Rapid maxillary expansion in pediatric patients with obstructive sleep apnea: an umbrella review
Abstract
Objective: To compare polysomnographic parameters with others from the literature in order to provide more accurate information about Rapid Maxillary Expansion (RME) for treating Obstructive Sleep Apnea (OSA) in children, through raising the question: Is RME a good option for treating OSA in children? Prevention of mouth breathing during children’s growth remains a challenge with significant clinical consequences. In addition, OSA induces anatomofunctional changes during the critical period of craniofacial growth and development.
Methods: The Medline, PubMed, EMBASE, CINAHL, Web of Science, SciELO and Scopus electronic databases were searched up to February 2021 for systematic reviews with meta-analysis in the English language. Among 40 studies on RME for treating OSA in children, we selected seven in which polysomnographic measurements of the Apnea-Hypopnea Index (AHI) had been made. Data were extracted and examined in order to clarify whether any consistent evidence exists for indicating RME as a treatment for OSA in children.
Results: We found no consistent evidence favoring RME for long-term treatment of OSA in children. All the studies presented considerable heterogeneity due to variability of age and length of follow-up.
Conclusion: Through this umbrella review, the need for methodologically better studies on RME is supported. Moreover, it can be considered that RME is not recommended for treating OSA in children. Further studies and more evidence identifying early signs of OSA are necessary in order to achieve consistent healthcare practice.
Introduction
Obstructive Sleep Apnea (OSA) is a complex and heterogeneous disorder1 characterized by episodes of complete or partial upper airway obstruction or sleep-related breathing disorder consisting of snoring, and by episodes of increased secondary respiratory effort, upper airway resistance and pharyngeal collapsibility during sleep, often resulting in gas exchange abnormalities and sleep disruption.2-5 This condition is present in 2%-5% of children and can occur at any age.2,3 It may be the most common sleep disorder.6 OSA in children is a severe disease involving diminished quality of life in many respects, such as neurocognitive and neuropsychomotor impairment, cardiovascular function implications and systemic diseases.5-11 This disorder affects children during critical brain development and craniofacial growth.10,12 Genetic influences and environmental stimuli can contribute to facial growth and neuromuscular compensation activity, in order to maintain upper airway patency.5 You have the opportunity to gather more in-depth information about obstructive sleep apnea treatment in our Online congress of Myofunctional Orthodontics and Functional Jaw Orthopedics.
Because of the complexity of OSA, a multidisciplinary healthcare team is required for better results from treatment to be obtained.6 Preventing OSA in children is still a challenge with regard to both multidisciplinary team attention and healthcare policies. Mouth breathing is one of the foremost clinical manifestations of OSA, and is accompanied by chronic snoring, increased respiratory effort and arousal,5,13-15 arising from anatomical and functional imbalance.5,7,14-18
Physiological respiratory function is one of the essential stomatognathic functions that require complex interactions of the central and peripheral nervous systems with the respiratory system.19 In neonates, respiratory control is relatively immature.20 The respiratory reflex is an innate reflex that depends on the level of maturation and function of different neuromuscular structures, which become established through physiological processes.
The act of breastfeeding establishes this reflex, which also involves other stomatognathic functions such as sucking and swallowing.18 These functions are essential for the growth and development of craniofacial structures in the first years of a child’s life.21
The number of episodes of obstructive apnea and hypopnea per hour of sleep, as assessed through the Apnea-Hypopnea Index (AHI) indicates the severity of OSA. Most laboratories define OSA in children as follows: mild, when in the range AHI > 1.5 (or AHI > 1-5; moderate, AHI > 5-10; or severe, AHI > 10.22,23
Early diagnosis and treatment of OSA may decrease morbidity; however, among children, this is frequently delayed.12 Polysomnographic studies need to form part of the screening, diagnosis and follow-up strategies because of the differences in characteristics between adult and pediatric OSA.12 Additionally, oximetry is one of the tools most used for preliminary evaluation and provides an abbreviated means for diagnosing OSA.23 The cross-culturally validated sleep disorders questionnaire is another sleep assessment tool for initial assessment of OSA children.24-26
The questionnaire is considered easy to use, is low-cost and is self-administered. Furthermore, some indexes such as the Baby ROMA index,27 which is needed for orthodontic screening among children from 2 to 6 years old, take into consideration systemic, skeletal, dental, and functional problems.
The pathophysiology of OSA in children is multifactorial and is divided into factors relating to the associated possibilities for craniofacial development in the upper airway.23
Narrowing of the upper airway and presence of neuromuscular disorders increase the risk of craniofacial abnormalities in children with OSA, and certain genetic conditions relating to structural elements lead to disharmony in craniofacial growth and development.6-9,28,29
Diagnosing and treating this breathing disorder in early life are possible.6,7,9,10,12 Moreover, early treatment is deemed necessary for prevention of harmful consequences, even though only a few studies have matched OSA with prevention in this population.6,8,30
Various therapies for OSA23,31-33 include adenotonsillectomy as the first-line treatment, with use of positive airway pressure devices, nasal devices, myofunctional therapy, sleep surgery and oral appliances.
With regard to oral appliance therapy, studies on orthodontic/facial orthopedic treatment have provided support for use of the Rapid Maxillary Expansion (RME) technique before midline fusion of the maxilla occurs. RME is an effective treatment for dental crowding and malocclusion in situations of a high arched or narrow hard palate, which is related to presence of OSA in children.7-10
The aims of this umbrella review were the following:
To provide a summary of existing research syntheses on RME interventions among children with OSA through evaluation of polysomnographic measurements, especially the Apnea-Hypopnea Index (AHI);
To provide data on prevention focusing on breastfeeding and stomatognathic function; and
To highlight future research necessities.
Methods Development
Through this study, it was sought to evaluate the effectiveness of RME as a treatment option for OSA, by compiling evidence from multiple research syntheses with polysomnographic measurements, including AHI and other outcomes.
We conducted the search strategy in February 2022 using the Patient-Intervention-Comparison-Outcome (PICO) strategy (Table 1). We included relevant studies through using a rigorous electronic search for the terms RME, OSA, AHI, children, systematic review, and meta-analysis.
Inclusion criteria
We included all systematic reviews with meta-analysis that assessed OSA in children aged 0-18 years, without gender restriction, who were treated with RME and for whom diagnoses were made using polysomnographic parameters, especially AHI; and for whom pre and post-treatment data and follow-up evidence were available.
Exclusion criteria
We established the language restriction of exclusively considering studies in English and excluded theoretical studies and opinions about the primary source of evidence.
Search strategy
An electronic database search to identify potentially relevant studies in the Web of Science, PubMed, Scopus, Embase, Cochrane, Epistemonikos, CINAHL and SciELO was conducted in February 2022. Boolean operators (‘‘OR’’ and ‘‘AND’’) were used to link search terms based on the PICO strategy. The English-language MeSH research terms used were the following: sleep-disordered breathing, obstructive sleep apnea, RME, children, pediatric, systematic review, and meta-analysis. Out of 40 systematic reviews with meta-analysis on use of RME for treating OSA in children, we selected eight studies on RME in children with OSA in which polysomnographic measurements including AHI were made. However, we then excluded one of these systematic reviews because it did not have a meta-analysis. The flow diagram for study selection is shown in Fig. 1.
Methodological quality
Initially, we analyzed the polysomnographic parameter out-comes from seven reviews on RME for their similarities and differences and applied a quality assessment. All the information collected is shown in Table 2 (Joanna Briggs Institute Reviewers’ Manual 2014). We discussed the qualitative evaluations of the articles retrieved for this study, to produce a consensus.
Systematically and independently, two reviewers (B, DF; and B, LF) conducted assessments and manually documented them with regard to each respective database: author, year of publication, title, study design, number of patients, age, methods, outcomes, results and conclusion. The two reviewers discussed their evaluations on qualitative articles in order to develop a consensus. In addition, a third reviewer (M-J, A-J) was consulted in order to validate and control the data in any event of disagreement. The two reviewers undertook several rounds of rereading each review and also searched through the bibliographies, to look for other studies that might not have been found in the initial search.
We summarized these characteristics and findings into a single question: Is RME an effective intervention for controlling the AHI in children with OSA? We sought to communicate all the evidence found through the present review to the multidisciplinary team that cares for children with OSA, so as to guide the team regarding good clinical-practice decision-making.
Results
The overall results from this review are described in Table 3.
All the studies presented considerable heterogeneity in their results from RME interventions among children with OSA, due to variability with regard to age and length of follow-up. Thus, there was significant heterogeneity in comparing populations.
Our synthesis, in Table 4, shows treatments and recommendations for best clinical practice, policies and future research, regarding the results from RME among children with OSA. The outcomes relating to AHI control among these children with OSA differed: one study showed that RME was efficacious,9 while another showed that RME may not be effective23 and all other studies6,7,14,33 demonstrated that RME might be effective. One study indicated that RME was an appropriate alternative for treating craniofacial abnormalities.35
Discussion
While RME is a well-accepted orthopedic procedure for managing structural and functional problems in the midface,29 upper airspace improvement and stability are the main long-term issues relating to treatments for OSA among children.
In addition, it is premature to speculate about use of RME as a treatment for nasal obstruction, given the significant risk of bias and high heterogeneity of results regarding improvement of OSA, especially with regard to long-term stability.6,9,33,34,36 At the same time, coadjuvant therapy among children with severe OSA, such as adenotonsillectomy in situations of a narrow maxilla, has been shown to provide improvement of the nasal airway dimensions and airflow.14,34,37 This may cloak the effects of RME because maxillary constriction may play a role in the etiology of OSA.36 Nevertheless, OSA may negatively affect a child for the rest of their life.7,36,38
We compared improvements in AHI achieved through RME interventions that were reported in selected systematic reviews with meta-analysis. We noted that there was a correlation between skeleton-related orofacial dysfunctions and presence of OSA among these children.7,33,40,41 Vale et al.35 recommended RME for treatment of OSA in children with craniofacial abnormalities. However, orthodontic and craniofacial abnormalities are often neglected in children with OSA.6,8 Meanwhile, Huynh et al.7 suggested that correcting craniofacial structure imbalances under the optimal conditions afforded by childhood growth may diminish snoring and OSA and would likely improve polysomnographic parameters such as AHI, oxygen saturation index, arousal index, upper airway volume or structures and sleep quality, especially over the short term (<3 years of follow-up). In addition, regarding the follow-up, Camacho et al.,33 Machado-Junior et al.6 and Quinzi et al.9 pointed out that there is a need for more long-term studies (>3 years of follow-up) and for more randomized clinical trials with long-term follow-up, in order to assess whether the effectiveness of this treatment is maintained throughout adulthood.6
Table 1 Framework for elaborating the PICO strategy.
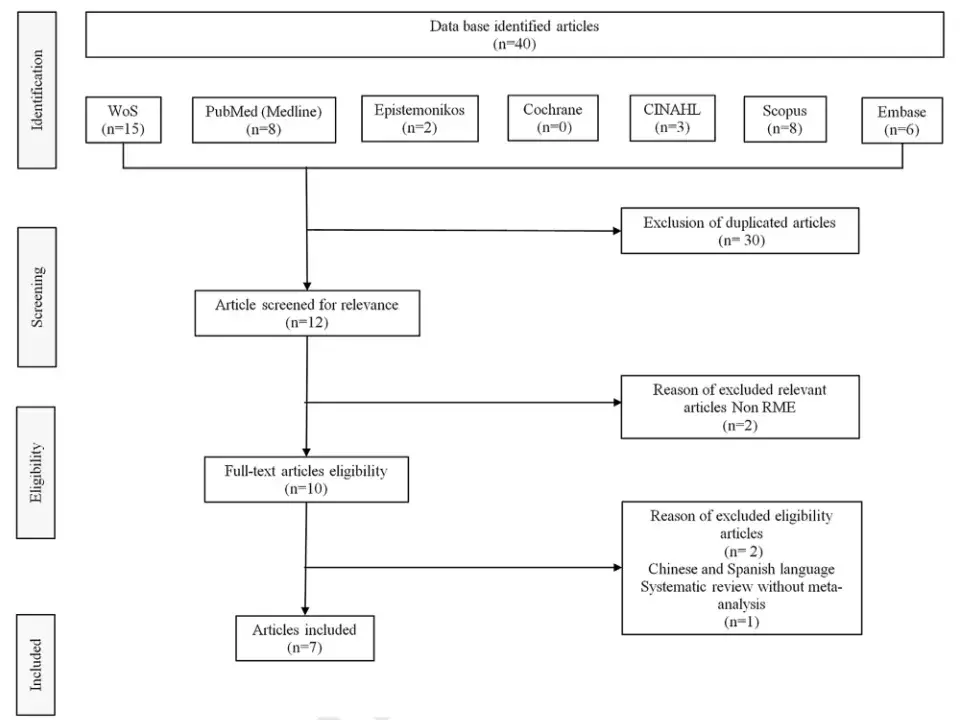 Figure 1 Flow diagram for study selection (Feb 2022).
Figure 1 Flow diagram for study selection (Feb 2022).
Lin et al.,23 Sánchez-Súcar et al.,14 Vale et al.,35 Calvo-Henriquez et al.37 and a recent systematic review39 came to similar conclusions regarding the heterogeneity of the results observed. They confirmed the importance of instituting standardized trial guidelines for research designs, to reduce bias and improve the inclusion and exclusion criteria. In this context, in future clinical trials, patient selection would likely benefit from including phenotypic approaches and personalized medicine, so as to gain understanding of therapeutic mechanisms and thereby improve diagnoses, prognoses and clinical management.42
We found a gap in the literature with regard to treatment plans. It needs to be considered that an adequate treatment plan stemming from early-stage diagnosis helps to identify respiratory disorders, reduce adverse health outcomes11,38 and prevent malocclusion.27 Treatments should focus on amending craniofacial development, given that there is a direct relationship between malocclusion and other OSA-related orofacial deformities, considering also that there is no robust scientific evidence to reach complete resolution of OSA.23
If it is supposed that a direct relationship exists between malocclusion and other OSA-related orofacial deformities, the question of what to do regarding treatments that do not correct craniofacial development arises. These treatment may include adenotonsillectomy, CPAP and other ineffective therapies.6,10 From the systematic reviews with accurate meta-analysis that we selected, there was no robust scientific evidence to support treatment of OSA patients with RME, surgically assisted RME or maxillomandibular surgical advancement.30,39
Trials that evaluate the latest technologies or combined therapies are also needed in order to identify the best treatment for pediatric OSA, for future networked meta-analysis.23 For this reason, we understand why there is discordance between the American Academy of Pediatrics43 and the European Respiratory Society4 with regard to recommending RME as a treatment for OSA in children. Importantly, our comprehensive survey showed that there is insufficient evidence of effectiveness regarding RME treatment.23,39,44 Thus, other challenges and perspectives regarding prognoses and optimal treatment among children with OSA need to be considered.48 These may include patient history and clinical sleep records,24 nocturnal pulse oximetry,23 OSA questionnaires25,26 and phenotypic markers.
Table 2 Critical appraisal checklist for systematic reviews and research synthesis (Joanna Briggs Institute Reviewers’ Manual 2014).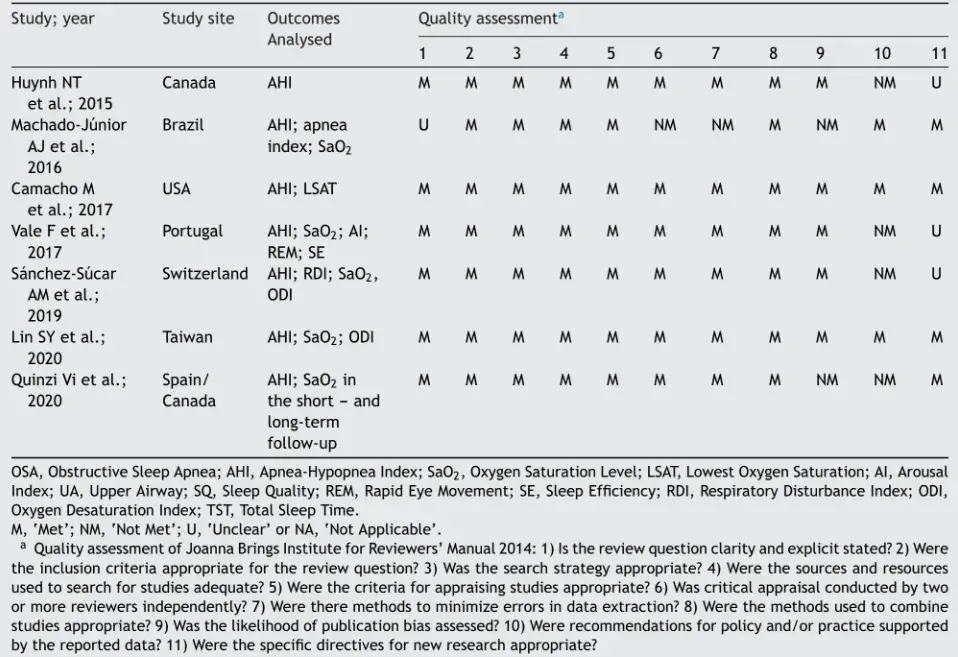
There is a need for more studies, especially with regard to public preventive healthcare policies for children with OSA. The links connecting breastfeeding action to pediatric sleep-disordered breathing,45 craniofacial growth and development in the postnatal period and first years of life46,47 need to be considered. The pediatric population under two years of age is a unique subgroup with a predisposition to upper airway obstruction with symptoms during wakefulness and requires age-appropriate interventions.11
Moreover, preventive treatment should act at the primary level of prevention, so as to improve anatomical form and systemic function and promote establishment of nasal breathing at the early stage of growth and development. Additionally, new studies should explore gaps in knowledge relating to long-term issues and orthopedic development of the stomatognathic system.
Strategic policies for preventive action to improve healthy breathing and for understanding stomatognathic system relationships and nasal breathing maturation function should be created, rather than working with installed mouth breathing with no apparent efficacy or effective treatment methods. Regarding the strengths of this review, we recommend that new studies should be conducted to explore the gaps in knowledge found in the literature.
Table 3 AHI findings from systematic reviews with meta-analysis on RME among children with OSA: an umbrella review.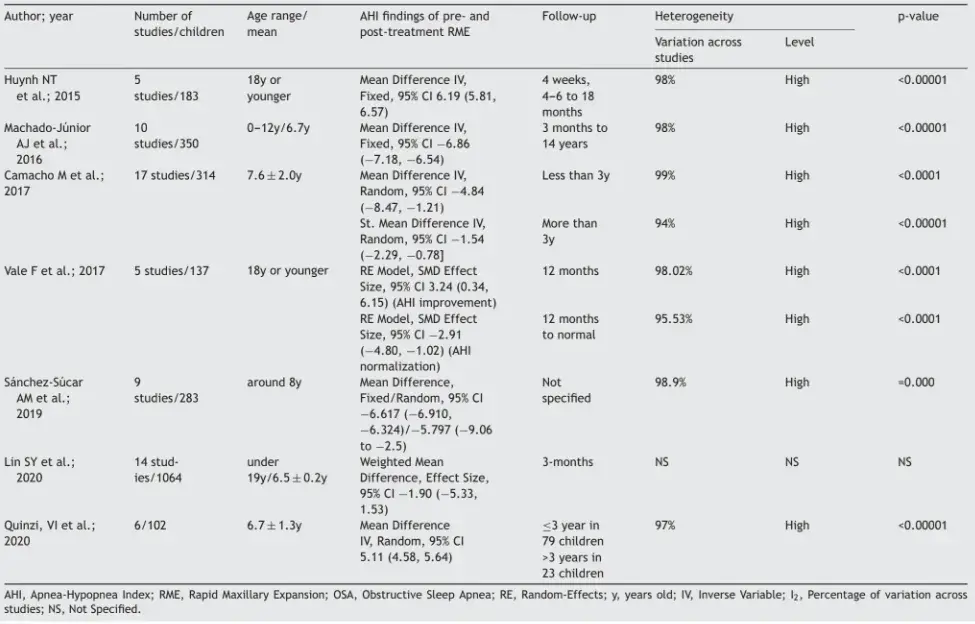
Table 4 Synthesis of findings from systematic reviews with meta-analysis on outcomes from RME for AHI control among children with OSA.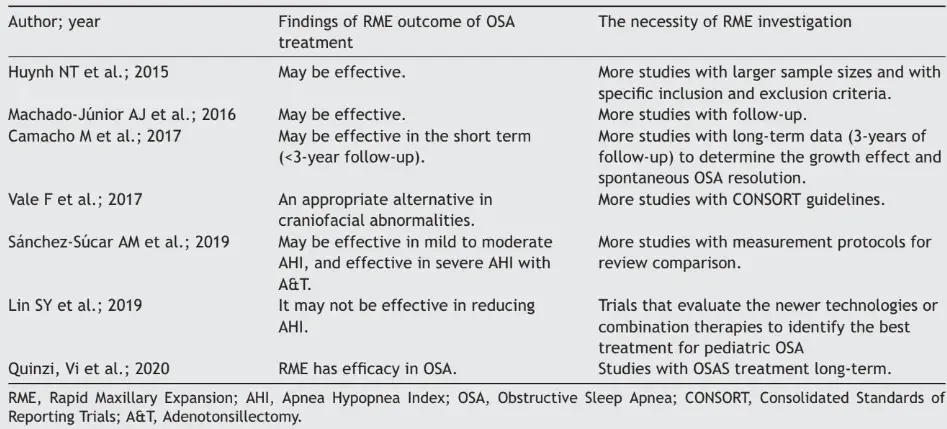
Conclusion
The conclusion from this umbrella review is that it is premature to speculate that RME forms a treatment for OSA in children. Because of the low quality of evidence and high heterogeneity between studies, we believe that RME treatment should not be recommended for children with OSA.
Clinical trial guidelines are needed in order to improve quality, avoid heterogeneity among studies and enable better outcomes. Management decisions should be linked to underlying phenotypes and consider outcomes other than the AHI.
Future strategic campaigns are needed to raise awareness among healthcare professionals regarding the best practices in relation to prevention of OSA among children. In addition, more evidence to make it possible to establish healthcare policies focusing on primary prevention of respiratory disorders should be obtained.
Further details about functional orthodontic treatment are accessible for you to learn on our website.
Future directions
Future long-term prospective research should prioritize methodological quality, so as to avoid selection bias through sample homogeneity, in terms of both patient age and length of treatment, with timely therapy. Overall, the present review indicated that preventive action to reestablish nasal breathing in the pediatric population is needed in order to avoid deviation from normal growth and development. In addition, the AHI and variables relating to clinical characteristics should be considered, including risk factors such as nasal obstruction and mouth breathing, anatomical and functional changes, craniofacial abnormalities, quality of life and cognitive and behavioral factors.
Authors
Denise Fernandes Barbosa, Laura Fernandes Bana, Maria Cristina Buta Michel, Miguel Meira e Cruz, Edilson Zancanella, Almiro José Machado Júnior
References
Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: A definition, examples, and evolution of approaches. Sleep Med Rev. 2017;35:113-23.
Rosen CL, Storfer-Isser A, Gerry Taylor H, Lester Kirchner H, Emancipator JL, Redline S. Increased behavioral morbidity in school-aged children with sleep-disordered breathing. Pediatrics. 2004;114:1640-8.
Sateia MJ. International classification of sleep disorders-third edition highlights and modifications. Chest. 2014;146:1387-94.
Kaditis AG, Luz M, Alvarez A, Boudewyns A, Alexopoulos EI, Ersu R, et al. Obstructive sleep disordered breathing in 2-to 18-year old children: diagnosis and management TASK FORCE REPORT ERS STATEMENT. Eur Respir J. 2016;47:69-94.
Katz ES, D’Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:253.
Machado-Júnior AJ, Signorelli LG, Zancanella E, Crespo AN. Randomized controlled study of a mandibular advancement appliance for the treatment of obstructive sleep apnea in children: a pilot study. Med Oral Patol Oral Cir Bucal. 2016;21:e403-7.
Huynh NT, Desplats E, Almeida FR. Orthodontics treatments for managing obstructive sleep apnea syndrome in children: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:84-94.
Bahammam SA. Rapid Maxillary Expansion for Obstructive Sleep Apnea among children-systematic review and meta-analysis. Sleep Sci. 2020;13:70-7.
Quinzi V, Saccomanno S, Manenti RJ, Giancaspro S, Coceani L, Marzo G. Efficacy of rapid maxillary expansion with or without previous adenotonsillectomy for pediatric obstructive sleep apnea syndrome based on polysomnographic data: a systematic review and meta-analysis. Appl Sci. 2020;10:6485.
Templier L, Rossi C, Miguez M, De la Cruz Pérez J, Curto A, Albaladejo A, et al. Combined surgical and orthodontic treatments in children with OSA: a systematic review. J Clin Med. 2020;9:2387.
Kaditis AG, Alvarez MLA, Boudewyns A, Abel F, Alexopoulos EI, Ersu R, et al. ERS statement on obstructive sleep disordered breathing in 1- to 23-month-old children. Eur Respir J. 2017;50:1700985.
Alsubie HS, BaHammam AS. Obstructive Sleep Apnoea: children are not little adults. Paediatr Respir Rev. 2017;21:72-9.
Villa MP, Brasili L, Ferretti A, Vitelli O, Rabasco J, Mazzotta AR, et al. Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep Breath. 2015;19:281-9.
Sánchez-Súcar A-M, Borja Sánchez-Súcar F-D, Almerich-Silla J-M, Paredes-Gallardo V, Montiel-Company J-M, García-San V, et al. Effect of rapid maxillary expansion on sleep apnea-hypopnea syndrome in growing patients. A meta-analysis. J Clin Exp Dent. 2019;11:759-67.
Vinha PP, De Mello-Filho FV. Evidence of a preventive effect of breastfeeding on obstructive sleep apnea in children and adults. J Hum Lact. 2017;33:448-53.
D’Onofrio L. Oral dysfunction as a cause of malocclusion. Orthod Craniofacial Res. 2019;22:43-8.
Yap B, Kontos A, Pamula Y, Martin J, Kennedy D, Sampson W, et al. Differences in dentofacial morphology in children with sleep disordered breathing are detected with routine orthodontic records. Sleep Med. 2019;55:109-14.
Savian CM, Bolsson GB, Botton G, Antoniazzi RP, Rocha RO, Zanatta FB, et al. Do breastfed children have a lower chance of developing mouth breathing? A systematic review and meta-analysis. Clin Oral Investig. 2021;25:1641-54.
Urfy MZ, Suarez JI. Breathing and the nervous system. Handb Clin Neurol. 2014;119:241-50.
Abu-Shaweesh JM. Maturation of respiratory reflex responses in the fetus and neonate. Semin Neonatol. 2004;9:169-80.
Sampallo-Pedroza RM, Cardona-López LF, Ramírez-Gómez KE. Description of oral-motor development from birth to six years of age. Rev Fac Med. 2014;62:593-604.
Marino A, Ranieri R, Chiarotti F, Villa MP, Malagola C. Rapid maxillary expansion in children with Obstructive Sleep Apnoea Syndrome (OSAS). Eur J Paediatr Dent. 2012;13:57-63.
Lin SY, Su YX, Wu YC, Chang JZC, Tu YK. Management of paediatric obstructive sleep apnoea: a systematic review and network meta-analysis. Int J Paediatr Dent. 2020;30:156-70.
Villa MP, Paolino MC, Castaldo R, Vanacore N, Rizzoli A, Miano S, et al. Sleep clinical record: an aid to rapid and accurate diagnosis of paediatric sleep disordered breathing. Eur Respir J. 2013;41:1355-61.
Incerti Parenti S, Fiordelli A, Bartolucci ML, Martina S, D’Antò V, Alessandri-Bonetti G. Diagnostic accuracy of screening questionnaires for obstructive sleep apnea in children: a systematic review and meta-analysis. Sleep Med Rev. 2021;57:101464.
Chan BC, Galland BC, Smith LA, Maessen SE, Haszard JJ, Schaughency EA, et al. Can sleep questionnaires predict outcome in children undergoing adenotonsillectomy for sleep disordered breathing? Aust J Otolaryngol. 2019;2, http://dx.doi.org/10.21037/ajo.2019.01.06.
Paolantonio EG, Ludovici N, Saccomanno S, La Torre G, Grippaudo C. Association between oral habits, mouth breathing and malocclusion in Italian preschoolers. Eur J Paediatr Dent. 2019;20:204-8.
Santana DMC, Nogueira VS, Lima SAM, Fernandes LPA, Weber SAT. The effect of rapid maxillary expansion in children: a meta- analysis. Braz J Otorhinolaryngol. 2022;88:907-16.
Katyal V, Pamula Y, Martin AJ, Daynes CN, Kennedy JD, Sampson WJ. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: systematic review and meta-analysis. Am J Orthod Dentofac Orthop. 2013;143:20-30.
Koretsi V, Eliades T, Papageorgiou SN. Oral interventions for obstructive sleep apnea. Dtsch Arztebl Int. 2018;115: 200-7.
Chuang LC, Hwang YJ, Lian YC, Hervy-Auboiron M, Pirelli P, Huang Y-S, et al. Changes in craniofacial and airway morphology as well as quality of life after passive myofunctional therapy in children with obstructive sleep apnea: a comparative cohort study. Sleep Breath. 2019;23:1359-69.
Koka V, De Vito A, Roisman G, Petitjean M, Pignatelli GRF, Padovani D, et al. Orofacial myofunctional therapy in obstructive sleep apnea syndrome: a pathophysiological perspective. Medicina (Kaunas). 2021;57:323.
Camacho M, Chang ET, Song SA, Abdullatif J, Zaghi S, Pirelli P, et al. Rapid maxillary expansion for pediatric obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2017;127:1712-9.
Niu X, Di Carlo G, Cornelis MA, Cattaneo PM. Three-dimensional analyses of short- and long-term effects of rapid maxillary expansion on nasal cavity and upper airway: a systematic review and meta-analysis. Orthod Craniofacial Res. 2020;23:250-76.
Vale F, Albergaria M, Carrilho E, Francisco I, Guimarães A, Caramelo F, et al. Efficacy of rapid maxillary expansion in the treatment of obstructive sleep apnea syndrome: a systematic review with meta-analysis. J Evid Based Dent Pract. 2017;17:159-68.
McNamara JA Jr, Lione R, Franchi L, Angelieri F, Cevidanes LHS, Darendeliler MA, et al. The role of rapid maxillary expansion in the promotion of oral and general health. Prog Orthod. 2015;16:33.
Calvo-Henriquez C, Capasso R, Chiesa-Estomba C, Liu SY, Martins-Neves S, Castedo E, et al. The role of pediatric maxillary expansion on nasal breathing. A systematic review and metanalysis. Int J Pediatr Otorhinolaryngol. 2020;135:110139.
Machado AJ, Zancanella E, Evangelisti M, Villa MP. OSAS treatments: is treating shape enough? Sleep Med. 2021;79:122-3.
Fernández-Barriales M, Lafuente-Ibánez ˜ De Mendoza I, Julián J, Pacheco A-F, Aguirre-Urizar JM. Rapid maxillary expansion versus watchful waiting in pediatric OSA: a systematic review. Sleep Med Rev. 2022;62:101609.
Machado AJ, Crespo AN, Pauna HF. Rapid maxillary expansion in pediatric patients with obstructive sleep apnea: current and future perspectives. Sleep Med. 2018;51:7-8.
Guilleminault C, Sullivan SS, Huang Y-S. Sleep-disordered breathing, orofacial growth, and prevention of obstructive sleep apnea. Sleep Med Clin. 2019;14:13-20.
Tan HL, Kaditis AG. Phenotypic variance in pediatric obstructive sleep apnea. Pediatr Pulmonol. 2021;56:1754-62.
Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576-84.
Gozal D, Tan H-L, Kheirandish-Gozal L. Treatment of obstructive sleep apnea in children: handling the unknown with precision. J Clin Med. 2020;9:888.
Ponce-Garcia C, Hernandez IA, Major P, Flores-Mir C. Association between breast feeding and paediatric sleep disordered breathing: a systematic review. Paediatr Perinat Epidemiol. 2017;31:348-62.
Remy F, Godio-Raboutet Y, Captier G, Bonnaure P, Adalian P, et al. Characterization of the perinatal mandible growth pattern: preliminary results. Surg Radiol Anat. 2030;40:667-679. Q5
Remy F, Godio-Raboutet Y, Captier G, Bonnaure P, Burgart P, Guyot L, et al. The hypoplasic mandible: what makes it different from the healthy child? Cleft Palate Craniofacial J. 2020;58, http://dx.doi.org/10.1177/10556656209723.
Randerath W, Bassetti CL, Bonsignore MR, Farre R, Ferini-Strambi L, Grote L, et al. Challenges and perspectives in obstructive sleep apnoea. Eur Respir J. 2018;52:1702616.
Dehlink E, Tan H-L. Update on paediatric obstructive sleep apnoea. J Thorac Dis. 2016;8:224-35.

/social-network-service/media/default/100259/d98c5154.jpg)
