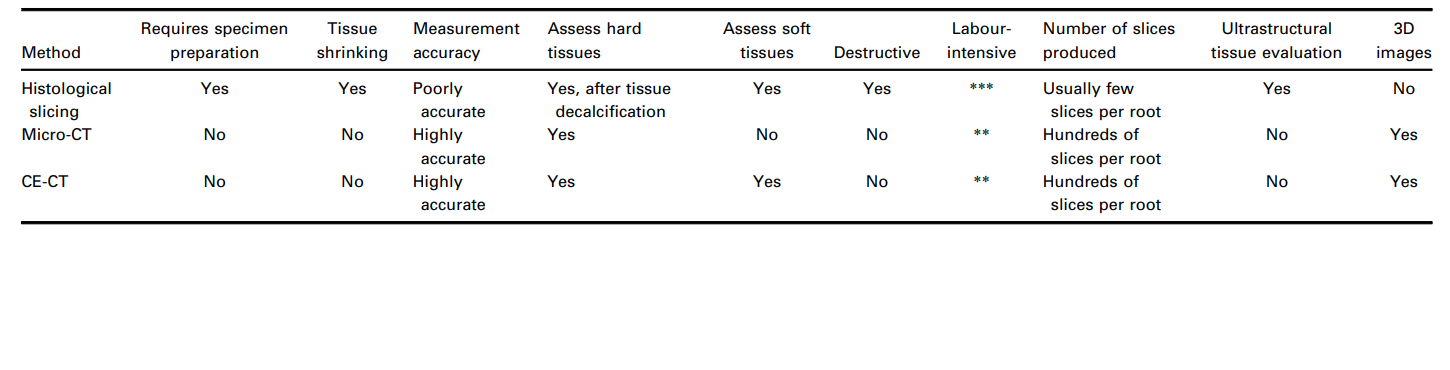
In intact teeth with vital pulps, normally used as a control in histological sectioning studies, the pulp tissue is attached to the entire perimeter of the root canal (De-Deus et al. 2010, 2011), whilst the tissue remnants in the experimental groups confirm which areas along the canal walls were not mechanically debrided or where irrigation protocols were ineffective. Since pulp remnants may serve as a substrate for bacteria and could negatively affect the quality of canal filling procedures (Ricucci et al. 2009, Ricucci & Siqueira 2011), it theoretically endorses this experimental variable, remaining pulp tissue, as an accepted surrogate end-point for the quality of debridement procedures within the root canal space. However, the processes and resultant workload for specimen preparation that embraces sectioning, staining, imaging and the final comprehensive histological assessment remains a cumbersome and labour-intensive technique. Specifically, in the context of endodontic laboratory research, the decalcification of mineralized tooth tissues is a time-consuming and complicated step and it is challenging to achieve high-quality specimens without damaging the pulp tissue. This becomes even more important as, in general, histological sectioning of decalcified hard tissues is prone to induce considerable tissue distortions, processing glitches and structural artefacts. For example, tissue shrinking by up to 3% occurs with bone tissues (Lane & Ráliš 1983, Henson et al. 1994), whilst dehydration of soft tissue can create shrinkage up to 11% (Rown et al. 2002). Thus, even with the useful insights available in the literature on this topic, it is of note that quantitative microscopy data from histological sectioning were derived from tissues that presumably shrunk during specimen preparation. Moreover, histological sectioning techniques invariably lead to the loss of specimens, rendering longitudinal experiments over time impossible. Hence, histology may be considered as an archaic method compared to the volumetric and quantitative approach achieved by nondestructive imaging methods (Table 2), even though to date it remains the only available experimental model that allows the simultaneous assessment of both mineralized hard and soft tissues of teeth at their ultrastructural level and, accordingly, is able to shed light on this important research area (De-Deus et al. 2008, 2010, 2011).
Several studies using nondestructive micro-CT technology have demonstrated that the preparation of root canal walls by endodontic instruments activated in either rotary or reciprocating motion is not ideal (Paqué et al. 2010, Paqué & Peters 2011, Versiani et al. 2013, De-Deus et al. 2015, Zuolo et al. 2018).
Mechanical preparation with these instruments is limited as they tend to prepare only the central aspects of root canals to create a round shape, leaving most of the buccal and lingual extensions of these complex spaces untouched, even when attempting lateral movements such as when using a brushing motion (Paqué et al. 2010, Paqué & Peters 2011, Versiani et al. 2013, De-Deus et al. 2015, Zuolo et al. 2018).
Although micro-CT can provide valuable and accurate measurements regarding the position and amount of dentine removed during canal preparation, it does not provide information on pulp tissue or microbial biofilms that may remain attached to the root canal walls, particularly in areas not reached by the mechanical action of the instruments, such as isthmus, fins, anastomoses and accessory canals (Versiani et al. 2013). This means that micro-CT has been essentially limited to the evaluation of the changes to the dentinal walls since, in its essence, micro-CT is unsuitable to image soft tissues as they are virtually ‘transparent’ for X-rays. This limitation is related to the inability of this bone research-derived radiographic method designed to depict denser elements such as calcium, to detect nonradiolucent soft tissues (Rüegsegger et al. 1996). However, there has been significant progress in micro-CT-based research in other biomedical areas, including different types of bench-top scanners, capture of phase-contrast information, more rapid and more effective scan acquisition protocols and reconstruction algorithms. Taken together, such developments can be exploited to image soft (noncalcified) tissues overcoming its inherent limitation. For that, specific soft tissue visualization enhancement can be achieved using radio-opaque contrast agents to attain X-ray attenuation, the so-called contrast-enhanced micro-CT technique (CE-CT). In short, CE-CT is suitable to assess heterogeneous tissues such as teeth.
Contrast agents are composed of specific chemical agents with a high molecular weight able to naturally bind to soft tissues to create ‘contrast’ in an effective way. The contrast agent used in the present study was the inorganic Lugol’s iodine (I2KI), which was first introduced by Metscher (2009a), who tested several sample fixation protocols and the staining potential of several commercially accessible compounds for a number of types of soft tissues. To date, it has been demonstrated that Lugol’s iodine has a high affinity for glycogen (Fennerty 1999) and targets epithelial cells and mouse soft tissue (Degenhardt et al. 2010, Baverstock et al. 2013). Thus, the potential of Lugol’s iodine to achieve pulp tissue imaging with micro-CT was tested in the current study. Through a series of trials with various impregnation protocols, teeth with conventional access cavities immersed in Lugol’s solution for 7 days allowed the effective impregnation of pulp tissue (Figures 3 and 7). However, two aspects regarding the use of the CE-CT method to analyse pulp remnants longitudinally after root canal irrigation with NaOCl solution may be of concern. The first aspect relates to the NaOCl reacting chemically with the iodine of the Lugol’s solution. Despite this interaction, Lugol’s solution did not significantly reduce the proteolytic ability of NaOCl under current conditions. The second concern was the potential of NaOCl to reduce the degree of contrast associated with the Lugol-impregnated pulp tissue. The radiopacity test revealed that Lugol’s solution was suitable for impregnating pulp tissue as the NaOCl did not interfere with its radiopacity. Taken together, these results confirmed that Lugol’s solution can be used as a contrast agent for testing pulp tissue as substrate for the analysis of NaOCl-based irrigation protocols. A further analysis focused on the validation of the Lugol’s solution in identifying pulp tissue properly. For that, paraffin-based histological sectioning was used to confirm whether the pulp tissue impregnated with Lugol’s solution was visible on micro-CT scans. An experimental approach was then developed to compare the histological sections with their corresponding images acquired from the micro-CT stacks, overcoming typical alignment problems in this type of correlative analysis. The results confirmed the correct identification of pulp tissue in the Lugol-impregnated micro-CT images and thus proved the quality of the impregnation protocol (Figures 4 and 5).
It is of note that, particularly for endodontic research using the remaining pulp tissue as an outcome parameter, the CE-CT approach has the clear advantage of not focusing on an ultrastructural detailed evaluation of the soft tissue. Instead, CE-CT easily enables quantitative assessment of the remaining pulp tissue as a whole in longitudinal (overtime) experiments (Figures 6 and 7). From a qualitative standpoint, hundreds of cross sections produced per tooth by CE-CT may render a better understanding of the close relationship between the internal anatomy of root canals, and mechanical shaping and irrigation protocols. This is because CE-CT delivers 3D high-resolution models, which contain true-to-life information on the dimensions, structural quantification and anatomic features of heterogeneous tissues, for example dentine and pulp tissue. At the same time, this method allows the evaluation of the preoperative distribution of the pulp tissue throughout the canal space before the experimental procedures even after 7 days without any fixation protocol. This is an important point as the amount and location of the pulp tissue may act as a confounding factor, affecting the outcome of the experiment. In this way, the use of the CE-CT method in teeth with vital pulps seems to be valid and reproducible, since pulp tissue was distributed along the entire root canal system in all sound teeth. Future studies using this innovative method should include the comparison of different irrigant solutions (inert vs active solutions) over time and preparation protocols on the dissolution/removal efficiency of pulp tissue from root canal systems. Further improvements on this method would also allow it to be applied in in vivo research using CBCT, for instance. At the moment, the present methodology requires the contrast agent to be in contact with pulp tissue for at least 7 days and, in an in vivo approach, it would also require pre- and postoperative tomographic imaging, which clearly needs to comply with acceptable ethical research principles. Definitely, the present protocol should be validated in vivo using CBCT. On the other hand, it can be safely applied in vivo by using noncarious and nonrestored teeth with vital pulps scheduled for extraction without pre- or postoperative scans. For example, after confirming the vital condition of the pulp by conventional tests, the chemomechanical protocol can be applied in situ and the contrast solution injected into the pulp canal space and the coronal access cavity restored to ensure the Lugol’s solution remains within the root canal space. Then, the tooth can be extracted, stored and evaluated through micro-CT imaging after one week.
In summary, the current study thus concentrates on providing preliminary but original evidence to support nondestructive longitudinal CE-CT studies using remaining pulp tissue as an outcome parameter. It was demonstrated that CE-CT combines, in a single method, the main advantages of micro-CT imaging technology (mineralized tissue evaluation) and traditional histological methods (nonmineralized tissue evaluation) to study root canal debridement procedures embracing the possibility of assessing, identifying and measuring those canal areas not affected by either mechanical preparation or irrigation protocols. Worthy of note is the fact that CE-CT allows mechanical canal preparation and irrigation protocols to be studied independently or the combined synergetic effect of chemical–mechanical procedures.
Conclusions
Lugol’s solution allowed the visualization of pulp tissue on micro-CT images. Lugol’s solution was unaffected by NaOCl in its radiopacity and did not interfere with the dissolution of the fixed and stained soft tissues. In practical terms, the contrast-enhanced micro-CT imaging technique with Lugol’s solution presented here allows the effect of chemical dissolution and the mechanical removal of pulp tissue by cleaning and shaping procedures to be evaluated independently or together making it a most useful technique in laboratory-based Endodontic research.
Authors: G. De-Deus, F. G. Belladonna, D. M. Cavalcante, M. Simões-Carvalho, E. J. N. L. Silva, J. C. A. Carvalhal, R. Q. Zamolyi, R. T. Lopes, M. A. Versiani, P. M. H. Dummer and M. Zehnder
References:
- Alfaro DP, Ruse ND, Carvalho RM, Wyatt CC (2015) Assessment of the internal fit of lithium disilicate crowns using micro-CT. Journal of Prosthodontics 24, 381–6.
- Baverstock H, Jeffery NS, Cobb SN (2013) The morphology of the mouse masticatory musculature. Journal of Anatomy 223, 46–60.
- Cunha AC, Marquezan M, Lima I, Lopes RT, Nojima LI, Sant’Anna EF (2015) Influence of bone architecture on the primary stability of different mini-implant designs. American Journal of Orthodontics and Dentofacial Orthopedics 147, 45–51.
- De-Deus G, Reis C, Beznos D, Gruetzmacher-de-Abranches AM, Coutinho-Filho T, Pacionrik S (2008) Limited ability of three commonly used thermoplasticised guttapercha techniques in filling oval-shaped canals. Journal of Endodontics 34, 1401–5.
- De-Deus G, Barino B, Quintella Zamolyi R et al. (2010) Suboptimal debridement quality produced by the single file F2 ProTaper technique in oval-shaped canals. Journal of Endodontics 36, 1897–900.
- De-Deus G, Souza EM, Barino B et al. (2011) The self-adjusting file optimizes debridement quality in oval-shaped root canals. Journal of Endodontics 37, 701–5.
- De-Deus G, Belladonna FG, Silva EJ et al. (2015) Micro-CT evaluation of non-instrumented canal areas with different enlargements performed by NiTi systems. Brazilian Dental Journal 26, 624–9.
- De-Deus G, Simões-Carvalho M, Belladonna FG et al. (2020) Creation of well-balanced experimental groups for comparative endodontic laboratory studies: a new proposal based on micro-CT and in silico methods. International Endodontic Journal 53, 974–85.
- Degenhardt K, Wright AC, Horng D, Padmanabhan A, Epstein JA (2010) Rapid 3d phenotyping of cardiovascular development in mouse embryos by micro-ct with iodine staining. Circulation: Cardiovascular Imaging 3, 314–22.
- Faulwetter S, Vasileiadou A, Kouratoras M, Dailianis T, Arvanitidis C (2013) Micro-computed tomography: Introducing new dimensions to taxonomy. ZooKeys 263, 1–45. Fedorov A, Beichel R, Kalpathy-Cramer J et al. (2012) 3D
- Slicer as an image computing platform for the quantitative imaging network. Magnetic Resonance Imaging 30, 1323– 41.
- Fennerty MB (1999) Tissue staining. Gastrointestinal Endoscopy Clinics of North America 4, 297–311.
- Gignac PM, Kley NJ (2014) Iodine-enhanced micro-CT imaging: methodological refinements for the study of the soft-tissue anatomy of post-embryonic vertebrates. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 322, 166–76.
- Gysi A, Röse C (1894) Sammlung von Mikrophotographien zur Veranschaulichung der mikroscopischen Struktur der Zähne des Menschen. Mikrophotographien der Zahnhistologie. Zürich: Schweiz.
- Hatton EH, Skillen WG, Moen OH (1928) Histologic findings in teeth with treated and filled root canals. Journal of the American Dental Association 15, 56.
- Henson MM, Henson OW Jr, Gewalt SL, Wilson JL, Johnson GA (1994) Imaging the cochlea by magnetic resonance microscopy. Hearing Research 75, 75–80.
- Heimel P, Swiadek NV, Slezak P et al. (2019) Iodine-enhanced micro-CT imaging of soft tissue on the example of peripheral nerve regeneration. Contrast Media G Molecular Imaging 2019, 1–15.
- Hertig G, Zehnder M, Woloszyk A, Mitsiadis T, Ivica A, Weber F (2017) Iodixanol as a contrast agent in a fibrin hydrogel for endodontic applications. Frontiers in Physiology 8, 152.
- Kölliker VA (1852) Handbuch der Gewebelehre des Menschen, 1st edn. Leipzig: W. Engelmann, p 405.
- Lacerda MFLS, Marceliano-Alves MF, P´erez AR et al. (2017) Cleaning and shaping oval canals with 3 instrumentation systems: a correlative micro-computed tomographic and histologic study. Journal of Endodontics 43, 1878–84.
- Lane & Ráliš (1983) Changes in dimensions of large cancellous bone specimens during histological preparation as measured on slabs from human femoral heads. Calcified Tissue International 35, 1–4.
- Metscher BD (2009a) Micro-CT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology 9, 11.
- Metscher BD (2009b) Micro-CT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Developmental Dynamics 238, 632–40.
- Paqué F, Balmer M, Attin T, Peters OA (2010) Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instruments: a micro-computed tomography study. Journal of Endodontics 36, 703–7.
- Paqué F, Peters OA (2011) Micro-computed tomography evaluation of the preparation of long oval root canals in mandibular molars with the self-adjusting file. Journal of Endodontics 37, 517–21.
- Pauwels E, Van Loo D, Cornillie P, Brabant L, Van Hoorebeke L (2013) An exploratory study of contrast agents for soft tissue visualization by means of high-resolution X-ray computed tomography imaging. Journal of Microscopy 250, 21–31.
- Ricucci D, Siqueira JF Jr, Bate AL, Pitt Ford TR (2009) Histologic investigation of root canal-treated teeth with apical periodontitis: A retrospective study from twenty-four patients. Journal of Endodontics 35, 493–502.
- Ricucci D, Siqueira JF Jr (2011) Fate of the tissue in lateral canals and apical ramifications in response to pathologic conditions and treatment procedures. Journal of Endodontics 36, 1–15.
- Rown MAB, Eed RBR, Enry RWH (2002) Effects of dehydration mediums and temperature on total dehydration time and tissue shrinkage. Journal of International Society for Plastination 17, 28–33.
- Rüegsegger P, Koller B, Müller R (1996) A microtomographic system for the nondestructive evaluation of bone architecture. Calcified Tissue International 58, 24–9.
- Siqueira JF Jr, Pérez AR, Marceliano-Alves MF et al. (2018) What happens to unprepared root canal walls: a correlative analysis using micro-computed tomography and histology/scanning electron microscopy. International Endodontic Journal 51, 501–8.
- Versiani MA, Leoni GB, Steier L et al. (2013) Micro-computed tomography study of oval-shaped canals prepared with the self-adjusting file, Reciproc, WaveOne, and Pro-Taper universal systems. Journal of Endodontics 39, 1060–6.
- Vogel AI (1978) A textbook of quantitative inorganic analysis including elementary instrumental analysis. 4th edn. London: Longmans, Green and Co., Ltd. p. 925.
- Walton RE (1976) Histologic evaluation of different methods of enlarging the pulp canal space. Journal of Endodontics 2, 304–11.
- Zehnder M (2006) Root canal irrigants. Journal of Endodontics 32, 389–98.
- Zuolo ML, Zaia AA, Belladonna FG et al. (2018) Micro-CT assessment of the shaping ability of four root canal instrumentation systems in oval-shaped canals. International Endodontic Journal 51, 564–71.
