The bruxism construct: From cut‐off points to a continuum spectrum
ABSTRACT
This commentary discusses the need to move on from the adoption of cut-off points for the definition of the presence/absence of bruxism and justifies the need to embrace an evaluation based on the continuum of jaw motor behaviours. Currently, the number of events per hour, as identified by polysomnography (PSG), is used to define the presence of sleep bruxism (SB). Whilst PSG still remains the indispensable equipment to study the neurophysiological correlates of SB, the scoring criteria based on a cut-off point are of questionable clinical usefulness for the study of oral health outcomes. For awake bruxism (AB), criteria for a definite diagnosis have never been proposed. Some goal-oriented strategies are proposed to identify bruxism behaviours that increase the risk of negative oral health outcomes (eg, tooth wear, muscle and/or temporomandibular joint [TMJ] pain, restorative complications). One possible strategy would embrace an improved knowledge on the epidemiology and natural variability of bruxism, even including study of the amount of PSG/SB and electromyography masticatory muscle activity (EMG/MMA) during sleep and the frequency/prevalence of bruxism behaviours during wakefulness that are needed to represent a risk factor for clinical consequences, if any. There should not be any preclusion about the diagnostic strategies to pursue that goal, and a combination of instrumental and non-instrumental approaches may even emerge as the best available option. Once data are available, large-scale, non-selected population samples representing the entire continuum of EMG/MMA activities are also needed, in the attempt to estimate untreated health risks in the population.
You have the opportunity to gather more in-depth information about diagnosis and treatment of bruxism in our Online congress of on evidence-based temporomandibular disorders and bruxism treatment.
INTRODUCTION
Bruxism is an umbrella term that groups together a broad spectrum of jaw muscle activities of different aetiology and clinical relevance. It might be a sign of underlying disorders, may represent a risk factor for clinical consequences or is possibly just a behaviour without any pathological relevance.1-3 This construct of bruxism formed the basis of a recently published updated consensus paper4 that revisited the definition and diagnostic grading proposed in 2013 by a bruxism expert panel.5
The diagnostic grading for bruxism suggests that instrumental assessment tools for sleep bruxism (SB) or real-time approaches for awake bruxism (AB) should be used to definitely establish the presence of the condition. This implies that, ideally, the existence of a threshold to identify bruxism as a risk factor for negative oral health outcomes could be evaluated, given its implications in terms of treatment-guiding principles. Current polysomnographic (PSG) criteria for SB assessment focus on the number of SB events,6,7 which is a parameter that is not necessarily related to clinical consequences and therefore requires an appraisal of its biological relevance. Moreover, a frequency or intensity threshold for a definite AB diagnosis has never been proposed.
This commentary discusses the need to avoid relying on a dichotomic approach to the presence/absence of bruxism for the study of its role as a possible risk factor for negative oral health outcomes, as well as the need to evaluate bruxism within the framework of a continuum of motor activities or experiences.
THE DICHOTOMY OF “DEFINITE” BRUXISM
The literature on SB has always relied on a dichotomic (ie, “definite”) approach to interpret PSG recordings and the classification of SB as present or absent, based on a set of clinically validated criteria.8 In short, two relatively small groups of young adults without major health concerns, with or without anamnestic report of SB (ie, teeth grinding sounds for five nights a week over the last six months) and clinical signs/symptoms (ie, tooth wear/shiny spots on restorations and/or masseter muscle pain/fatigue in the morning and/or masseter hypertrophy upon palpation), were compared for sleep features in a laboratory setting.6 All masticatory EMG potentials with at least 20% of the maximum voluntary contraction (MVC) amplitude were considered SB events, and all bursts of jaw muscles activity (eg, including swallowing and other non-SB activities) were recorded. The best diagnostic cut-off criteria for predicting the clinical diagnosis, viz., belonging to the bruxism or control group (specificity and sensitivity >80%), were as follows: (a) more than 4 bruxism episodes per hour, (b) more than 6 bruxism bursts per episode and/or 25 bruxism bursts per hour of sleep and (c) at least 2 episodes with grinding sounds. Further elaborations of the same criteria led to the proposal of three SB frequency categories.7 It was noteworthy that the authors recommended further testing before the adoption of such sleep research criteria as a clinical tool.
Despite the authors’ cautionary statements about their intention to propose these criteria as screening tools, it must be pointed out the PSG/SB criteria have been progressively introduced in clinical research as the reference standard to diagnose definite SB. In addition, the current PSG/SB cut-point (eg, more than four bruxism episodes per hour of sleep) has been frequently used to purportedly assess the association of SB with potential consequences. Based on current knowledge, as discussed in the recent updated consensus paper,4 such approach should no longer be considered optimal for establishing the status of clinically relevant SB or non-SB.
There are both methodological and clinical reasons for this. Methodological issues include circularity problems in the criteria used to establish the cut-point.1 In fact, the cut-point was selected as the best compromise to detect individuals with a reported history of tooth grinding and clinical signs and symptoms. By definition, this created a tautology increasing the probability, artificially, of a relationship between PSG criteria purposefully set at a cut-point designed to maximally match a certain definition of SB and the self- or partner-reported bruxism and clinical signs that were used to define the very same PSG criteria. In addition, the cut-point was established in a super-selected study sample, excluding individuals with conditions known for their possible association with SB (eg, sleep apnoea and other sleep disorders, amongst others). From a methodological viewpoint, the fact that the PSG/SB criteria were proposed for use only as a screening tool for research purpose would explain the absence of clear-cut correlations with potential consequences in a less-protected clinical setting.9,10 Moreover, the presence or absence of SB has even become a reference outcome for meta-analysis data,10 with the introduction of potential interpretation bias for readers.11
Such improper use is reflected in the data from studies that have found a substantial prevalence of high-frequency SB at population level. For instance, work on the validation of portable devices for home EMG measurements using the proposed PSG scoring criteria for EMG/SB events,12 as well as investigations on the relationship between SB and clinical signs/symptoms, 13-15 found average SB episodes/hr values around or above the threshold for diagnosing high-frequency SB.
As an additional point, it should be mentioned that PSG/SB criteria reflect a bruxism construct based on the identification of rhythmic masticatory muscle activity (RMMA) proper of sleep arousals. Such motor phenomena are only a portion of the spectrum of masticatory muscle activities that an individual can exhibit during sleep. These activities have been included in the recently updated bruxism consensus paper.4 Some of the activities (eg, prolonged, low-to-moderate intensity teeth clenching or mandible bracing) are not counted as SB events by the automatic software elaborating PSG/EMG traces, despite their possible higher relevance for the onset of muscle fatigue than short-lasting, phasic events typical of RMMA. The potential time-variant nature of RMMA/SB at the individual level on a nightly basis is another factor that would help to explain the poor clinical validity of the current instrumental assessment,16 especially in terms of treatment need for an affected individual.17
These considerations are by no means intended to dismantle the role of PSG, which still remains the standard of reference to study the neurophysiological correlates of sleep-time masticatory muscle activity and the presence of SB events within the context of sleep arousal.4 The recommendation is that more elaborated strategies for interpreting masseter EMG signals are needed for a better understanding of the clinical correlates.3 It should also be noted that the concept of a threshold is often strongly linked to the assumption of a linear relationship between the measure of MMA/EMG and the purported consequences of that activity (Figure 1A).18,19 However, there could be other and more complex relationships (Figure 1B). They could depend on the health outcomes and the presence of concurrent factors as well as on the specific measures of EMG/MMA. Amongst these, parameters such as cumulated activity (ie, area under time—EMG curve), relative rest time and amplitude probability distribution function could be theoretically more related to the onset of clinical consequences than frequency measures (Figure 1C). The implication is that more and better research into the significance of clinical indicators of a high or a low SB/MMA is needed, with particular focus on the complexity of changes in the pain experience over time (Figure 2).
As for AB, the difficulty of patient compliance when undergoing hour-long recordings of jaw muscle activity during wakefulness is key to the incomplete knowledge on this condition's epidemiological characteristics and clinical management.20 It is likely that future developments in this field will derive from the spread of real-time self-reported techniques, viz., ecological momentary assessment (EMA—also called experience sampling methodology [ESM]). Smartphone technology offers promising potentials for implementing EMA/ESM diffusion in the field of AB assessment, though it does need to be understood that this relies on subjective reports of a behaviour, which is different from the objective measurement approach of current SB investigations.
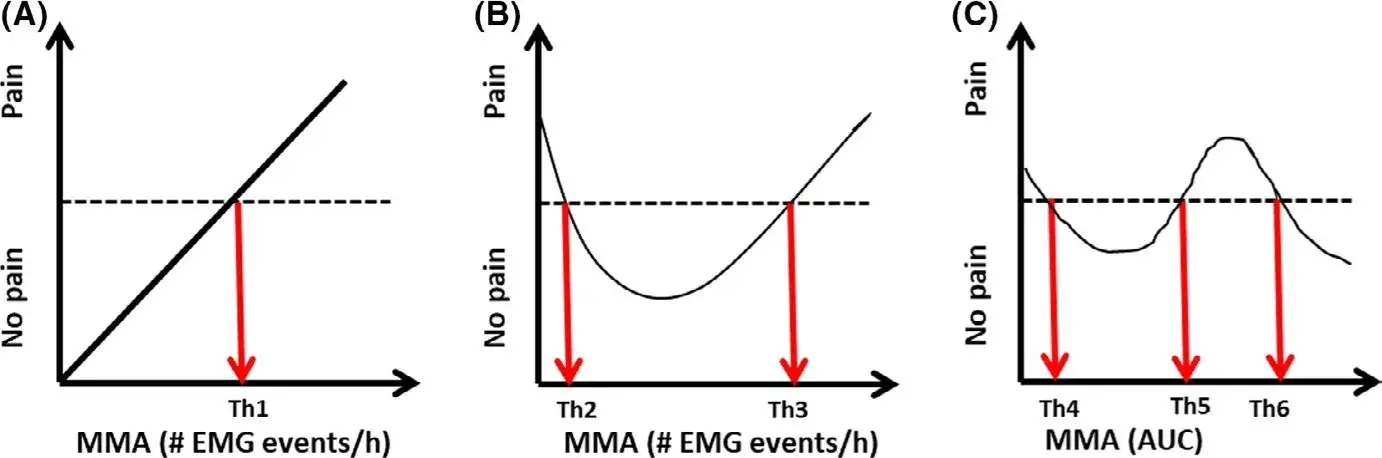 FIGURE 1 Theoretical relationship between measures of Masticatory Muscle Activity (MMA) and a possible consequence, for example pain. A, illustrates a traditional linear relationship where a threshold (Th1) can be defined as the borderline between normal and pathophysiological activity (pain/no pain). However, the relationship could be more complex as illustrated in B, where now 2 thresholds would be important to identify (Th2 and Th3). The examples in A and B may relate to frequency measures of MMA but not to other measures of MMA, for example area under the time—EMG curve (AUC), which then would lead to more thresholds (Th4-6) (C)
FIGURE 1 Theoretical relationship between measures of Masticatory Muscle Activity (MMA) and a possible consequence, for example pain. A, illustrates a traditional linear relationship where a threshold (Th1) can be defined as the borderline between normal and pathophysiological activity (pain/no pain). However, the relationship could be more complex as illustrated in B, where now 2 thresholds would be important to identify (Th2 and Th3). The examples in A and B may relate to frequency measures of MMA but not to other measures of MMA, for example area under the time—EMG curve (AUC), which then would lead to more thresholds (Th4-6) (C)
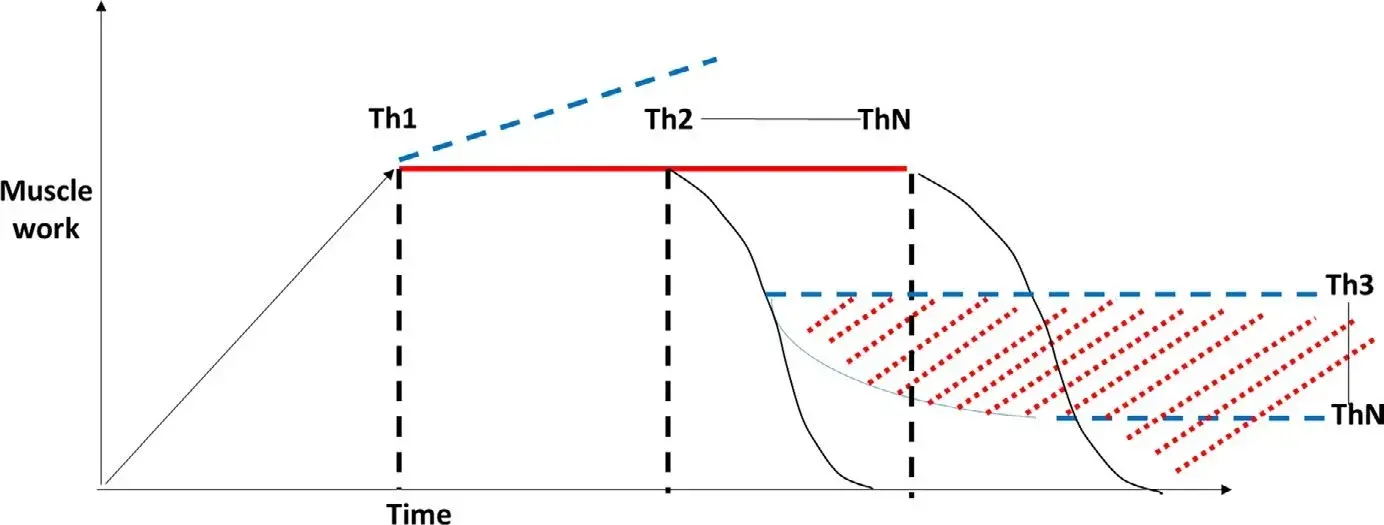 FIGURE 2 Theoretical relationship between the amount of muscle work (y axis) over time (x axis) and pain. Even if it is likely that prolonged muscle activity (eg, MMA AUC) may lead to muscle fatigue and symptoms when the individual threshold is reached (Th1), based on the integrated pain-adaptation model and the activity is not likely to increase further (blue dashed line).35 If pain emerges, after a certain period of time (individual thresholds—Th2-ThN) a decrease in muscle activity due to exhaustion may be observed. If pain becomes chronic (red dotted area), a lower activity is present but still sufficient to maintain a nociceptive input (Th3-ThN)
FIGURE 2 Theoretical relationship between the amount of muscle work (y axis) over time (x axis) and pain. Even if it is likely that prolonged muscle activity (eg, MMA AUC) may lead to muscle fatigue and symptoms when the individual threshold is reached (Th1), based on the integrated pain-adaptation model and the activity is not likely to increase further (blue dashed line).35 If pain emerges, after a certain period of time (individual thresholds—Th2-ThN) a decrease in muscle activity due to exhaustion may be observed. If pain becomes chronic (red dotted area), a lower activity is present but still sufficient to maintain a nociceptive input (Th3-ThN)
HOW TO IDENTIFY CLINICALLY RELEVANT BRUXISM
Within this framework, some goal-oriented strategies can be proposed to better identify bruxism behaviours that increase or reduce the risk for clinical consequences (eg, tooth wear, muscle and/or temporomandibular joint [TMJ] pain, restorative complications).
One possible strategy would be to increase knowledge on all aspects of bruxism epidemiology, with particular regard to strong data collection on night-to-night and day-to-day variability. An agenda which focuses on trying various ways of measuring and scoring bruxism in the attempt to relate it to oral health outcomes is also a priority. Within these premises, identifying the amount of PSG/SB and EMG/MMA during sleep and the frequency/prevalence of bruxism behaviours during wakefulness that may be expected in an average, otherwise healthy individual, even if potentially unrealistic, are another potential goal for clinical research studies.
Studying the epidemiology
The absence of values for the range of bruxism activity that is not associated with clinical consequences and the paucity of data on bruxism fluctuation on nightly or daily basis severely limit advances in current knowledge and interpretation of research findings.
Currently available data on SB at the general population level are hard to interpret, since most are based on case-control studies, viz., investigations including a pre-selected sample of probable or possible bruxers and a control sample of non-bruxers. The scarce literature on open samples of non-selected individuals shows that the number of bruxism episodes/hr ranges between 4 and 5 on average, when a threshold of 10% MVC is adopted to identify SB episodes14,15 and exceeds 10 when a threshold set at twice the baseline EMG activity value is adopted.21
Unfortunately, none of these data is directly comparable to the above-described standard of reference criteria, which provide a 20% MVC threshold to identify SB events.6 It is noteworthy that when such a threshold was used in a large epidemiological paper, a 7.4% prevalence for SB in the general population was recorded.22 All data, however, are derived from single-night investigations. Thus, little is actually known on the time-variant nature of SB phenomena, which should instead be part of any attempt to relate bruxism with health outcomes.16
As for AB, recent findings suggest that healthy volunteers engage in some awake bruxism behaviours (ie, teeth clenching, jaw bracing, teeth grinding, teeth contact) in 28.3% of real-time answers to EMA stimuli over one week.23 However, the huge amount of observations that can be gathered with EMA-based real-time collection (ie, number of patients x number of reports/day x number of observation days) proved challenging, leaving researchers with a huge database to analyse. The common-sense strategy adopted so far has been to report the mean frequency of each AB behaviour at the study population level, as well as the proportion of individuals who reported the behaviour at least once a day. The frequencies were calculated daily on an individual basis, and individual frequencies used to calculate an average of the study population on a daily basis. At the end of the seven-day observation period, the mean frequency of each condition was assessed both for each subject and for the study population. Data were reported as mean values of the seven-day span per each condition. At the patient level, the most prevalent behaviour was teeth contact, reported at least once a day by a minimum of 39.1% and a maximum of 52.2% of individuals.
All in all, such values offer the best available compromise to capture epidemiological data on AB at the general population level, but the above-reported data on frequency (ie, average percentage of positive answers amongst the total of received alerts over one week at the population level) and prevalence (ie, proportion of individuals reporting the behaviour at least once a day) should be carefully interpreted when comparisons are made with future study findings, before any generalisations are made.
Definition of bruxism event
The choice of the most suitable bruxism scoring system to measure risk for oral health outcomes is crucial for future studies. This implies that the definition of a bruxism event itself in terms of EMG features should be carefully appraised. Current standard criteria identify an SB event when the EMG amplitude of masseter muscle activity exceeds 20% of MVC. Events can be phasic, tonic or mixed, based on their duration, with a predominance of the phasic ones likely due to the discard of second-to-minute long tonic contractions that should mirror teeth clenching or mandible bracing.24
Different thresholds have been adopted in other studies, the most diffused of which are 10% of MVC and twice the baseline EMG amplitude. In another study, EMG amplitude during swallowing was set as the threshold to cut off background EMG noises and to measure the total work (µV x S) produced by each muscle during sleep time.25 Other orofacial motor events (OME) that may also occur have recently been categorised with respect to the features of the RMMA that are typical of SB.21 Such differences possibly resemble the different equipment (ie, full audio/video PSG vs EMG; multi- vs single-channel) and setting (ie, sleep laboratory vs other environments). Nonetheless, correlational data with respect to the original instruments are lacking for most devices, with minor exceptions, thus making it difficult to compare the different scoring systems and even to create proportional measures for conversion of data from one instrument to another.
Based on the above, it seems reasonable that agreement on a new, broader construct of bruxism itself should be reached. Early works conceptualised SB within the framework of microarousals during sleep.26 They refer to the term sleep bruxism to indicate EMG events that occur at the end of a cascade of arousal-related neurovegetative signs and are accompanied by teeth grinding sounds in at least 50% of cases.27 PSG with audiovisual (AV) recordings is the standard of reference to detect the so-defined SB.
Nonetheless, the low availability of sleep laboratory settings for studying SB emerged as a critical factor to limit consistent data gathering in multiple centres. The diffusion of simplified devices for home usage has shifted the attention to the EMG traces.28 This has led to a widened bruxism construct, up to being an umbrella term for the spectrum of masticatory muscle activities (MMA) exceeding the above-described EMG thresholds, independently of their relation with PSG-detected microarousals. These ambulatory or home recording strategies, based on EMG tracking only, may lead to a possible overestimation of SB with respect to the PSG/SB scoring criteria.29 This underscores the need for high-quality studies to better identify the most suitable scoring criteria for any determined oral health outcomes. The adoption of a combined EMG/ECG recording has been shown valid to approximate a definite PSG/SB diagnosis with >90% accuracy,12 thus possibly overcoming the above shortcomings.
However, in the light of their possible different relationships with the clinical consequences, it is suggested that PSG/SB events are differentiated from EMG/MMA events whenever possible in future research.
For AB, whilst the theoretical reference standard is real-time EMG, problems with patient compliance suggested the introduction of real-time subject-based reporting (ie, EMA/ESM).5 This point has been emphasised in the updated consensus paper, but it should be noted that further testing is needed.4 Indeed, even if close in time to the experience, the subjectivity of self-report may introduce some intra- and interindividual reliability bias. An accurate definition of awake bruxism behaviours and the calibration of the examiners who introduce the EMA strategy to patients are a reasonable strategy to provide the most solid evidence on the topic. In support of this, a recent paper showed a very low coefficient of intra-individual variability for the absence of AB over one week.23
Identifying possible clinical consequences
Until now, the literature has not been able to provide an identification of the amount of bruxism behaviour that is associated with negative oral health outcomes. This should be a priority for future studies. Besides, there are no evidence-based suggestions on the amount of additive bruxism that can be observed when associated factors are present (eg, obstructive sleep apnoea [OSA], gastroesophageal reflux disease [GERD], other sleep disorders), or when secondary bruxism due to known causative factors is observed (eg, bruxism-enhancing drugs, primary motor disorders).
Currently available information on possible clinical consequences is equivocal. For instance, some studies have shown that the SB index is higher in individuals with than without tooth wear,14,15,30,31 whilst other authors did not find different single-channel EMG measures between individuals with different degrees of tooth wear.32 Moreover, there are no elements to relate the SB index with pain in the TMJs or jaw muscles, since no differences in the SB activity of individuals with and without pain have been described.9,13,19,33
Such findings on the SB-pain relationship are in line with the postulates of the pain-adaptation model.19,34,35 More importantly, they also accord with the above considerations on the limits of current PSG/SB criteria based on RMMA and do not exclude the possible role of some other SB activities (eg, clenching, bracing) as a risk factor for muscle pain. Indeed, a plausible hypothesis is that even if prolonged clenching-related muscle work can lead to fatigue and pain, a reduced EMG activity due to muscle fibre exhaustion and protective adaptation could be expected after pain onset, thus further complicating the study of the bruxism-pain relationship in terms of risk measurement (see Figure 2). Such a hypothesis may also explain the inconsistent findings about a higher14 or equal15 SB activity in individuals with reported muscle fatigue compared with those without it. It is also supported by findings on the reduced EMG amplitude during MVC when pain is present.36,37 Yet again, other findings suggest that even very long-lasting, low-level static contraction of the jaw closing muscles will lead to a progressive decrease in jaw muscle symptoms, indicating adaptation and not sensitiation to repeated bouts of jaw muscle activity.38
Thus, possible alternative options to identify SB individuals at higher risk of pain have been proposed over the years. They range from cluster analysis identifying moderate bruxers7 to the assessment of increased background EMG activity.39 A multiple-point observation accounting for time-related activity as well as measuring the amount of muscle work seem plausible strategies to probe deeper into the prediction of clinical consequences at the individual level.16,25
The measurement of risk is further complicated by the multitude of factors that interact in a biological model and that can moderate the effects of bruxism positively or negatively. Examples of the latter are represented by poorly lubricated low-PH oral environments that greatly increased the observed enamel wear due to tooth grinding,40 or by the unfavourable muscle force vectors described in the orthodontic and maxillofacial surgery literature that predispose hyperdivergent individuals to TMJ disorders.41
As for the associated conditions, limited data are available to support a high (ie,>13 episodes/hr) SB index in individuals with confirmed SB and OSA,42 whilst knowledge is also lacking on the possible amount of additive bruxism associated with other conditions or risk factors. An exception is represented by some preliminary data of higher periodic limb movement during sleep (PLMS) in sleep bruxers than in healthy controls.43
Finally, it must be noted that a gap still exists between the clinically drawn hypotheses and their confirmation in the research setting as far as the clinical consequences of AB are concerned. EMA approaches may enlighten some issues, but their agreement with real-time EMG activity should be tested in the near future.44 The recently gathered data in healthy young adults at the general population level should constitute a comparison parameter for future studies on selected populations with purported associated factors and/or clinical consequences.23
If you enjoyed reading the article and would like to explore the bruxism topic further, we encourage you to enroll in our course "Encyclopedia of Bruxism and Sleep Apnea".
CONCLUSIONS
It is now clear that the theoretical concept of a cut-point for bruxism as a risk factor for any specific consequence is not biologically sustainable. Too many factors interact at an individual basis, and the effects depend on the type and amount of muscle work and the host response. This is not only the case for SB, but also for AB. The bruxism construct so far was based on a yes/no dichotomy that is needed for screening purposes in the research setting, as in the case of the valuable PSG studies on the SB neurophysiological correlates, but it is not a valid option to increase knowledge in the clinical setting.
Future studies should preferably be based on the measurement of the amount of bruxism behaviour that increases (or reduces) the probability of any health outcomes.
As a first step, identification of the level of bruxism activities that amount to actual risk factors with clinical consequences might tentatively be established, also taking account of the fluctuations in bruxism behaviours over time, both within a night or a day and between nights or days. There should not be any preclusion about the diagnostic strategies to pursue that goal, and a combination of instrumental and non-instrumental approaches may even emerge as the best available option. Once data are available, large-scale population samples representing the entire continuum of EMG/MMA activities are also needed, in the attempt to estimate untreated health risks in the population.
Author's information
Daniele Manfredini
Jari Ahlberg
Peter Wetselaar
Peter Svensson
Frank Lobbezoo
References
Raphael KG, Santiago V, Lobbezoo F. Is bruxism a disorder or a behavior? Rethinking the international consensus on defining and grading of bruxism. J Oral Rehabil. 2016;43:791-798.
Raphael KG, Santiago V, Lobbezoo F. Bruxism is a continuously distributed behavior, but disorder decisions a dichotomous (Response to letter by Manfredini, De Laat, Winocur & Ahlberg (2016)). J Oral Rehabil. 2016;43:802-803.
Manfredini D, De Laat A, Winocur E, Ahlberg J. Why not stop looking at bruxism as a black/white condition? Aetiology could be unrelated to clinical consequences. J Oral Rehabil. 2016;43:799-801.
Lobbezoo F, Ahlberg J, Raphael KG et al. International consensus on the assessment of bruxism: report of a work in progress. J Oral Rehabil. 2018;45(11):837-844.
Lobbezoo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013;40:2-4.
Lavigne GJ, Rompré PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996;75:546-552.
Rompré PH, Daigle‐Landry D, Guitard F, Montplaisir JY, Lavigne GJ. Identification of a sleep bruxism subgroup with a higher risk of pain. J Dent Res. 2007;86:837-842.
Svensson P, Arima T, Lavigne G, Castrillon E. Sleep bruxism: Definition, prevalence, classification, etiology and consequences. In Kryger, MH, Roth, T, Dement, WC eds. Principles and Practice of Sleep Medicine, 6th edn. Philadelphia, PA: Elsevier; 2017:1423-1426.
Raphael KG, Sirois DA, Janal MN, et al. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012;143:1223-1231.
Casett E, Conti Réus J, Stuginski‐Barbosa J, et al. Validity of different tools to assess sleep bruxism: a meta-analysis. J Oral Rehabi. 2017;44:722-734.
Manfredini D, Greene CS, Ahlberg J, De Laat A, Lobbezoo F, Klasser GD Evidence-based dentistry or meta-analysis illness? A commentary on current publishing trends in the field of bruxism and temporomandibular disorders. J Oral Rehabil. 2018;46(1):1-4.
Castroflorio T, Deregibus A, Bargellini A, Debernardi C, Manfredini D. Detection of sleep bruxism: comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J Oral Rehabil. 2014;41:163-169.
Rossetti LM, Rossetti PH, Conti PC, de Araujo CR. Association between sleep bruxism and temporomandibular disorders: a polysomnographic pilot study. Cranio. 2008;26:16-24.
Yoshizawa S, Suganuma T, Takaba M, et al. Phasic jaw motor episodes in healthy subjects with or without clinical signs and symptoms of sleep bruxism: a pilot study. Sleep Breath. 2014;18:187-193.
Castroflorio T, Bargellini A, Rossini G, Cugliari G, Deregibus A, Manfredini D. Agreement between clinical and portable EMG/ECG diagnosis of sleep bruxism. J Oral Rehabil. 2015;42:759-764.
van der Zaag J, Lobbezoo F, Visscher CM, Hamburger HL, Naeije M. Time-variant nature of sleep bruxism outcome variables using ambulatory polysomnography: Implications for recognition and therapy evaluation. J Oral Rehabil. 2008;35:577-584.
Manfredini D, Ahlberg J, Winocur E, Lobbezoo F. Management of sleep bruxism in adults: a qualitative systematic literature review. J Oral Rehabil. 2015;42:862-874.
Svensson P, Jadidi F, Arima T, Baad‐Hansen L.Pain and bruxism. In: Paesani D ed. Bruxism – Theory and Practice. Berlin, Germany: Quintessence; 2010:309-324.
Svensson P, Jadidi F, Arima T, Baad‐Hansen L, Sessle BJ. Relationships between craniofacial pain and bruxism. J Oral Rehabil. 2008;35:524-547.
Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain. 2013;27:99-110.
Yamaguchi T, Abe S, Rompré PH, Manzini C, Lavigne GJ. Comparison of ambulatory and polysomnographic recording of jaw muscle activity during sleep in normal subjects. J Oral Rehabil. 2012;39:2-10.
Maluly M, Andersen ML, Dal-Fabbro C, et al. Polysomnographic study of the prevalence of sleep bruxism in a population sample. J Dent Res. 2013;92:97S-103S.
Bracci A, Djukic G, Favero L, Salmaso L, Guarda-Nardini L, Manfredini D. Frequency of awake bruxism behaviors in the natural environment. A seven-day, multiple-point observation of real time report in healthy young adults. J Oral Rehabil. 2018;45:423-429.
Lavigne GJ, Guitard F, Rompré PH, Montplaisir JY. Variability in sleep bruxism activity over time. J Sleep Res. 2001;10:237-244.
Manfredini D, Fabbri A, Peretta R, Guarda-Nardini L, Lobbezoo F. Influence of psychological symptoms on home-recorded sleep-time masticatory muscle activity in healthy subjects. J Oral Rehabil. 2011;38:902-911.
Macaluso GM, Guerra P, Di Giovanni G, Boselli M, Parrino L, Terzano MG. Sleep bruxism is a disorder related to periodic arousals during sleep. J Dent Res. 1998;77:565-573.
Lavigne GJ, Huynh N, Kato T, et al. Genesis of sleep bruxism: motor and autonomic-cardiac interactions. Arch Oral Biol. 2007;52:381-384.
Manfredini D, Ahlberg J, Castroflorio T, Poggio CE, Guarda‐Nardini L, Lobbezoo F. Diagnostic accuracy of portable instrumental devices to measure sleep bruxism: a systematic literature review of polysomnographic studies. J Oral Rehabil. 2014;41:836-842.
Stuginski‐Barbosa J, Porporatti AL, Costa YM, Svensson P, Conti PC. Diagnostic validity of the use of a portable single-channel electromyography device for sleep bruxism. Sleep Breath. 2016;20:695-702.
Abe S, Yamaguchi T, Rompré PH, De Grandmont P, Chen YJ, Lavigne GJ. Tooth wear in young subjects: a discriminator between sleep bruxers and controls? Int J Prosthodont. 2009;22:342-350.
Wetselaar P, Lobbezoo F. The tooth wear evaluation system: a modular clinical guideline for the diagnosis and management planning of worn dentitions. J Oral Rehabil. 2016;43:69-80.
Jonsgar C, Hordvik PA, Berge ME, Johansson AK, Svensson P, Johansson A. Sleep bruxism in individuals with and without attrition-type tooth wear: an exploratory matched case-control electromyographic study. J Dent. 2015;43:1504-1510.
Yachida W, Castrillon EE, Baad-Hansen L, et al. Craniofacial pain and jaw-muscle activity during sleep. J Dent Res. 2012;91:562-567.
Lund J, Donga R, Widmer CG, Stohler CS. The pain‐adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683-694.
Murray GM, Peck CC. Orofacial pain and jaw muscle activity: a new model. J Orofac Pain. 2007;21:263-278; discussion 279–88.
Manfredini D, Cocilovo F, Favero L, Ferronato G, Tonello S, Guarda-Nardini L. Surface electromyography of jaw muscles and kinesiographic recordings: diagnostic accuracy for myofascial pain. J Oral Rehabil. 2011;38:791-799.
Manfredini D, Cocilovo F, Stellini E, Favero L, Guarda-Nardini L. Surface electromyography findings in unilateral myofascial pain patients: comparison of painful vs. non painful sides. Pain Med. 2013;14:1848-1853.
Takeuchi T, Arima T, Ernberg M, Yamaguchi T, Ohata N, Svensson P. Symptoms and physiological responses to prolonged, repeated, low-level tooth-clenching in humans. Headache. 2015;55:381-394.
Raphael KG, Janal MN, Sirois DA, et al. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J Oral Rehabil. 2013;40:883-891.
Kaidonis JA, Richard LC, Townsend GC, Tansley GD. Wear of human enamel: a quantitative in vitro assessment. J Dent Res. 1998;77:1983-1990.
Manfredini D, Segù M, Arveda N, et al. Temporomandibular joint disorders in patients with different facial morphology. a systematic review of the literature. J Oral Maxillofac Surg. 2016;74:29-46.
Saito M, Yamaguchi T, Mikami S, et al. Temporal association between sleep apnea-hypopnea and sleep bruxism events. J Sleep Res. 2014;23:196-203.
van der Zaag J, Naeije M, Wicks DJ, Hamburger HL, Lobbezoo F. Time-linked concurrence of sleep bruxism, periodic limb movements, and EEG arousals in sleep bruxers and healthy controls. Clin Oral Investig. 2014;18:507-513.
Castrillon EE, Ou K‐L, Wang K, Zhang J, Zhou X, Svensson P. Sleep bruxism: an updated review of an old problem. Acta Odontol Scand. 2016;74:328-334.