The development of the Standardised Tool for the Assessment of Bruxism (STAB): An international road map
ABSTRACT
This paper summarises the background reasoning and work that led to the selection of the items included in the Standardised Tool for the Assessment of Bruxism (STAB), also introducing the list of items. The instrument is currently being tested for face validity and on-field comprehension. The underlying premise is that the different motor activities included in the bruxism spectrum (e.g. clenching vs. grinding, with or without teeth contact) potentially need to be discriminated from each other, based on their purportedly different aetiology, comorbidities and potential consequences. Focus should be on a valid impression of the activities' frequency, intensity and duration. The methods that can be used for the above purposes can be grouped into strategies that collect information from the patient's history (subject-based), from the clinical assessment performed by an examiner (clinically based) or from the use of instruments to measure certain outcomes (instrumentally based). The three strategies can apply to all aspects of bruxism (i.e. status, comorbid conditions, aetiology and consequences). The STAB will help gathering information on many aspects, factors and conditions that are currently poorly investigated in the field of bruxism. To this purpose, it is divided into two axes. Axis A includes the self-reported information on bruxism status and potential consequences (subject-based report) together with the clinical (examiner report) and instrumental assessment (technology report). Axis B includes the self-reported information (subject-based report) on factors and conditions that may have an etiological or comorbid role for bruxism. This comprehensive multidimensional assessment system will allow building predictive model for clinical and research purposes.
You have the opportunity to gather more in-depth information in our Online congress on evidence-based temporomandibular disorders and bruxism treatment.
INTRODUCTION
The definition of bruxism has evolved considerably over the past few years. From the perspective of the dental practitioner, bruxism has predominately been considered the act of grinding the teeth while asleep. With the evolution of knowledge concerning both the sleep correlates and the muscle activities that may equally be present also during wakefulness, the bruxism construct has progressively changed. The frequent clinical observations on the absence of clear-cut relationships between bruxism and its purported consequences (e.g. tooth wear, temporomandibular disorders) has led to the proposal of conceptualising bruxism as a ‘behaviour’. Thereby, the bruxism construct has shifted from a pathology or disorder to a motor behaviour (hence the neutral term ‘muscle activity’ in the definitions of bruxism) that may even have potential physiological or protective relevance.
This progressive evolution of the concept of bruxism is reflected in the changes that were introduced in the most recent definition, which separates sleep and awake bruxism and explicitly states that bruxism is not necessarily a disorder. Hence, sleep bruxism (SB) is defined in a recent consensus paper as a masticatory muscle activity (MMA) during sleep that is characterised as rhythmic (phasic) or non-rhythmic (tonic) and that is not a movement disorder or a sleep disorder in otherwise healthy individuals. Awake bruxism (AB), based on the same construct, is defined as a masticatory muscle activity during wakefulness that is characterised by repetitive or sustained tooth contact and/or by bracing or trusting of the mandible, and, as with SB, not a movement disorder in otherwise healthy individuals.
Within these premises, the need to prepare a multidimensional evaluation system emerged. Two invitation-only workshops were held during the 2018 and 2019 General Session & Exhibition of the International Association for Dental Research (IADR) meetings in London and Vancouver. Participants of these IADR closed meetings were divided into two groups to develop a comprehensive, multidimensional evaluation system for bruxism. The original aim was to define a multiaxial instrument for the assessment of bruxism status, etiological/risk/associated factors and conditions, and consequences, based on different evaluation domains (i.e. subject-based, clinically based, and instrumentally based assessment). The work of the two groups, that led to the identification of different domains for assessment, is summarised in a paper that presented the general framework of the Standardised Tool for the Assessment of Bruxism (STAB) published in the Journal of Oral Rehabilitation.
The premise was that comprehensive data gathering is needed to define bruxism status and its comorbid conditions, aetiology and consequences. As muscle activities, both sleep and awake bruxism require a thorough assessment that could be based on a combination of subject-based, clinically based and instrumentally based assessment aiming to identify bruxism status, comorbidities, aetiology and consequences.
The next step was a closed meeting held online in March 2020, in lieu of the cancelled IADR General Session & Exhibition meeting that was scheduled in Washington, DC. From the panel discussion, some general recommendations for the selection of the STAB items emerged, with particular focus on the decision to keep the STAB as brief as possible and to use already existing and validated, complete instruments for a STAB toolkit, whenever available. Further, it was decided that minor adaptations of existing instruments (e.g. time span for a certain question) or the preparation of lists of items that are currently not included in existing instruments (e.g. list of medications, list of potential prosthetic complications) should be considered.
After this, another closed meeting was held online as a pre-meeting Satellite Symposium in occasion of the IADR General Session & Exhibition scheduled in July 2021. During that meeting, further discussions on the format and items that are to be included for the STAB were held. Following the meeting and several rounds of mail exchanges, a preliminary STAB was drafted by the chairmen of the project (DM, JA, FL) and submitted to the external review of invited experts who were not part of the original core group of STAB developers. After two rounds of adjustments, the STAB was finalised. Figure 1 summarises the main steps that led to the STAB finalisation.
This paper summarises the background reasoning and work that led to the selection of the items included in the STAB. Subsequently, the list of items will be introduced. The instrument is currently being tested for face validity and on-field comprehension. The full version, also co-authored by all experts involved in the external review process, will be presented with explanatory notes in a separate paper.
 FIGURE 1 Road map to the preparation of STAB.
FIGURE 1 Road map to the preparation of STAB.
BACKGROUND
The current evidence suggests that bruxism is a behaviour that is not suitable to be evaluated in terms of the simple dichotomy ‘present versus absent’. The different motor activities included in the bruxism spectrum (e.g. clenching vs. grinding, with or without teeth contact) potentially need to be discriminated from each other. The reason for this need is that the aetiology, comorbidities and potential consequences could be different between the different motor activities. Thus, a better description of the bruxism continuum is needed, with focus on a valid impression of the activities' frequency, intensity and duration. As pointed out in the 2018 consensus paper, this leads to the question of how to assess the full range of the bruxism construct (i.e. its status, comorbid conditions, aetiology and consequences) in a reliable, valid and feasible way. In both the clinical and research setting, this could help not only with understanding when bruxism is a harmless behaviour but also with identifying the conditions in which it is associated with negative or even positive health outcomes. Several recent commentary papers have conceptualised the complexity of the emerging bruxism construct.
The methods that can be used for the above purposes can be grouped into strategies that collect information from the patient's history (subject-based), from the clinical assessment performed by an examiner (clinically based) or from the use of instruments to measure certain outcomes (instrumentally based). Since the usage of self-report, clinical assessment and instrumental assessment can apply to all aspects of bruxism (i.e. status, comorbid conditions, aetiology and consequences), these approaches are described below in general terms.
Subject-based strategies require the patient to self-report the condition under study. In fact, self-reported assessment of sleep and awake bruxism is the most used tool in both research and clinical practice. It can be based on open interviews or structured questionnaires, which is the ideal approach to gather data for large-size epidemiological studies on bruxism as well as to get deeper into the correlation and comorbidity with other conditions (e.g. psychological disorders, gastroesophageal reflux disease, obstructive sleep apnoea) and potential consequences (e.g. pain, headache, teeth sensitivity, non-painful muscle symptoms) that are also self-reported by the patients. Efforts are currently being made to enhance the possibility of obtaining reliable self-reported data on AB due to the use of ecological momentary assessment (EMA) approaches, whilst strategies to obtain valid self-reports of SB are less easy to standardise.
Clinically based strategies encompass the evaluation of the potential clinical signs observed by the examiner and symptoms reported by the patient that may purportedly be associated with AB and SB, thus representing an indicator of bruxism status based on the presence of potential consequences. Amongst those, the presence of masseter hypertrophy, indentations on the tongue or lip, the presence of mucosal ridges (i.e. linea alba) on the inner cheek, and the evaluation of damage to restorations, implants, dental hard tissues or mechanical tooth wear should be listed.
Similarly, comorbidities and etiological factors may also be evaluated clinically. Importantly, it should be noted that for some of the above signs (e.g. when is a masseter muscle hypertrophic? When is the tongue actually indented?) validated evaluation criteria do still not exist. Instrumentally based strategies are currently available for both AB and SB. During wakefulness, electromyography (EMG) recordings or other direct measures of muscle activity may provide information on the presence of bruxism behaviours. Importantly, it should be noted that from a technical viewpoint this category may also include EMA strategies, which are essentially advanced self-reported strategies taking advantage of the use of smartphones and other technological devices. Concerning SB, EMG of jaw muscle activities may be complemented with other measures to study the comorbid conditions as well as the etiological factors and clinical consequences. The standard of reference technique in this field is polysomnography (PSG), which ideally requires a sleep laboratory environment, with (Type I) or without audio/video recordings (Type II), but is also available for home recordings with portable devices with multiple (Type III) (i.e. polygraph) or single recording channels (Type IV). Below, an insight into the assessment of bruxism status, comorbid conditions, aetiology and consequences is briefly summarised.
Bruxism status
As mentioned earlier, approaches for assessing bruxism status can be distinguished into subject-, clinically or instrumentally based. Amongst the former two, all the strategies that may be used to collect self-reported information (e.g. questionnaires, structured interviews, narrative oral history) and data gathered with the clinical examination are included. Instrumental approaches include all the armamentarium of methods and devices that are currently used to measure the masseter muscle activity with EMG, either alone or as part of polysomnography.
Only scarce literature is available concerning the correlation between these three possible categories of bruxism assessment strategies. Most studies are built upon the classical concept that PSG should be considered the standard of reference for SB assessment, which was also suggested in the first consensus paper on bruxism definition and then re-conceptualised in the updated paper. While there is no doubt that PSG is fundamental to understand the physiology/pathophysiology and sleep-related comorbid conditions of SB and the distribution of SB activities within the framework of sleep architecture, it is now clear that the current PSG-based research diagnostic criteria for SB offer just a glimpse of the bruxism puzzle. Indeed, such registrations are based on the count of arousal-related events, leading to a dichotomic evaluation of SB presence/absence that is not suitable to identify any clear-cut relationship with potential comorbid conditions, etiological factors and consequences. Based on that, the development of EMG/PSG analysis software that allows achieving a valid discrimination of the different muscle activities and a quantification of total muscle work during hour-long EMG recordings is recommended. Furthermore, little is known concerning the best possible strategy to assess AB, which is less studied than sleep-related phenomena. To a certain extent, this has led to the re-evaluation of subject-based and clinically based strategies.
Thus, an important scope of the bruxism status assessment is to phenotype bruxism via quantitative evaluation (e.g. bruxism time index, bruxism work index) and qualitative evaluation (e.g. for sleep bruxism: arousal-related events, isolated short-lasting events, elevated background activity, tonic prolonged activity; for awake bruxism: tonic prolonged activity, short-lasting events).
Concerning the STAB, one of the assumptions to propose assessment strategies is that none of currently available approaches can be considered the absolute standard of reference for the assessment of a bruxism status.
Comorbid conditions
Many conditions may increase masticatory muscle activities during wakefulness and sleep. However, a number of these do not literally fit in the bruxism definition that describes bruxism activities as behaviours, viz., ‘in otherwise healthy individuals’. Bearing in mind that the concurrent report of SB and AB in the same individual is quite frequent, the comorbidities of sleep bruxism are better recognised. Many of these have been previously listed in the preliminary STAB paper by Manfredini et al.
Sleep-related disorders such as sleep disordered breathing, gastroesophageal reflux disease67 and periodic limb movements are probably the conditions that have been found most frequently to associate with sleep bruxism. Whether these conditions are secondary or share etiological factors with bruxism remains unclear, although evidence for a true, arousal-related association between jaw-closing muscle activity and obstructive sleep apnoea is accumulating.71 The association between sleep bruxism and temporomandibular disorders (TMD) is, however, controversial, due to the general tendency to show an association in self-reported studies but difficulty to confirm this instrumentally.
The reasons for the inconsistent literature findings have recently been elaborated in a dedicated scoping review.11 Nonetheless, as a general remark, when present, TMDs are commonly considered as a potential consequence rather than an etiological or a comorbid factor for bruxism. Finally, it should be noted that many of the aforementioned and other sleep-related conditions are interlinked in a complex comorbidity network, including the so-called dental sleep disorders, viz., sleep-related breathing disorders, gastrooesophageal reflux, mandibular movement disorders, oral moistening disorders and oro-facial pain25 as well as other conditions such as restless legs syndrome/periodic limb movements, insomnia, REM behaviour disorder, Parkinson's disease and epilepsy. For the purposes of STAB, existing questionnaires and items are selected to screen for those conditions.
Etiological factors
Etiological factors may be genetic and/or environmental. Indirect evidence for the role of genetic factors in a disorder comes mainly from two study designs in humans, namely family studies and twin studies. Familial aggregation is found when the occurrence is higher in first degree relatives, such as parents. Familial aggregation by itself is not evidence for genetic influences, as children may learn from the experiences and behaviours of the parents, they may share the same environmental risk factors, or parents with bruxism may seek care for their children more readily than parents who are non-bruxers. Without further information, genetic and shared environmental effects cannot be distinguished using nuclear family (parents and children) data. For example, an association with sleep bruxism was found for only one variant (rs6313) out of 13 polymorphisms in four candidate genes. The minor allele frequencies of rs6313 vary between populations as well. Thus, like many disorders and diseases, also bruxism runs in families, and likely has a genetic component. However, as yet, we do not have strong evidence about the genes that are responsible for developing bruxism, also because of the different bruxism types and aetiologies that are currently included under the generic umbrella term ‘bruxism’. The genetic component can vary with age and environmental circumstances. However, a recent nationwide twin study in Finland discussed the etiological factors of bruxism and showed that both sleep and awake bruxism have a genetic component but that they also share several significant environmental (additive) correlates, which are more or less permanently present.
Amongst the potential etiological factors, emerging evidence suggests that a list of drugs that may exacerbate or attenuate bruxism should be constantly updated and monitored by all clinicians and researchers involved in the field. In addition to that, psychological factors must also be kept in mind when assessing bruxism aetiology, even if further studies on their actual role as triggers that may lead to the use the jaw muscles as a stress relief organ versus a ‘simple’ role of associated factors are needed. In particular, whilst the bruxism-psyche relationship is likely complex for SB due to the complexity of sleep architecture and the many comorbid sleep disorders, there is increasing evidence of an association between psychological factors (e.g. anxiety traits, stress sensitivity) and AB. All the above factors may play a clinically important role as they may act in the thin zone between harmless and potentially harmful bruxism behaviours. For the STAB purposes, all these factors are investigated via screening items and questionnaires.
Consequences
Over the years, bruxism has been associated with a host of potential consequences, which can be evaluated via any of the three above described methods (i.e. self-report, clinical assessment, instrumental evaluation). Traditionally, focus was given to the consequences with negative impact on the individual, mostly due to overloading by the repetitive masticatory muscle activities that characterise bruxism. Such consequences can be classified depending on the affected structures: dental hard tissues (e.g. mechanical wear of enamel and dentine; cracks and fractures of dental crowns and roots), dental restorations and implants (e.g. fractures and failures), periodontal tissues (e.g. increased mobility due to widening of periodontal cleft) and musculoskeletal tissues (e.g. pain and dysfunction of masticatory muscles and temporomandibular joints).
Because of the paucity of data in the literature and the evolution of bruxism concepts that may mandate re-evaluation of previous findings, for most of these potential consequences of bruxism high certainty evidence of a relationship is either lacking or is hampered by conflicting data. The need for more and better primary research articles, which should be privileged with respect to inconclusive meta-analyses of poor data, has been recently advocated and will be part of the research agenda for further STAB development and use. However, despite these uncertainties that characterise the potential negative consequences of bruxism, they are important from a clinical point of view, because these are the problems with which a patient reports to the dentist with a request for help; bruxism itself, being a muscle activity, does not call for action from the patient's point of view. Hence, the potential negative consequences of bruxism thus justify and even necessitate assessment. More recently, focus has shifted from the negative consequences to the potential positive consequences of bruxism. Already for a longer time, bruxism has been considered a relief factor for psychological stress and even as an expression of aggression.
Nowadays, other potential positive health outcomes of bruxism have been suggested in the literature, such as prevention or restoration of upper airway collapse in obstructive sleep apnea, neutralising pH drops in gastro-oesophageal reflux patients by increasing salivary flow rate, reducing oral dryness by mechanically stimulating salivary flow rate via masticatory muscle activity-induced parotid gland massage, improving mineral bone density by traction of the jaw muscles on their bony origins and insertions within the masticatory system,94 and even preventing of slowing down cognitive decline by improving the perfusion of blood in the brain due to chewing-like masticatory muscle activities. Since it is important to allow these potential positive consequences of bruxism to occur, the presence of the mentioned conditions should also be assessed as part of a comprehensive bruxism assessment. It will be a clinical challenge to find the best possible balance for each individual patient between the potential negative and positive consequences of bruxism.
OVERVIEW OF STAB ITEMS
The STAB is divided into two axes. Axis A includes the self-reported information on bruxism status and potential consequences (subject-based report) together with the clinical (examiner report) and instrumental assessment (technology report). Axis B includes the self-reported information (subject-based report) on factors and conditions that may have an etiological or comorbid role for bruxism.
In addition to the full version STAB, two additional instruments are under development. A Bruxism Screener instrument (BruxScreen) has been pilot-tested for comprehension and face validity96 and a toolkit version of the STAB (STAB toolkit) will be available to expand the evaluation of specific factors or conditions using a dedicated full questionnaire/instrument.
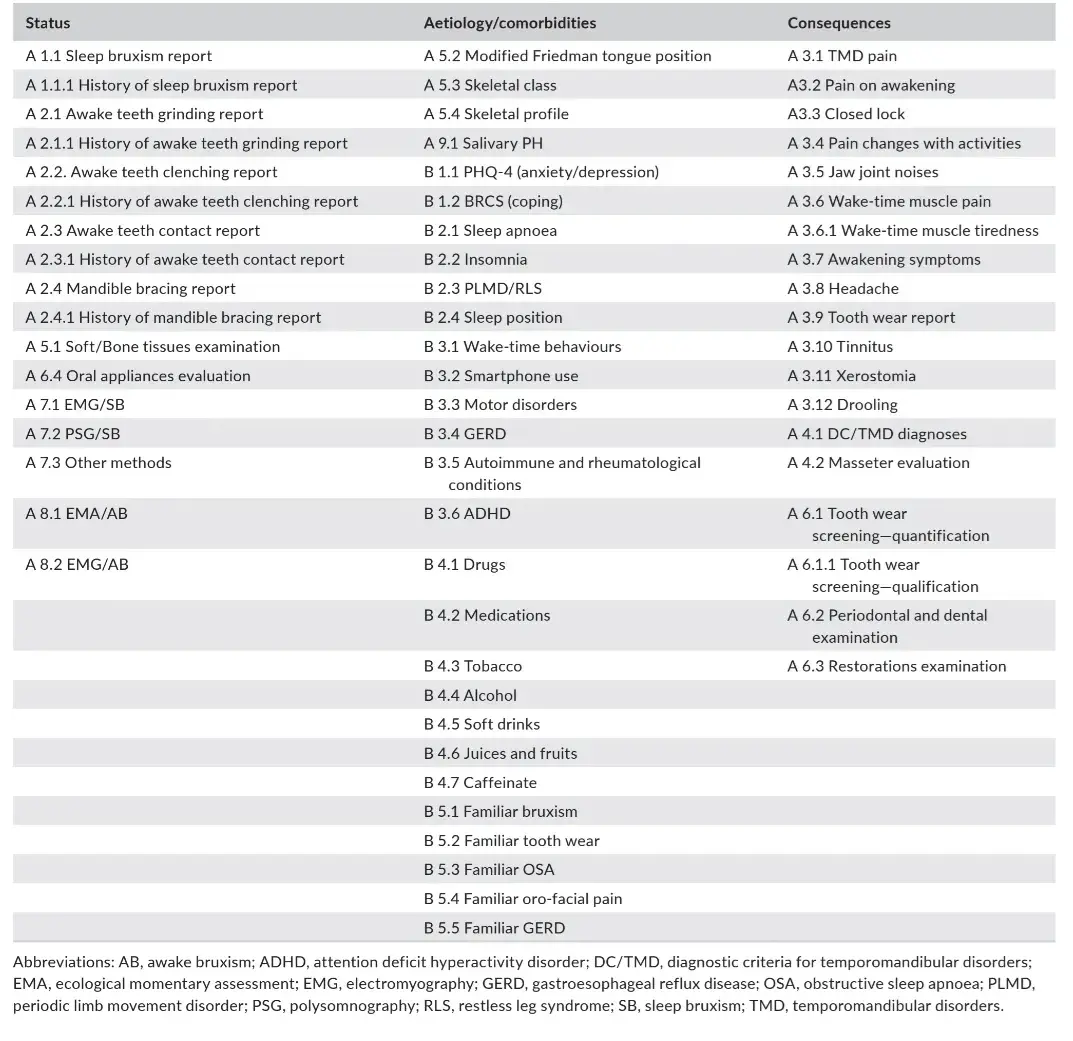
Concerning the STAB full version, a list of items that have been selected for inclusion in the instrument is provided below, divided per topic (Table 1). The specific items with an explanatory manual will be provided in a dedicated paper that is currently under preparation.
TABLE 1 STAB items divided per their scope: evaluation of bruxism status, aetiology and comorbid conditions or bruxism consequences.
3.1 Bruxism status
The following items were selected for the evaluation of bruxism status.
3.1.1 Axis A
A1 Sleep bruxism report (subject-based report)
To assess this domain, two items are proposed, with a question selected from the Oral Behaviour Checklist (OBC)97 to report on the current/last month habit of clenching or grinding the teeth when asleep. The same reports are asked for history:
A1.1 Current SB question
A1.1.1 History of SB question
A2 Awake bruxism report (subject-based report)
To assess this domain, four items are proposed, with questions selected from the OBC to report on the current/last month habits of teeth grinding, teeth clenching, teeth contact, mandible bracing. History of the same conditions are investigated by using questions based on the same formulation.
A2.1 Current awake teeth grinding question
A2.1.1 History of awake teeth grinding report
A2.2 Current awake teeth clenching question
A2.2.1 History of awake teeth clenching report
A2.3 Current awake teeth contact question
A2.3.1 History of awake teeth contact report
A2.4 Current awake mandible bracing question
A2.4.1 History of awake mandible bracing report
A5 Intra-oral tissues examination (examiner's report)
As part of the intra-oral clinical assessment, the evaluation of the presence of some signs (i.e. linea alba, lip impression, tongue scalloping, tongue traumatic lesion, alveolar bone exostosis) is proposed to evaluate the bruxism status.
A5.1 Soft/bone tissues
A6 Teeth and restoration examination (examiner's report)
As part of the clinical assessment concerning teeth and restorations, the evaluation of marks on oral appliances (if hard resin splint is worn by the patient) is suggested.
A6.4 Oral appliance evaluation
A7 Instrumental assessment of sleep bruxism (technology report)
As part of the instrumental assessment of bruxism status, criteria are suggested based on the proposals of a Sleep Bruxism Task Force.
A7.1 EMG
A7.2 PSG53
A7.3 Other methods (e.g. smartphone application scores for grinding sounds)
A7.4 Appliance with sensor (Optional)
A8 Instrumental assessment of awake bruxism (technology report)
As part of the instrumental assessment of awake bruxism status, evaluation with smartphone-based EMA and EMG is suggested.
A8.1 EMA
A8.2 EMG
Criteria for the interpretation of wake-time EMG will be suggested based on the proposals of an Awake Bruxism Task Force.
3.2 Bruxism comorbid conditions and aetiology
The items that were selected for the evaluation of comorbid conditions and bruxism aetiology are grouped together below.
3.2.1 Axis A
A5 Intra-oral tissues and facial profile (examiner's report)
As part of this evaluation domain, tongue position is evaluated. Skeletal class and profile are optional items that will be included in the STAB toolbox kit.
A5.2 Modified Friedman tongue position
A5.3 Skeletal class—sagittal profile (optional item)
A5.4 Skeletal profile—genial angle (optional item)
A9 Additional instruments (technology report)
Intra-oral acidity evaluation included as an optional item.
A9.1. Intra-oral acidity (optional item)
3.2.2 Axis B
B1 Psychosocial assessment (subject-based report)
As part of this evaluation domain, anxiety and depression screening items are included based on the Patient Health Questionnaire along with the Brief Resilient Coping Scale as a coping evaluation instrument.106B1.1 Anxiety and depression screening
B1.2 Coping
B2 Concurrent sleep-related conditions assessment (subject-based report)
Within this evaluation domain, screening questions on sleep-related conditions that are potentially associated with bruxism are proposed, with focus on sleep apnoea (STOP-BANG questionnaire), insomnia, periodic limb movement disorders and restless leg syndrome (Sleep Disorder Questionnaire).108 An item on sleep position from the OBC is also included.
B2.1 Sleep apnoea screening
B2.2 Insomnia screening
B2.3 Periodic limb movement disorder and restless leg syndrome screening
B2.4 Oral behaviours—sleep position
B3 Concurrent non-sleep conditions assessment (subject-based report)
Within this evaluation domain, screening questions on non-sleep-related conditions that are potentially associated with bruxism are proposed, with focus on oral behaviours during waking hours (Oral Behaviours Checklist—OBC), smartphone use, concurrent diagnoses of motor disorders based on the International Network for Orofacial pain and Related disorders Methodology (INfORM) recommendations, gastroesophageal reflux disease screening (GERD-Q),110 autoimmune diseases, attention deficit hyperactive disorders.
B3.1 Oral behaviours—activities during waking hours
B3.2 Smartphone use (optional?)
B3.3 Oro-facial motor disorders
B3.4 Gastroesophageal reflux disease screening
B3.5 Autoimmune or connective tissue disorders screening
B3.6 Attention deficit hyperactivity disorder
B4 Prescribed medications and use of substances assessment (subject-based report)
In this section, the patient is asked about his/her use of drugs, medications and substances that are known for their potential exacerbating or attenuating role on bruxism and its consequences.
B4.1 Drugs
B4.2 Medications
B4.3 Tobacco
B4.4. Alcohol
B4.5 Soft drinks
B4.6 Juices and fruits
B4.7 Caffeine
B5 Additional factors assessment (subject-based report)
In this section, the patient is asked to report about history of bruxism and other related conditions in the family.
B5.1 Familiar bruxism screening
B5.2 Familiar tooth wear screening
B5.3 Familiar obstructive sleep apnoea screening
B5.4. Familiar oro-facial pain screening
B5.5 Familiar GERD screening
3.3 Bruxism consequences
The following items were selected for the evaluation of bruxism consequences.
3.3.1 Axis A
A3 Patient's complaints (subject-based report)
Amongst the potential consequences, TMD and jaw muscle symptoms (TMD pain screener and other items from the diagnostic criteria for TMD [DC/TMD], non-painful symptoms),111–113 headache (from the DC/TMD), tooth wear (from the Tooth Wear Evaluation System), tinnitus (from the DC/TMD), xerostomia (from the Xerostomia Inventory)115 and drooling (Radboud Oral Motor inventory for Parkinson—ROMP)116 is investigated via the following items:
A3.1 TMD pain
A3.2 Pain or stiffness on awakening
A3.3 Closed lock
A3.4 Pain change with activities
A3.5 Jaw joint noises
A3.6 Wake-time muscle pain
A3.6.1 Wake-time muscle tiredness or fatigue
A3.7 Awakening symptoms question
A3.8 Headache
A3.9 Tooth wear
A3.10 Tinnitus
A3.11 Xerostomia
A3.12 Drooling
A4 Joints and muscles (examiner's report)
A4.1 TMD diagnoses
As part of the clinical assessment, the examiner should confirm the presence of one or multiple DC/TMD diagnoses112 and evaluate masseter hypertrophy.
A4.1.1 Pain disorders
A4.1.2 Right TMJ disorders
A4.1.3 Left TMJ disorders
A4.2. Masseter muscle hypertrophy
A6 Teeth and restorations (examiner's report)
As part of the clinical assessment concerning teeth and restorations, the evaluation of tooth wear from both a quantitative and qualitative perspective is suggested based on the Tooth Wear Evaluation System. Also, periodontal screening and dental examination as well as an evaluation of restorations is suggested to evaluate mobility, thermal sensitivity, discomfort on biting, teeth fractures, lost/broken fillings, scratched restorations, ceramic fractures, mobile implants, implant fractures and/or implant screw loosening.
A6.1. Tooth wear quantification
A6.1.1 Tooth wear qualification
A6.2 Periodontal and dental examination
A6.3 Restorations
DISCUSSION
Given the emerging complexity of the bruxism picture, a stackable assessment grading seems not recommendable at the current stage of knowledge. Gathering as much data as possible on the indicators of bruxism status, an integrated evaluation of the potential etiological and comorbid factors and conditions as well as its clinical consequences, emerged as the best available strategy to create a multidimensional evaluation system for bruxism. Within this premise, the items that are currently included in the full STAB version have been listed above and are summarised in Table 1. The items of the middle column (i.e. bruxism status) represent the core part of the STAB. A screening instrument have been developed to identify individuals who may benefit from administration of the full STAB.96 If a patient is not positive for the bruxism status, there is no need to move onto the time-demanding full version of the STAB.
A flowchart for STAB administration based on the patients' access to dental office is suggested in Figure 2. The starting point for a clinical assessment is when a patient of a specific phenotype (e.g. pain, tooth wear) presents in the clinic. The phenotype is determined by a range of factors shaped by the central nervous system and linked to different comorbidities. In order to conduct a comprehensive assessment, the clinician will ‘zoom out’ from the symptoms per se, and incorporate symptoms, signs and comorbidities in relation to diagnosis and aetiology. The assessment will combine the patients' self-report, collated through questionnaires and oral interview, with a clinical assessment, including the necessary components related to the phenotype. This clinical assessment may be complemented with technology by means of instrumental assessment, again in relation to the patient's phenotype. By combining these different levels of assessment, we can provide precision medicine targeted to specific phenotypes whilst adjusted for the individual. For the patient in whom bruxism is part of the above assessment, for example as a potential etiological factor for pain and/or tooth wear, the full STAB might be considered. Based on that, possible STAB workflows can also be figured out. A Bruxism Screener instrument96 can be used to screen for bruxism in case of suspected bruxism status. The working hypothesis is that status has to be confirmed with the endorsement of specific items. Thus, when the BruxScreen leads to bruxism suspicion, the clinician should verify positiveness to one or more selected STAB items to confirm the bruxism status. This path can be viewed as the confirmation process from suspicion to status. When the status is confirmed, a comprehensive evaluation of the comorbid conditions, etiological factors and clinical consequences is needed, thus leading to the administration of the full STAB version (Figure 3).
The next step for further STAB refinement provides the design of on-field studies for the identification of clinical algorithms to predict the presence of bruxism, its aetiology and comorbid conditions, based on the ‘entry point’ to the full STAB, viz., the presence of purported bruxism signs and symptoms. Besides, research using cross-sectional as well as, especially, longitudinal designs can be performed to answer important questions like ‘In which cases is bruxism associated with certain consequences and in which ones it is not?’ and ‘In which cases is bruxism associated with a known aetiology (i.e. secondary bruxism)?’. With time, enough data will be available to mine them with artificial intelligence strategies. Some example models of prediction based on the trajectory aetiology-status-consequences can be hypothesised, viz., knowing either the presence of a certain bruxism activity or its aetiology or its consequence(s), the other aspects can be predicted. For instance, a certain individual with myofascial pain (bruxism consequences) might be predicted to have a certain (awake) bruxism behaviour (bruxism status) that is associated with a certain psychological profile (bruxism aetiology). Another individual with a certain respiratory disorder (bruxism aetiology or comorbid condition) might be predicted to have a certain sleep bruxism pattern (bruxism status) that is associated with a certain degree of tooth wear or rate of prosthodontic complications (bruxism complications).
The definition and evaluation of bruxism status is critical for the use of the STAB for clinical and research purposes. The final goal is to phenotype bruxism status via quantitative (e.g. bruxism time index, bruxism work index) and qualitative evaluation (e.g. for sleep bruxism: arousal-related events, isolated short-lasting events, elevated background activity, tonic prolonged activity; for awake bruxism: tonic prolonged activity, short-lasting events). As an important additional remark, it must be noticed that this is especially important as to improve collaboration with medical doctors in the assessment, prevention and treatment of bruxism: the STAB project efforts will provide them with tools for their clinics and referral.
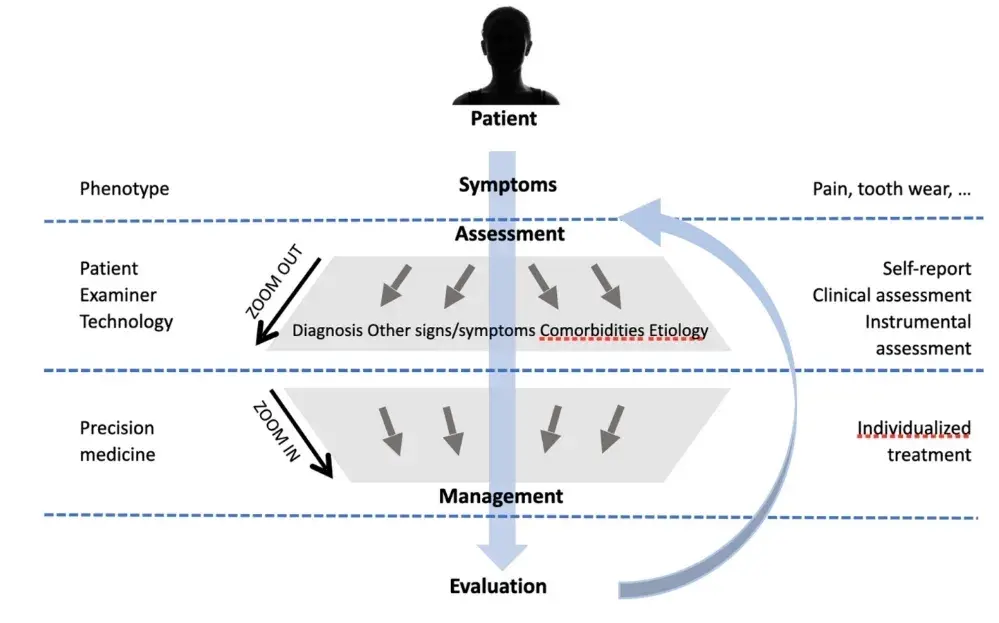 FIGURE 2 Schematic overview of the patient assessment, starting with symptoms related to the specific phenotype, such as pain or tooth wear. The examiner will then ‘zoom out’ in order to conduct a comprehensive assessment that combines the patients' self-report with the appropriate clinical assessment. Technology can then be utilised for instrumental assessment that can provide the full picture. By combining the signs and symptoms with the tentative diagnosis, comorbidities and possible aetiology, the clinician will ‘zoom in’ and develop an individualised treatment plan that focuses on the individual patient as well as on the management of the specific phenotype. After evaluation, the process may be repeated as necessary.
FIGURE 2 Schematic overview of the patient assessment, starting with symptoms related to the specific phenotype, such as pain or tooth wear. The examiner will then ‘zoom out’ in order to conduct a comprehensive assessment that combines the patients' self-report with the appropriate clinical assessment. Technology can then be utilised for instrumental assessment that can provide the full picture. By combining the signs and symptoms with the tentative diagnosis, comorbidities and possible aetiology, the clinician will ‘zoom in’ and develop an individualised treatment plan that focuses on the individual patient as well as on the management of the specific phenotype. After evaluation, the process may be repeated as necessary.
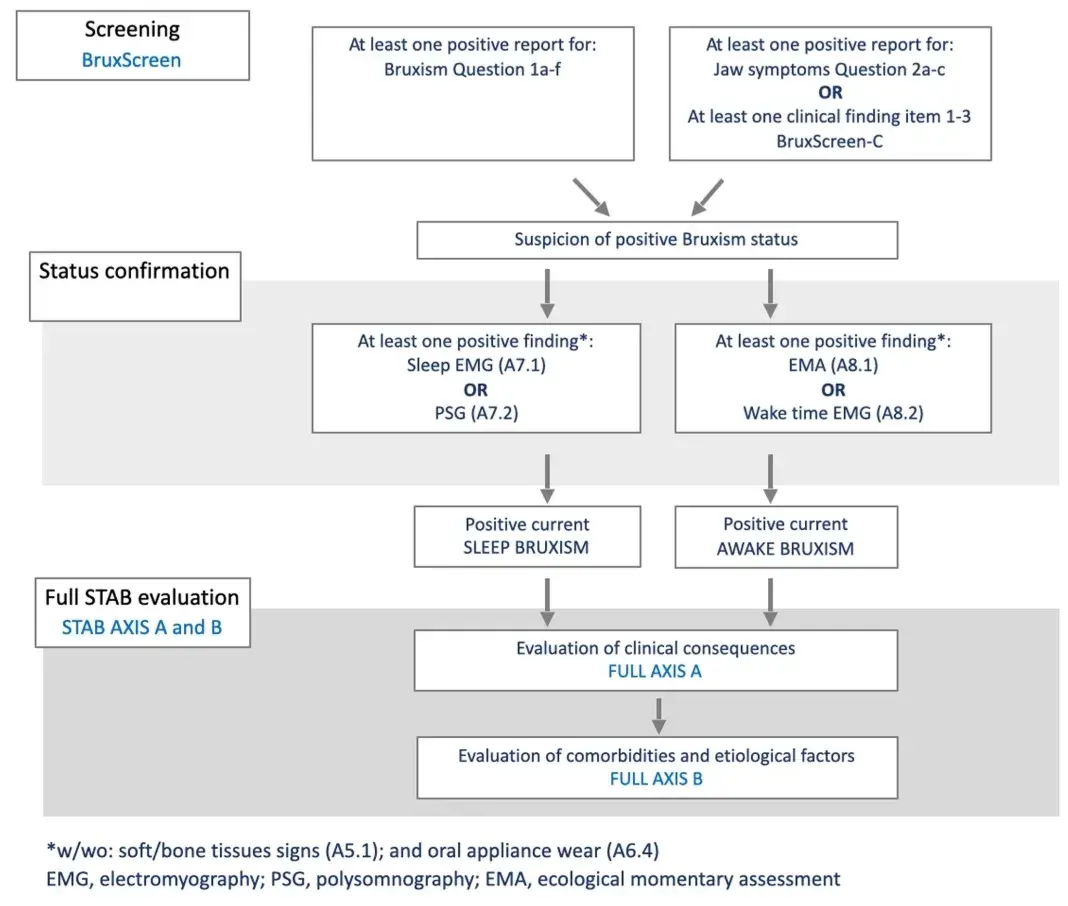 FIGURE 3 STAB flow—The BruxScreen instrument is used screen for bruxism. In case of suspected bruxism status (positive symptoms/signs), status is confirmed with the endorsement of specific items.
FIGURE 3 STAB flow—The BruxScreen instrument is used screen for bruxism. In case of suspected bruxism status (positive symptoms/signs), status is confirmed with the endorsement of specific items.
If bruxism is confirmed, aetiology, comorbid conditions and clinical consequences is evaluated (i.e. full STAB). In case of specific goal for clinical or research purposes, a STAB toolkit version will be available to allow the selection of specific questionnaires.
If you enjoyed reading the article and would like to explore bruxism diagnosis and treatment protocols further, we encourage you to enroll in our course "Encyclopedia of Bruxism and Apnea".
CONCLUSIONS
In this paper, the road map that led to the proposal of the Standardised Tool for the Assessment of Bruxism has been presented, along with the list of items that have been selected after several rounds of debate between the core authors and with expert external reviewers. The instrument will help gathering information on many aspects, factors and conditions that are currently poorly investigated in the field of bruxism. For this reason, a stackable grading or a dichotomic evaluation (yes/no) have been replaced with a comprehensive multidimensional assessment system that will allow building predictive model for clinical and research purposes.
AUTHOR'S INFORMATION
Daniele Manfredini
Jari Ahlberg
Ghizlane Aarab
Alessandro Bracci
Justin Durham
Alona Emodi-Perlman
Dominik Ettlin
Luigi M. Gallo
Birgitta Häggman-Henrikson
Michail Koutris
Ingrid Peroz
Peter Svensson
Peter Wetselaar
Frank Lobbezoo
REFERENCES
Lobbezoo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013;40:2-4.
Lobbezoo F, Ahlberg J, Wetselaar P, et al. International consensus on the assessment of bruxism: report of a work in progress. J Oral Rehabil. 2018;45:837-844.
Manfredini D, Ahlberg J, Wetselaar P, Svensson P, Lobbezoo F. The bruxism construct: from cut-off points to a continuum spectrum. J Oral Rehabil. 2019;46:991-997.
Manfredini D, Ahlberg J, Lobbezoo F. Bruxism definition: past, present, and future – what should a prosthodontist know? J Prosthet Dent. 2021. doi:10.1016/j.prosdent.2021.01.026
Rompré PH, Daigle-Landry D, Guitard F, Montplaisir JY, Lavigne GJ. Identification of a sleep bruxism subgroup with a higher risk of pain. J Dent Res. 2007;86:837-842.
Svensson P, Jadidi F, Arima T, Baad-Hansen L, Sessle BJ. Relationships between craniofacial pain and bruxism. J Oral Rehabil. 2008;35:524-547.
Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e26-e50.
Raphael KG, Sirois DA, Janal MN, et al. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012;143:1223-1231.
Baad-Hansen L, Thymi M, Lobbezoo F, Svensson P. To what extent is bruxism associated with musculoskeletal signs and symptoms? A systematic review. J Oral Rehabil. 2019;46(9):845-861.
Manfredini D, Lombardo L, Visentin A, Arreghini A, Siciliani G. Correlation between sleep-time masseter muscle activity and tooth Wear: an electromyographic study. J Oral Facial Pain Headache. 2019;33(2):199-204.
Manfredini D, Lobbezoo F. Sleep bruxism and temporomandibular disorders: a scoping review of the literature. J Dent. 2021;111:103711.
Raphael KG, Santiago V, Lobbezoo F. Is bruxism a disorder or a behavior? Rethinking the international consensus on defining and grading of bruxism. J Oral Rehabil. 2016;43:791-798.
Manfredini D, Guarda-Nardini L, Marchese-Ragona R, Lobbezoo F. Theories on possible temporal relationships between sleep bruxism and obstructive sleep apnea events. An expert opinion. Sleep Breath. 2015;19:1459-1465.
Manfredini D, De Laat A, Winocur E, Ahlberg J. Why not stop looking at bruxism as a black/white condition? Aetiology could be unrelated to clinical consequences. J Oral Rehabil. 2016;43:799-801.
Svensson P, Lavigne G. Clinical bruxism semantics beyond academic debates: normo- and patho-bruxism as a new proposal. J Oral Rehabil. 2020;47:547-548.
Colonna A, Cerritelli L, Lombardo L, et al. Temporal relationship between sleep-time masseter muscle activity and apnea-hypopnea events: a pilot study. J Oral Rehabil. 2022;49:47-53.
Manfredini D, Ahlberg J, Aarab G, et al. Towards a Standardised Tool for the Assessment of Bruxism (STAB) – overview and general remarks of a multidimensional bruxism evaluation system. J Oral Rehabil. 2020;47:549-556.
Gallo LM, Palla S. Activity recognition in long-term electromyograms. J Oral Rehabil. 1995;22(6):455-462.
Gallo LM, Lavigne G, Rompré P, Palla S. Reliability of scoring EMG orofacial events: polysomnography compared with ambulatory recordings. J Sleep Res. 1997;6:259-263.
Thymi M, Lobbezoo F, Aarab G, et al. Signal acquisition and analysis of ambulatory electromyographic recordings for the assessment of sleep bruxism: a scoping review. J Oral Rehabil. 2021;48:846-871.
Lavigne G, Kato T, Herrero Babiloni A, et al. Research routes on improved sleep bruxism metrics: toward a standardised approach. J Sleep Res. 2021;30:e13320.
Lobbezoo F, Ahlberg J, Aarab G, Manfredini D. Why using 'harmless behaviour', 'risk factor' and 'protective factor' as terms describing the various possible consequences of bruxism is still the best option. J Oral Rehabil. 2021;48:762-763.
Manfredini D, Landi N, Fantoni F, Segù M, Bosco M. Anxiety symptoms in clinically diagnosed bruxers. J Oral Rehabil. 2005;32:584-588.
Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain. 2009;23:153-166.
Lobbezoo F, Aarab G, Wetselaar P, Hoekema A, de Lange J, de Vries N. A new definition of dental sleep medicine. J Oral Rehabil. 2016;43:786-790.
Li Y, Yu F, Niu L, et al. Associations among bruxism, gastroesophageal reflux disease, and tooth Wear. J Clin Med. 2018;7:417.
Ahlberg J, Wiegers JW, van Selms MKA, et al. Oro-facial pain experience among symphony orchestra musicians in Finland is associated with reported stress, sleep bruxism and disrupted sleep-independent of the instrument group. J Oral Rehabil. 2019;46:807-812.
Martynowicz H, Gac P, Brzecka A, et al. The relationship between sleep bruxism and obstructive sleep apnea based on polysomnographic findings. J Clin Med. 2019;8:1653.
van Selms M, Kroon J, Tuomilehto H, et al. Self-reported sleep bruxism among Finnish symphony orchestra musicians: associations with perceived sleep-related problems and psychological stress. Cranio. 2020;1-8. doi:10.1080/08869634.2020.1853310
Ahlberg J, Piirtola M, Lobbezoo F, et al. Correlates and genetics of self-reported sleep and awake bruxism in a nationwide twin cohort. J Oral Rehabil. 2020;47:1110-1119.
Colonna A, Guarda-Nardini L, Ferrari M, Manfredini D. COVID-19 pandemic and the psyche, bruxism, temporomandibular disorders triangle. Cranio. 2021;1-6. doi:10.1080/08869634.2021.1989768
Kuang B, Li D, Lobbezoo F, et al. Associations between sleep bruxism and other sleep-related disorders in adults: a systematic review. Sleep Med. 2022;89:31-47.
Manfredini D, Cantini E, Romagnoli M, Bosco M. Prevalence of bruxism in patients with different research diagnostic criteria for temporomandibular disorders (RDC/TMD) diagnoses. Cranio. 2003;21:279-285
Manfredini D, Winocur E, Guarda-Nardini L, Lobbezoo F. Self-reported bruxism and temporomandibular disorders: findings from two specialised centres. J Oral Rehabil. 2012;39:319-325.
van Selms MK, Muzalev K, Visscher CM, Koutris M, Bulut M, Lobbezoo F. Are pain-related temporomandibular disorders the product of an interaction between psychological factors and self-reported bruxism? J Oral Facial Pain Headache. 2017;31:331-338.
Botelho J, Machado V, Proença L, et al. Relationship between self-reported bruxism and periodontal status: findings from a cross-sectional study. J Periodontol. 2019;91:1049-1056. doi:10.1002/JPER.19-0364
Thymi M, Shimada A, Lobbezoo F, Svensson P. Clinical jaw-muscle symptoms in a group of probable sleep bruxers. J Dent. 2019;85:81-87.
Wetselaar P, Manfredini D, Ahlberg J, et al. Associations between tooth wear and dental sleep disorders: a narrative overview. J Oral Rehabil. 2019;46:765-775.
Shimada A, Castrillon EE, Svensson P. Revisited relationships between probable sleep bruxism and clinical muscle symptoms. J Dent. 2019;82:85-90.
Vieira KRM, Folchini CM, Heyde MDVD, Stuginski-Barbosa J, Kowacs PA, Piovesan EJ. Wake-up headache is associated with sleep bruxism. Headache. 2020;60:974-980.
Kothari SF, Visser M, Timmerman K, et al. Painful and non-painful symptoms evoked by experimental bracing and thrusting of the mandible in healthy individuals. J Oral Rehabil 2021;48:1004-1012.
Bracci A, Djukic G, Favero L, Salmaso L, Guarda-Nardini L, Manfredini D. Frequency of awake bruxism behaviors in the natural environment. A seven-day, multiple-point observation of real time report in healthy young adults. J Oral Rehabil. 2018;45:423-429.
Zani A, Lobbezoo F, Bracci A, et al. Smartphone-based evaluation of awake bruxism behaviours in a sample of healthy young adults: findings from two university centres. J Oral Rehabil. 2021;48:989-995.
Marbach JJ, Raphael KG, Dohrenwend BP, Lennon MC. The validity of tooth grinding measures: etiology of pain dysfunction syndrome revisited. J Am Dent Assoc. 1990;120:327-333.
Raphael KG, Janal MN, Sirois DA, et al. Validity of self-reported sleep bruxism among myofascial temporomandibular disorder patients and controls. J Oral Rehabil. 2015;42:751-758.
Yachida W, Arima T, Castrillon EE, Baad-Hansen L, Ohata N, Svensson P. Diagnostic validity of self-reported measures of sleep bruxism using an ambulatory single-channel EMG device. J Prosthodont Res. 2016;60:250-257.
Restrepo C, Manfredini D, Castrillon E, et al. Diagnostic accuracy of the use of parental-reported sleep bruxism in a polysomnographic study in children. Int J Paediatr Dent. 2017;27:318-325.
Ohlmann B, Rathmann F, Bömicke W, Behnisch R, Rammelsberg P, Schmitter M. Validity of patient self-reports and clinical signs in the assessment of sleep bruxism based on home-recorded electromyographic/electrocardiographic data. J Oral Rehabil. 2022;49:720-728. doi:10.1111/joor.13327
Koyano K, Tsukiyama Y, Ichiki R, Kuwata T. Assessment of bruxism in the clinic. J Oral Rehabil. 2008;35:495-508.
Kitagawa K, Kodama N, Manda Y, Mori K, Furutera H, Minagi S. Effect of masseter muscle activity during wakefulness and sleep on tooth wear. J Prosthodont Res. 2021;66:551-556. doi:10.2186/jpr.JPR_D_21_00171
Ramanan D, Palla S, Bennani H, Polonowita A, Farella M. Oral behaviours and wake-time masseter activity in patients with masticatory muscle pain. J Oral Rehabil. 2021;48:979-988.
Zani A, Lobbezoo F, Bracci A, Ahlberg J, Manfredini D. Ecological momentary assessment and intervention principles for the study of awake bruxism behaviors, part 1: general principles and preliminary data on healthy young Italian adults. Front Neurol. 2019;10:169.
Lavigne GJ, Rompré PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996;75:546-552.
Castroflorio T, Deregibus A, Bargellini A, Debernardi C, Manfredini D. Detection of sleep bruxism: comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J Oral Rehabil. 2014;41:163-169.
Manfredini D, Ahlberg J, Castroflorio T, Poggio CE, Guarda-Nardini L, Lobbezoo F. Diagnostic accuracy of portable instrumental devices to measure sleep bruxism: a systematic literature review of polysomnographic studies. J Oral Rehabil. 2014;41:836-842.
Lobbezoo F, Aarab G, Ahlers MO, et al. Consensus-based clinical guidelines for ambulatory electromyography and contingent electrical stimulation in sleep bruxism. J Oral Rehabil. 2020;47:164-169.
Li D, Aarab G, Lobbezoo F, Arcache P, Lavigne GJ, Huynh N. Accuracy of sleep bruxism scoring based on electromyography traces of different jaw muscles in individuals with obstructive sleep apnea. J Clin Sleep Med. 2022;18:1609-1615. doi:10.5664/jcsm.9940
Paesani DA, Lobbezoo F, Gelos C, Guarda-Nardini L, Ahlberg J, Manfredini D. Correlation between self-reported and clinically based diagnoses of bruxism in temporomandibular disorders patients. J Oral Rehabil. 2013;40:803-809.
Castroflorio T, Bargellini A, Rossini G, Cugliari G, Deregibus A, Manfredini D. Agreement between clinical and portable EMG/ECG diagnosis of sleep bruxism. J Oral Rehabil. 2015;42:759-764.
Casett E, Réus JC, Stuginski-Barbosa J, et al. Validity of different tools to assess sleep bruxism: a meta-analysis. J Oral Rehabil. 2017;44:722-734.
Dias R, Vaz R, Rodrigues MJ, Serra-Negra JM, Bracci A, Manfredini D. Utility of smartphone-based real-time report (ecological momentary assessment) in the assessment and monitoring of awake bruxism: a multiple-week interval study in a Portuguese population of university students. J Oral Rehabil. 2021;48:1307-1313.
Emodi-Perlman A, Manfredini D, Shalev T, et al. Awake bruxism-single-point self-report versus ecological momentary assessment. J Clin Med. 2021;10(8):1699.
Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain. 2013;27:99-110.
Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil. 2008;35:476-494.
Wetselaar P, Vermaire EJH, Lobbezoo F, Schuller AA. The prevalence of awake bruxism and sleep bruxism in the Dutch adolescent population. J Oral Rehabil. 2021;48:143-149.
Mayer P, Heinzer R, Lavigne G. Sleep bruxism in respiratory medicine practice. Chest. 2016;149:262-271.
Abe S, Yamaguchi T, Rompré PH, De Grandmont P, Chen YJ, Lavigne GJ. Tooth wear in young subjects: a discriminator between sleep bruxers and controls? Int J Prosthodont. 2009;22:342-350.
Kato T, Yamaguchi T, Okura K, Abe S, Lavigne GJ. Sleep less and bite more: sleep disorders associated with occlusal loads during sleep. J Prosthodont Res. 2013;57:69-81.
van der Zaag J, Naeije M, Wicks DJ, Hamburger HL, Lobbezoo F. Time-linked concurrence of sleep bruxism, periodic limb movements, and EEG arousals in sleep bruxers and healthy controls. Clin Oral Investig. 2014;18:507-513.
Zhang Y, Lu J, Wang Z, et al. Companion of oral movements with limb movements in patients with sleep bruxism: preliminary findings. Sleep Med. 2017;36:156-164.
Aarab G, Arcache P, Lavigne GJ, Lobbezoo F, Huynh N. The effects of mandibular advancement appliance therapy on jaw-closing muscle activity during sleep in patients with obstructive sleep apnea: a 3–6 months follow-up. J Clin Sleep Med. 2020;16:1545-1553.
Hublin C, Kaprio J, Partinen M, Koskenvuo M. Sleep bruxism based on self-report in a nationwide twin cohort. J Sleep Res. 1998;7:61-67.
Abe K, Shimakawa M. Genetic and developmental aspects of sleep-talking and teeth-grinding. Acta Paedopsychiatr 1966;33:339-344.
Abe Y, Suganuma T, Ishii M, et al. Association of genetic, psychological and behavioral factors with sleep bruxism in a Japanese population. J Sleep Res. 2012;21:289-296.
Lobbezoo F, Visscher CM, Ahlberg J, Manfredini D. Bruxism and genetics: a review of the literature. J Oral Rehabil 2014;41:709-714.
Lobbezoo F, Visscher CM, Koutris M, Wetselaar P, Aarab G. Bruxism in dentists' families. J Oral Rehabil. 2018;45:657-658.
de Baat C, Verhoeff M, Ahlberg J, et al. Medications and addictive substances potentially inducing or attenuating sleep bruxism and/or awake bruxism. J Oral Rehabil. 2021;48:343-354.
Pierce CJ, Chrisman K, Bennett ME, Close JM. Stress, anticipatory stress, and psychologic measures related to sleep bruxism. J Orofac Pain. 1995;9:51-56.
Manfredini D, Landi N, Romagnoli M, Bosco M. Psychic and occlusal factors in bruxers. Aust Dent J. 2004;49:84-89.
Emodi Perlman A, Lobbezoo F, Zar A, Friedman Rubin P, van Selms MK, Winocur E. Self-reported bruxism and associated factors in Israeli adolescents. J Oral Rehabil. 2016;43:443-450.
Manfredini D, Arreghini A, Lombardo L, et al. Assessment of anxiety and coping features in bruxers: a portable electromyographic and electrocardiographic study. J Oral Facial Pain Headache. 2016;30:249-254.
Emodi-Perlman A, Manfredini D, Shalev T, Bracci A, Frideman-Rubin P, Eli I. Psychosocial and behavioral factors in awake bruxism-self-report versus ecological momentary assessment. J Clin Med. 2021;10:4447.
Manfredini D, Visscher CM, Guarda-Nardini L, Lobbezoo F. Occlusal factors are not related to self-reported bruxism. J Orofac Pain. 2012;26:163-167.
Manfredini D, Poggio CE, Lobbezoo F. Is bruxism a risk factor for dental implants? A systematic review of the literature. Clin Implant Dent Relat Res. 2014;16:460-469.
Jonsgar C, Hordvik PA, Berge ME, Johansson AK, Svensson P, Johansson A. Sleep bruxism in individuals with and without attrition-type tooth wear: an exploratory matched case-control electromyographic study. J Dent. 2015;43:1504-1510.
Manfredini D, Ahlberg J, Mura R, Lobbezoo F. Bruxism is unlikely to cause damage to the periodontium: findings from a systematic literature assessment. J Periodontol. 2015;86:546-555.
Zhou Y, Gao J, Luo L, Wang Y. Does bruxism contribute to dental implant failure? A systematic review and meta-analysis. Clin Implant Dent Relat Res. 2016;18:410-420.
Kapagiannidou D, Koutris M, Wetselaar P, Visscher CM, van der Zaag J, Lobbezoo F. Association between polysomnographic parameters of sleep bruxism and attrition-type tooth wear. J Oral Rehabil. 2021;48:687-691.
Manfredini D, Greene CS, Ahlberg J, De Laat A, Lobbezoo F, Klasser GD. Evidence-based dentistry or meta-analysis illness? A commentary on current publishing trends in the field of temporomandibular disorders and bruxism. J Oral Rehabil. 2019;46:1-4.
Glaros AG, Rao SM. Effects of bruxism: a review of the literature. J Prosthet Dent. 1977;38:149-157.
Gouw S, de Wijer A, Bronkhorst EM, Kalaykova SI, Creugers NHJ. Association between self-reported bruxism and anger and frustration. J Oral Rehabil. 2019;46:101-108.
Ohmure H, Oikawa K, Kanematsu K, et al. Influence of experimental esophageal acidification on sleep bruxism: a randomized trial. J Dent Res. 2011;90:665-671.
Miyawaki S, Katayama A, Tanimoto Y, et al. Salivary flow rates during relaxing, clenching, and chewing-like movement with maxillary occlusal splints. Am J Orthod Dentofacial Orthop. 2004;126:367-370.
Casazza E, Ballester B, Philip-Alliez C, Raskin A. Evaluation of mandibular bone density in bruxers: the value of panoramic radiographs. Oral Radiol. 2022. doi:10.1007/s11282-022-00612-3
Verhoeff MC, Lobbezoo F, van Leeuwen AM, Schuller AA, Koutris M. Oral health-related quality of life in patients with Parkinson's disease. J Oral Rehabil. 2022;49:398-406.
Lobbezoo F, Ahlberg J, Aarab G, et al. The bruxism screener (BruxScreen): development, pilot testing, and face validity. J Oral Rehabil. in submission.
Markiewicz MR, Ohrbach R, McCall WD Jr. Oral behaviors check-list: reliability of performance in targeted waking-state behaviors. J Orofac Pain. 2006;20:306-316.
Van Der Zaag J, Lobbezoo F, Visscher CM, Hamburger HL, Naeije M. Time-variant nature of sleep bruxism outcome variables using ambulatory polysomnography: implications for recognition and therapy evaluation. J Oral Rehabil. 2008;35:577-584.
Manfredini D, Fabbri A, Peretta R, Guarda-Nardini L, Lobbezoo F. Influence of psychological symptoms on home-recorded sleep-time masticatory muscle activity in healthy subjects. J Oral Rehabil. 2011;38:902-911.
Colonna A, Lombardo L, Siciliani G, et al. Smartphone-based application for EMA assessment of awake bruxism: compliance evaluation in a sample of healthy young adults. Clin Oral Investig. 2020;24:1395-1400.
Nykänen L, Manfredini D, Lobbezoo F, et al. Ecological momentary assessment of awake bruxism with a smartphone application requires prior patient instruction for enhanced terminology comprehension: a multi-center study. J Clin Med. 2022;11:3444.
Colonna A, Noveri L, Ferrari M, Bracci A, Manfredini D. Electromyographic assessment of masseter muscle activity: a proposal for a 24hr recording device with preliminary data. J Clin Med. 2022. Accepted for publication.
Bracci A, Lobbezoo F, Hangman-Henrikson B, et al. Current knowledge and future perspectives on awake bruxism assessment: expert consensus recommendations. J Clin Med. 2022;11:5083.
Laharnar N, Herberger S, Prochnow LK, et al. Simple and unbiased OSA prescreening: introduction of a new morphologic OSA prediction score. Nat Sci Sleep. 2021;13:2039-2049.
Kroenke K, Spitzer RL, Williams JB, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50:613-621.
Sinclair VG, Wallston KA. The development and psychometric evaluation of the brief resilient coping scale. Assessment. 2004;11:94-101.
Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
Douglass AB, Bornstein R, Nino-Murcia G, et al. The sleep disorders questionnaire. I: creation and multivariate structure of SDQ. Sleep. 1994;17:160-167.
Peck CC, Goulet JP, Lobbezoo F, et al. Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J Oral Rehabil. 2014;41:2-23.
Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther.2009;30:1030-1038.
van Grootel RJ, van der Glas HW, Buchner R, de Leeuw JR, Passchier J. Patterns of pain variation related to myogenous temporomandibular disorders. Clin J Pain. 2005;21:154-165.
Schiffman E, Ohrbach R, Truelove E, et al. International RDC/TMD consortium network, international association for dental research; orofacial pain special interest group, International Association for the Study of Pain. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network* and orofacial pain special interest group. J Oral Facial Pain Headache. 2014;28:6-27.
Lövgren A, Häggman-Henrikson B, Visscher CM, Marklund S, Lobbezoo F, Wänman A. Jaw pain and dysfunction in the general population. Eur J Pain. 2016;20:532-540.
Wetselaar P, Lobbezoo F. The tooth wear evaluation system: a modular clinical guideline for the diagnosis and management planning of worn dentitions. J Oral Rehabil. 2016;43:69-80.
Thomson WM, Chalmers JM, Spencer AJ, Williams SM. The xerostomia inventory: a multi-item approach to measuring dry mouth. Community Dent Health. 1999;16:12-17.
Kalf JG, Bloem BR, Munneke M. Diurnal and nocturnal drooling in Parkinson's disease. J Neurol. 2012;259:119-123.