Physicochemical Properties and Microshear Bond Strength of Experimental Self-adhesive Resin Cements to Dentin or Yttria-stabilized Tetragonal Zirconia Polycrystal
Purpose: To determine the degree of conversion (DC), physicochemical properties, and microshear bond strength (μSBS) of experimental self-adhesive resin cements (SARCs) to dentin and yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) ceramic.
Materials and Methods: Dual-curing cements were formulated with UDMA, HEMA, bis-GMA, and TEG-DMA as the organic matrix. 2MP (bis 2-(methacryloyloxy)-ethyl-phosphate) and GDMAP (1,3-glycerol dimethacrylate phosphate) were added to impart self-adhesive characteristics. A control group was formulated without self-etch methacrylates. Silanized particles were incorporated. Photoactivation was carried out using an LED light-curing unit (1200 mW/cm2) for 20 s. Infrared spectroscopy assessed the DC immediately and after 24 h. pH was analyzed in real time and recorded after 48 h. Water sorption (Wsp), water solubility (Wsl), and film thickness measurements followed ISO 4049. μSBS of the cements to dentin and Y-TZP was evaluated immediately and after 3 months of water storage. Y-TZP was also tested without a surface treatment and after tribochemical silica coating with subsequent application of a silane agent. The fractures patterns were classified as adhesive, cohesive, and mixed. Data were submitted to analyses of variance and Tukey’s tests (α = 0.05).
Results: Control (91.7%) and 2MP (92.0%) groups generated the highest DC after 24 h. 2MP (pH = 3.6) showed the lowest pH followed by GDMAP (pH = 4.7) and control (pH = 6.4) after 48 h. The control cement exhibited lower Wsp (41.0 μg/mm3) and Wsl (4.3 μg/mm3) than the other groups. Film thickness was statistically similar (p = 0.266) for all cements. Control (27.0 MPa) and GDMAP (24.1 MPa) showed higher μSBS to dentin than 2MP (13.7 MPa) after water storage. Mixed fractures were predominant in dentin. For all cements, the μSBS to Y-TZP was < 3.0 MPa after water storage, independent of the surface treatment.
Conclusion: The results of DC, pH, Wsp and Wsl were material dependent. Only the film thickness was statistically similar for all groups. The cement formulated with GDMAP maintained the bond strengths to dentin even after aging. However, none of the groups were able to generate satisfactory bond strength to Y-TZP, independent of the surface treatment.
Esthetic demands by patients in association with adhesive techniques have led to the widespread use of resin cements instead of conventional acid-base luting materials such as zinc phosphate or glass ionomers. In addition, lower hydrolytic degradation and higher mechanical properties of resin cements favored the establishment of a new adhesive luting protocol. Moreover, the relatively high bond strength observed between resin-based cements and dental and prosthetic substrates enables minimally invasive procedures, increasing the popularity of these materials among clinicians.
Cementation techniques using resin-based materials usually involve previous treatment of the prosthetic intaglio surface and the application of an adhesive on the dental substrate. An advantage of the adhesive protocol using resin-based cements is that the prosthetic-tooth unit after cementation behaves as a single body. This structure favors more homogeneous stress distribution of occlusal loads, allowing greater restoration longevity. Moreover, control over polymerization in light- or dual-curing resin cements has established this class of material as the first choice for luting dental prostheses. However, an important drawback of this class of luting agent is the high sensitivity of the adhesive technique. The multiple steps (eg, acid etching, dentin moisture control, surface treatment of the prosthesis) increase the incidence of operative failures, which may jeopardize the success of the restorative treatment. There are additional details about adhesion in dentistry that you can obtain in our course "Modern adhesion".
Self-adhesive resin cements (SARCs) have been developed to optimize the cementation procedure and reduce the incidence of clinical errors. The simplification of the cementation technique is related to the presence of a self-etch methacrylate in SARCs. These functional monomers contain phosphate or carboxylic groups which are responsible for chemical interactions between the cement and the dental and prosthetic substrates. Currently, a wide variety of adhesive monomers is available in commercial SARCs. One of the most studied and well established is 10-methacryloxydecyl dihydrogen phosphate (10-MDP). However, the literature is still contradictory relative to the adhesive potential of SARCs to dental tissues and acid-resistant ceramics (eg, Y-TZP).
A recent study provided information regarding the polymerization behavior of experimental SARCs formulated with different self-etch methacrylates (2MP and GDMAP) associated with a salt as third component of the activation system. Those authors reported that the addition of this salt increased the DC, mainly in the first minutes of selfcuring. However, the physicochemical properties of the cements and the bond strength to different substrates were not evaluated. Therefore, the aim of the present study was to formulate experimental resin cements with 2MP or GDMAP and compare them with a control cement (without self-etch methacrylate) in terms of degree of C=C conversion (DC), pH, water sorption (Wsp), water solubility (Wsl), and film thickness. The μSBS of the cements to dentin and Y-TZP was also analyzed immediately and after 3 months of water storage.
The research hypotheses were that: a) the results of DC, pH, Wsp, Wsl, and film thickness would be similar for all tested resin cements; b) all the experimental resin cements would show similar μSBS results to dentin or Y-TZP after aging.
MATERIALS AND METHODS
Components
The monomers urethane dimethacrylate (UDMA, Esstech; Essington, PA, USA), bisphenol-A-glycidyl methacrylate (bis-GMA, Evonik; Essen, Germany), triethylene glycol dimethacrylate (TEG-DMA, Esstech), 2-hydroxyethyl methacrylate (HEMA, Sigma-Aldrich; St Louis, MO, USA) and bis 2-(methacryloyloxy) ethyl phosphate (2MP, Sigma-Aldrich) were used as provided by the manufacturers. 1,3-glycerol dimethacrylate phosphate (GDMAP) was synthesized as previously described. Silanized barium borosilicate glass particles (Esstech, average size 2 μm) were used as fillers. The photosensitizer camphorquinone (CQ, Esstech) and the co-initiator ethyl 4-(dimethylamino) benzoate (EDAB, Sigma-Aldrich) were added to render photosensitive properties. Benzoyl peroxide (BPO, Sigma-Aldrich), N-N-dihydroxyethyl-p-toluidine (DHPT, Sigma-Aldrich), and benzenesulfinic acid sodium salt 98% (BAS, Sigma-Aldrich) were also incorporated to yield a dual-curing mechanism. Heavy water (deuterium oxide [D2O], Sigma-Aldrich) was used as an ionizing vehicle of the adhesive monomers.
Composition of the Experimental Resin Cements
Experimental SARCs were formulated with 2MP or GDMAP, and a control group was formulated without adhesive monomers. A paste-to-paste system (base/catalyst) was produced for each experimental cement. The composition of the cements is described in Table 1, and the formulation was based on recently published studies.4,26 Equal volumes of base and catalyst pastes were mixed for 10 s. Photoactivation procedures were carried out using an LED light-curing unit (Radii cal, SDI; Bayswater, Victoria, Australia) with an irradiance of 1200 mW/cm2 for 20 s. The LED irradiance was measured with a hand-held radiometer (LED radiometer, Demetron-Kerr; Orange, CA, USA) before each test. The summary of the research methodology is illustrated in Fig 1.
Table 1. Composition of the experimental cements at final weight (wt%)
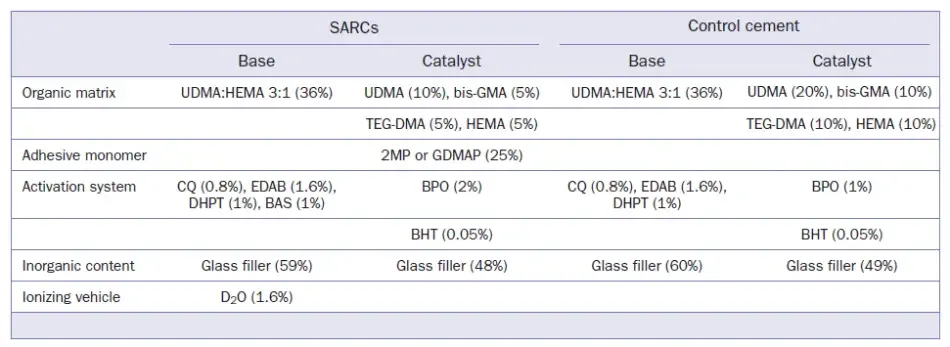
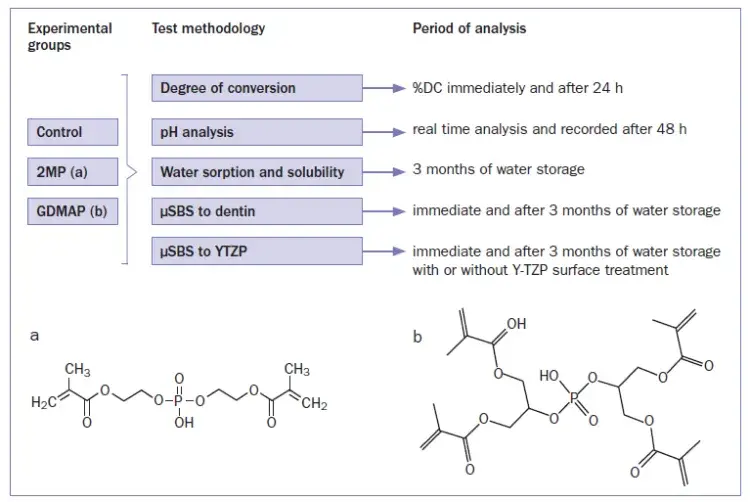 Fig 1. Summary of the research methodology and chemical structures of 2MP (a) and GDMAP (b).
Fig 1. Summary of the research methodology and chemical structures of 2MP (a) and GDMAP (b).
Degree of C=C Conversion
DC was determined by near-infrared spectroscopy (NIR, Vertex 70, Bruker Optik; Ettlingen, Germany) configured with a white light source supplemented by a potassium bromide (KBr) beam splitter and mercury cadmium telluride (MCT) detector. Spectra were obtained with 32 scans and 4 cm-1 resolution. Three specimens (n = 3) were produced using a silicone mold (Ø = 7.0 mm x height = 0.8 mm) positioned between two glass blades. A mylar strip was placed between the cement and the blades to inhibit contact with oxygen. Four spectra of the unpolymerized material were obtained. The photoactivation procedure was performed at controlled temperatures (23°C-25°C). DC was analyzed immediately and after 24 h. The calculation was performed considering the intensity of C=C stretching vibration (peak height) at 1635 cm-1. The symmetric ring stretching at 1608 cm-1 was used as an internal standard. DC was calculated by the formula:
 pH Analysis
pH Analysis
Three resin cement disks (n = 3) were produced with specific dimensions (Ø = 10.0 mm x height = 2.0 mm) using a prefabricated metal mold. Each disk was photoactivated for 20 s. The specimens were individually stored in 10 ml distilled water. The pH of the cements was evaluated in real time using a pH meter (Metrohm; Herisau, Switzerland) and recorded after 48 h.
Water Sorption and Solubility
Six specimens (n = 6) were produced using a prefabricated metal mold (Ø = 15.0 mm x height = 1.0 mm). The diameter and height of the disks were measured with a digital caliper with 1-μm accuracy (Mitutoyo; Kanagawa, Japan) at four equidistant points from the center. The volume (V) was calculated in mm3 using the average of the diameter and the height. Specimens were then stored in a desiccator with silica gel in a furnace at 37°C for 24 h, and weighed in an analytical balance (Adventurer, Ohaus; Parsippany, NJ, USA) with 0.1-mg accuracy. This cycle was repeated until a constant mass (variation less than ± 0.2 mg; M1) was obtained. After determining M1, all specimens were individually immersed in tubes with 10 ml distilled water and stored in an oven at 37°C. The disks were weighed until constant mass was reached (variation less than ± 0.2 mg; M2). Before the weighing procedures, each specimen was washed in distilled water and dried with absorbent paper. After M2, the same drying process described for M1 was repeated until a new constant mass was obtained (variation less than ± 0.2 mg; M3). The results of M1, M2, and M3 as well as volume were entered into the following equations:
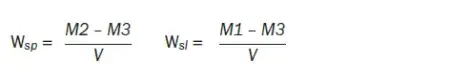 Film Thickness
Film Thickness
The thickness of two glass plates (10 mm each) in contact was measured with a digital caliper (Mitutoyo) with 1-μm accuracy. The base and catalyst pastes were mixed and the cement was positioned centrally between the plates. A constant load of 150 N was applied using a hydraulic press machine (model MPH-15, Marcon; Marília, Brazil) for 3 min. The load was then removed and the cements were photoactivated. The combined thickness of the glass plates and cement was measured. Film thickness (n = 5) was determined as a function of the difference between the measurements made before and after insertion of the resin cement.
Preparation of Dentin Specimens
After approval of the study protocol by the research ethics committee, University of São Paulo, School of Dentistry, Brazil (protocol 736.003), 72 human third molars were embedded in epoxy resin and wet ground on the occlusal surface, exposing 4 mm of mid-depth dentin 2.0 to 2.5 mm from the pulp. The flat dentin was wet polished with 600-grit SiC abrasive papers for 60 s in an automatic polishing machine (Ecomet 3000; Uzwil, Switzerland). Four Tygon tubes (internal diameter = 0.8 mm, height = 0.5 mm) were fixed on dentin surface using sticky wax. The mixed resin cements were inserted into each Tygon tube. For the control group, the dentin surfaces were previously treated with phosphoric acid for 15 s (Condac 37%, FGM Produtos Odontológicos; Joinville, Brazil) and adhesive (Single Bond 2, 3M Oral Care; St Paul, MN, USA). Polyester strips and glass slides were placed on the top of the cylinders to press out the excess cement and inhibit contact with oxygen. After that, the resin cements were left to self-cure for 3 min and then photoactivated as previously described.
Preparation of Y-TZP Blocks
Pre-sintered Y-TZP blocks (In-ceram YZ, VITA Zahnfabrik; Bad Säckingen, Germany) were sectioned with a diamond disk mounted in a precision cutter (Isomet 1000, Buehler; Lake Bluff, IL, USA). A total of 144 blocks with dimensions of 6 x 6 x 3 mm were produced. Final sintering was carried out in a Zyrcomat furnace (VITA Zahnfabrik) following the manufacturer’s protocol. The blocks were embedded in black phenolic resin and wet-polished in an automatic machine (EcoMet 3000). Half of the blocks were sandblasted with 30-μm silica-coated alumina particles (Cojet, 3M Oral Care) at a distance of 10 mm and pressure of 2.8 bar, followed by application of a silane coupling agent (Silane Ceramic Primer, 3M Oral Care). Y-TZP surface treatment was performed according to the respective manufacturer’s recommendations; this dispensed with the application of an acidic primer before the SARCs application, since the resin cements already contained an adhesive monomer. The other half of the blocks did not undergo any surface treatment. Afterwards, Tygon tubes (dimensions as above) were fixed on the surface of Y-TZP blocks using sticky wax, and the same bonding and photoactivation procedures described for the dentin specimens were followed.
Bond Strength Test and Fracture Patterns
μSBS (n = 12) was performed in a universal testing machine (Kratos IKCL 3; Cotia, São Paulo, Brazil) after 24 h or 3 months of wet storage at 37°C. Dentin and Y-TZP specimens were fitted into a metal matrix perpendicular to the long axis of the testing machine. A 2-mm-diameter stainless-steel orthodontic wire (Morelli Orthodontics; Sorocaba, Brazil) was attached to the upper extension of the load cell of the universal machine. It was simultaneously in contact with the lower semicircle of the cylinders and with the surface of the dentin or Y-TZP. The μSBS test was then performed at a speed of 0.5 mm/min. The fractured surfaces were evaluated using a stereomicroscope (Olympus SZ61; Tokyo, Japan) at 40X magnification. The failure modes were classified as adhesive, cohesive, or mixed.
Statistical Analysis
Data were submitted to normality and homoscedasticity tests. pH, Wsp, Wsl and film thickness results were submitted to one-way ANOVA. Results of DC and μSBS to dentin were analyzed by two-way ANOVA, and μSBS to Y-TZP by three-way ANOVA. All pairwise multiple comparisons were carried out using Tukey’s post-hoc test (α = 0.05).
RESULTS
Degree of C=C conversion
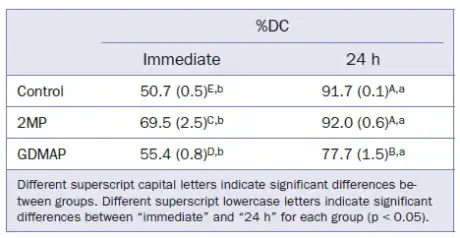
DC data are shown in Table 2. All groups showed an increase in DC after 24 h. In terms of immediate results, 2MP exhibited the highest DC and the control group showed the lowest DC. 2MP and control groups showed higher DC after 24 h in comparison with the group containing GDMAP.
Table 2. Means (SD) for degree of C=C conversion (%DC)
 pH Analysis
pH Analysis
The pH as a function of time is shown in Fig 2, and the results after 48 h are depicted in Table 3. 2MP and GDMAP showed lower pHs from the beginning of the analysis. After 48 h, the control group showed the highest pH, followed by GDMAP and 2MP.
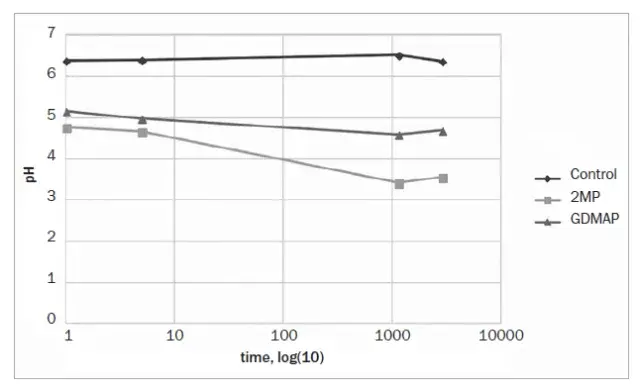 Fig 2. Results of pH at different periods (minutes)
Fig 2. Results of pH at different periods (minutes)
Table 3. Means (standard deviations) for pH, water sorption/solubility, and film thickness
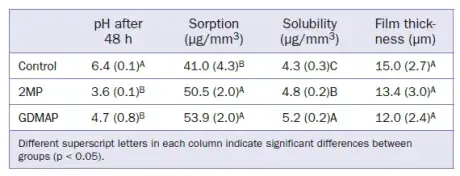 Sorption, Solubility, and Film Thickness
Sorption, Solubility, and Film Thickness
Wsp, Wsl, and film thickness are shown in Table 3. The material composition significantly affected (p < 0.05) both Wsp and Wsl. However, film thickness was not affected by the material composition (p = 0.266). GDMAP showed the highest Wsp and Wsl in comparison to the other experimental cements.
Microshear Bond Strength to Dentin and Y-TZP
The μSBS results for the different resin cements to dentin are shown in Table 4. All groups showed a significant decrease in bond strength after aging, except GDMAP. This group maintained the values after aging, being statistically similar to the control group.
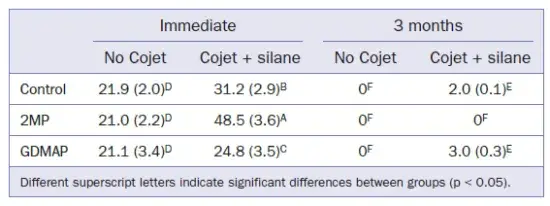
The μSBS results of resin cements to Y-TZP are shown in Table 5. None of the experimental groups was able to generate satisfactory bond strength after water storage, regardless of the surface treatment applied.
Table 4. Means (SD) for microshear bond strength to dentin (MPa) for the different aging conditions
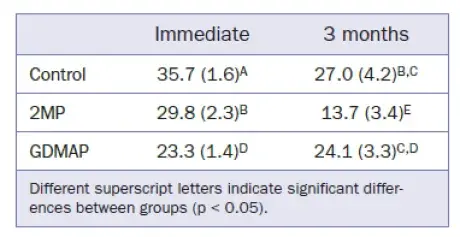 Table 5. Means (SD) for microshear bond strength to Y-TZP (MPa) for the different aging conditions
Table 5. Means (SD) for microshear bond strength to Y-TZP (MPa) for the different aging conditions
 Fracture Patterns
Fracture Patterns
The failure mode at the cement-dentin interface was mixed (100%) for all experimental groups, regardless of the period analyzed (Fig 3). The predominant mode for the experimental cement/Y-TZP interfaces was also mixed (100%) for specimens tested immediately. However, the fracture mode was adhesive (100%) for aged specimens (Figs 4 and 5).
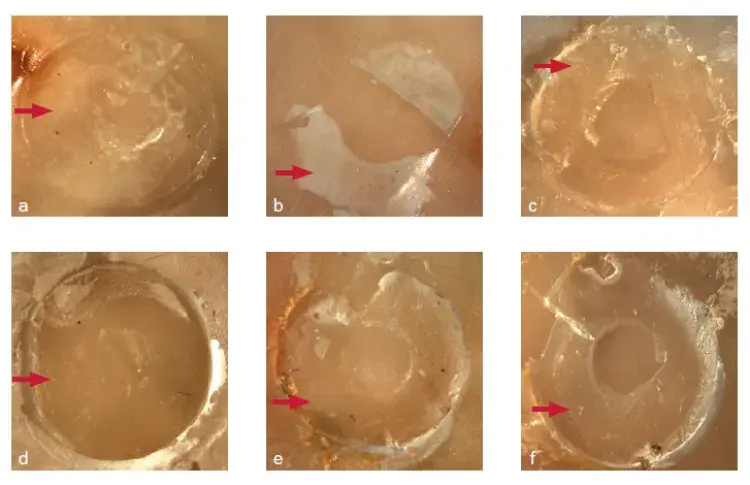 Fig 3. Fracture pattern of SARC/dentin interfaces: immediate and after aging (control: a and d; 2MP: b and e; GDMAP: c and f). Red arrows indicate residual cement at the surface after fracture in dentin or Y-TZP.
Fig 3. Fracture pattern of SARC/dentin interfaces: immediate and after aging (control: a and d; 2MP: b and e; GDMAP: c and f). Red arrows indicate residual cement at the surface after fracture in dentin or Y-TZP.
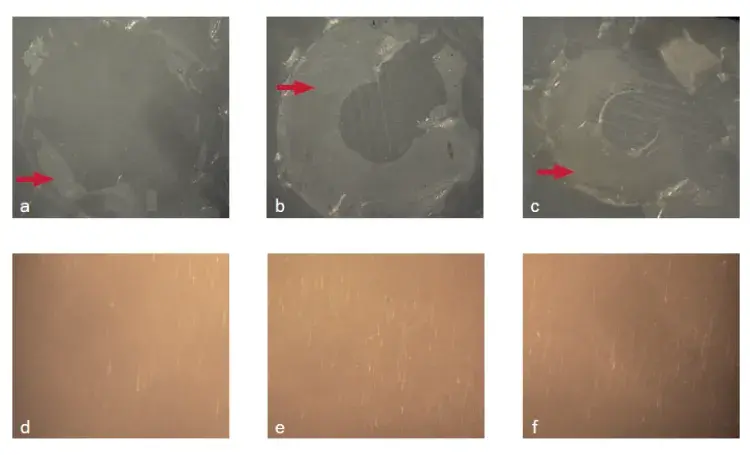 Fig 4. Fracture pattern of cement/Y-TZP interfaces without surface treatment: immediate and after aging (control: a and d; 2MP: b and e; GDMAP: c and f). Red arrows indicate residual cement at the surface after fracture in dentin or Y-TZP.
Fig 4. Fracture pattern of cement/Y-TZP interfaces without surface treatment: immediate and after aging (control: a and d; 2MP: b and e; GDMAP: c and f). Red arrows indicate residual cement at the surface after fracture in dentin or Y-TZP.
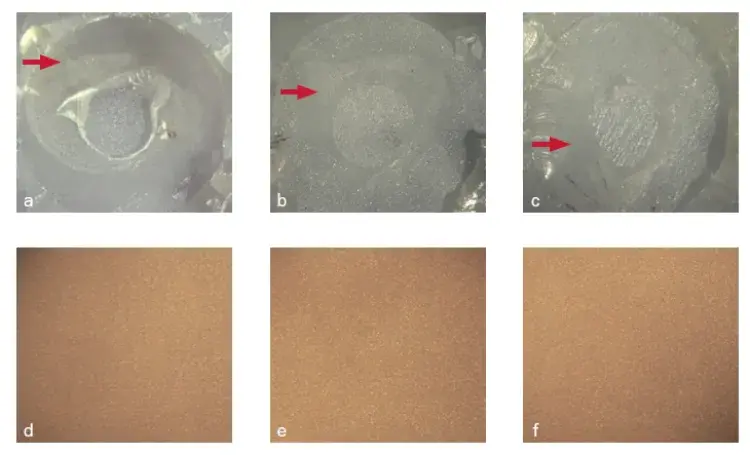 Fig 5. Fracture pattern of cement/Y-TZP interfaces with surface treatment: immediate and after aging (control: a and d; 2MP: b and e; GDMAP: c and f). Red arrows indicate residual cement at the surface after fracture in dentin or Y-TZP.
Fig 5. Fracture pattern of cement/Y-TZP interfaces with surface treatment: immediate and after aging (control: a and d; 2MP: b and e; GDMAP: c and f). Red arrows indicate residual cement at the surface after fracture in dentin or Y-TZP.
DISCUSSION
The present study evaluated DC, pH, hydrolytic degradation, and film thickness of experimental resin cements. Additionally, the bond strength of these cements to dentin and Y-TZP was assessed after different storage times. The first research hypothesis – that the results of DC, pH, Wsp, Wsl and film thickness would be similar for all experimental resin cements – was rejected, as material composition affected these properties. The second research hypothesis, that all resin cements would show similar μSBS to dentin or Y-TZP after aging, was also rejected.
It is well established that the composition of resin-based cements significantly affects the material properties. In the current investigation, the experimental cements showed a DC above 77% after 24 h (Table 2), which is in agreement with previous reports2,19 but contradicts results from studies that showed DC < 50% for commercial SARCs. These contradictory results may be associated with the material composition and other factors, such as test method, light source used, and the photoactivation time.
Regarding the activation system, the dual-curing mode is most appropriate for luting procedures in which light transmission is limited. In the present work, all groups showed an increase of DC after 24 h (Table 2). These results were expected due to the self-curing mechanism that remains active over time.28 On the other hand, 2MP reached similar immediate and final DC in comparison to the control group. This result is different from that presented in previous reports which showed higher DC for conventional resin cements than for SARCs.29 SARC polymerization is negatively affected by the interaction between amine and an adhesive monomer. Despite these results, the 2MP group was not affected by this interaction and reached similar immediate and final DC in comparison to the control group. It is hypothesized that the high DC observed for 2MP could be related to its molecular form, which has high flexibility and therefore shows high intermolecular interaction during the polymerization process.
In addition to the inherent flexibility of the 2MP structure, the salt BAS also contributed to the high DC observed for the SARCs. The addition of this component and its derivatives to experimental SARCs could inhibit the amine inactivation by the adhesive monomer, increasing the DC of the material. A previous study also evaluated the effect of a similar component (p-toluenesulfonic acid sodium salt 95%) in experimental SARCs and found a similar increase of DC specifically in the first minutes after starting the polymerization reaction. Additionally, it has been reported that this type of salt tends to react with the adhesive monomers, producing free radicals that initiate the polymerization reaction and increase the final DC of SARCs.
Despite the results observed for 2MP, the resin cement formulated with GDMAP did not reach a DC higher than 80% (Table 2), even with added BAS. GDMAP contains a greater number of reactive methacrylate sites available for polymerization. During the early stages of polymerization, GDMAP tends to exhibit a high polymerization rate, which results in fast growth of the polymer chains. Such a reaction increases the initial viscosity of the cement and consequently limits the diffusion of the free radicals within the polymer network. Due to this limitation, GDMAP resin-based cements are not able to achieve high degrees of conversion.
SARCs do not require any type of previous treatment of dental substrates because they contain adhesive monomers. However, the relatively low pH of these monomers can significantly affect their shelf life. The proportion of adhesive monomers incorporated in the SARCs should be high enough to promote proper interaction with dental and prosthetic substrates, but as low as possible to avoid polymerization problems for the cement. In the present study, pH real-time analysis (Fig 2) showed lower values for 2MP and GDMAP, and a pH close to neutral for the control group. These results corroborate those of other studies, which demonstrated lower pH for SARCs in comparison to conventional resin cements. Notwithstanding, the literature shows a great variation in pHs for commercial SARCs, ranging from 2.4 to 7.0. A neutralization reaction commonly occurs when SARCs contact dental tissues, increasing the pH. It seems that the absence of this process on the present pH test had a fundamental influence on the final pH values. It is important to note that neutralization can occur when SARCs are in contact with the dental substrate; this reaction does not occur in the parts of the cement that are farther away from the interface.
In the present study, groups containing 2MP and GDMAP did not achieve neutral pH (7.0), and alternative methods could be suggested. In an attempt to simulate the neutralization of SARCs in contact with the dental substrate, a previous study tested the effect of different concentrations of calcium hydroxide in experimental SARCs. The authors indicated that the presence of this component resulted in a better control of the neutralization reaction, but the presence of calcium hydroxide decreased the mechanical properties of the cement. Considering commercially available materials, RelyX Unicem (3M Oral Care) is the only SARC that could achieve a pH close to neutral, which is probably related to the presence of calcium hydroxide in this cement.
The ISO recommends that the film thickness of a cement should be less than 25 μm for acid-base reaction materials and should not exceed 50 μm for resin cements. The results of the present study (Table 3) are in accordance with these values and corroborate with a previous report, since all tested groups showed a film thickness lower than 15 μm. In the present study, it is noteworthy that there was a decrease in the initial resin-cement viscosity due to the addition of adhesive monomer. However, the final film thickness of the materials was not significantly affected in relation to the control group.
The literature indicates that conventional resin cements have lower water sorption and solubility than do SARCs. This could be associated with the high hydrophilicity of adhesive monomers. Moreover, the water added to the formulation of SARCs acts as an ionizing vehicle for the adhesive monomer and tends to increase water sorption in these materials. In the present study, the control group showed lower solubility followed by 2MP and GDMAP (Table 3). The solubility observed for GDMAP might be related to its lower DC in relation to that measured for the other groups. It is well known that low DC increases the number of unreacted components that are leached during he water storage period.
Regarding the bonding characteristics of resin cements to dentin, the control group showed higher immediate values than did the other groups (Table 4). Similar results were obtained for commercially available cements, which demonstrated superior bond strength for the conventional resin cement Variolink II (Ivoclar Vivadent) (μSBS = 39.2 MPa) in relation to the SARC RelyX Unicem (3M Oral Care) (μSBS = 18.4 MPa). In the present study, the bond strength of the control group remained non-significantly higher than that of the GDMAP group after water storage. 2MP showed the lowest bond strength to dentin, confirming their high susceptibility to hydrolytic degradation.
The interaction between SARCs and dentin results in a thinner or no hybrid layer in relation to that observed for resin cements placed after a pre-treatment (phosphoric acid and/or adhesive). It has been suggested that the bond strength to dentin could be increased by secondary chemical bonds developed between functionality groups (eg, phosphoric acid) and the calcium present in hydroxyapatite. 3 GDMAP may have established a more stable bond to hydroxyapatite than that obtained by 2MP. The mixed fracture pattern observed in dentin agrees with previous reports of such patterns being predominant after μSBS testing.
The immediate μSBS of resin cement to Y-TZP was superior in comparison to the μSBS after aging (Table 5). All experimental groups showed immediate μSBS > 20 MPa, regardless of the presence of surface treatment. This has been reported as a clinically acceptable bond strength. However, these results do not reflect the behavior of the cement/Y-TZP interface after long-term storage. After aging, all groups showed adhesive failures and very low bond strengths (Table 5). One study suggested the possibility of chemical bonding between the adhesive monomer present in SARCs and the inert surface of Y-TZP. However, the low bond strengths observed suggest that such a chemical interaction is very weak and unstable in the experimental cement tested. The application of MDP-acidic primers has been strongly recommended by several authors to increase the bond strength between acid-resistant ceramic and SARCs.
After surface treatment (Cojet+Silane), the immediate bond strength of the resin cement to Y-TZP was higher, but low bond strengths with premature debonding were observed after long-term storage. Some studies reported an increase in bond strength for acid-resistant ceramics that had undergone tribochemical treatment associated with the application of MDP-primer. This type of surface treatment enhanced the bond strength of the cement to Y-TZP due to the bonding ability of the MDP and the increased wetting ability of the cement in the presence of a primer. Therefore, the chemical bond of the acidic monomer added to the SARCs may have been overestimated. Mixed failure was observed for the formulated cements on Y-TZP when tested immediately, irrespective of thesurface treatment. On the other hand, adhesive failures were observed after aging (Figs 4 and 5). These results are in accordance with the literature, which demonstrated adhesive fracture as the most common failure mode, indicating low interaction of the cement with the Y-TZP surface.
You have the opportunity to gather more in-depth information about aesthetic treatment protocols on our website.
CONCLUSIONS
Considering that the SARCs were used in the absence of the MDP primer, it can be concluded that:
the DC was dependent on resin-cement composition;
2MP and GDMAP showed lower pH and higher Wsp and Wsl than did the control;
the film thickness was similar for the resin cements tested;
GDMAP showed similar μSBS to dentin in comparison to the control group after aging;
none of the groups exhibited satisfactory μSBS to Y-TZP after aging, regardless of the surface treatment.
List of authors:
Pedro Paulo Albuquerque Cavalcanti de Albuquerque
Marcela Ferraz de Barros Duarte
Marina Barreto Pereira Moreno
Luis Felipe Schneider
Rafael Ratto Moraes
Paulo Francisco Cesar
Leonardo Eloy Rodrigues Filho
REFERENCES
1. Abo-Hamar SE, Hiller KA, Jung H, Federlin M, Friedl KH, Schmalz G. Bond strength of a new universal self-adhesive resin luting cement to dentin and enamel. Clin Oral Investig 2005;9:161–167.
2. Aguiar TR, Di Francescantonio M, Arrais CA, Ambrosano GM, Davanzo C, Giannini M. Influence of curing mode and time on degree of conversion of one conventional and two self-adhesive resin cements. Oper Dent 2010; 35:295–299.
3. Al-Assaf K, Chakmakchi M, Palaghias G, Karanika-Kouma A, Eliades G. Interfacial characteristics of adhesive luting resins and composites with dentine. Dent Mater 2007;23:829–839.
4. Albuquerque PPAC, Rodrigues EC, Schneider LF, Moraes RR, Cesar PF, Rodrigues Filho LE. Effect of an acidic sodium salt on the polymerization behavior of self-adhesive resin cements formulated with different adhesive monomers. Dent Mater 2018;34:359–366.
5. Attia A. Bond strength of three luting agents to zirconia ceramic - Influence of surface treatment and thermocycling. J Appl Oral Sci 2010;19: 388–395.
6. Butler S, Linke B, Torrealba Y. Effect of MDP-based primers on the luting agent bond to Y-TZP ceramic and to dentin. BioMed Res Int 2018;1–6.
7. Carvalho RM, Manso AP, Geraldeli S, Tay FR, Pashley DH. Durability of bonds and clinical success of adhesive restorations. Dent Mater 2012;28: 72–86.
8. Christensen GJ. Why use resin cements? J Am Dent Assoc 2010;141: 204–206.
9. D’Alpino PH, Silva MS, Vismara MV, Di Hipolito V, Miranda Gonzalez AH, de Oliveira Graeff CF. The effect of polymerization mode on monomer conversion, free radical entrapment, and interaction with hydroxyapatite of commercial self-adhesive cements. J Mech Behav Biomed Mater 2015; 46:83–92.
10. Ferracane JL, Stansbury JW, Burke FJ. Self-adhesive resin cements - chemistry, properties and clinical considerations. J Oral Rehabil 2011;38:295–314.
11. Guo X, Peng Z, Spencer P, Wang Y. Effect of initiator on photopolymerization of acidic, aqueous dental model adhesives. J Biomed Mater Res Part A 2009;90:1120–1127.
12. Han L OA, Fukushima M, Okiji T. Evaluation of physical properties and surface degradation of self-adhesive resin cements. J Dent Mater 2007; 26:906–914.
13. Hitz T, Stawarczyk B, Fischer J, Hammerle CH, Sailer I. Are self-adhesive resin cements a valid alternative to conventional resin cements? A laboratory study of the long-term bond strength. Dent Mater 2012;28:1183–1190.
14. Ikemura K, Endo T, Kadoma Y. A review of the developments of multi-purpose primers and adhesives comprising novel dithiooctanoate monomers and phosphonic acid monomers. J Dent Mater 2012;31:1–25.
15. Ikemura K, Endo T. A review of our development of dental adhesives — Effects of radical polymerization initiators and adhesive monomers on adhesion. Dent Mater J 2010;29:109–121.
16. Inokoshi M, De Munck J, Minakuchi S, Van Meerbeek B. Meta-analysis of bonding effectiveness to zirconia ceramics. J Dent Res 2014;93:329–334.
17. Inokoshi M, Kameyama A, De Munck J, Minakuchi S, Van Meerbeek B. Durable bonding to mechanically and/or chemically pre-treated dental zirconia. J Dent 2013;41:170–179.
18. International Organization for Standardization. ISO 4049: Dentistry polymer-based filling, restorative and luting materials. Geneva, 2000.
19. Jang Y, Ferracane JL, Pfeifer CS, Park JW, Shin Y, Roh BD. Effect of insufficient light exposure on polymerization kinetics of conventional and selfadhesive dual-cure resin cements. Oper Dent 2017;42:1–9.
20. Kim HJ, Bagheri R, Kim YK, Son JS, Kwon TY. Influence of curing mode on the surface energy and sorption/solubility of dental self-adhesive resin cements. Materials 2017;10;129:1–13.
21. Lad PP, Kamath M, Tarale K, Kusugal PB. Practical clinical considerations of luting cements. A review. Int J Dent Oral Health 2013;6:116–120.
22. Leal FB, Madruga FC, Prochnow EP, Lima GS, Ogliari FA, Piva E, Moraes RR. Effect of acidic monomer concentration on the dentin bond stability of self-etch adhesives. Int J Adhes Adhes 2011;31:571–574.
23. Li J, Shibuya I, Teshima I, Nemoto K, Nishiyama N. Development of dual curing type experimental composite resin cement for orthodontic bonding – Effect of additional amount of accelerators on the mechanical properties. J Dent Mater 2009;28:401–408.
24. Liu Q, Meng X, Yoshida K, Luo X. Bond degradation behavior of self-adhesive cement and conventional resin cements bonded to silanized ceramic. J Prosthet Dent. 2011;105:177–184.
25. Luhrs AK, Guhr S, Gunay H, Geurtsen W. Shear bond strength of self-adhesive resins compared to resin cements with etch and rinse adhesives to enamel and dentin in vitro. Clin Oral Investig 2010;14:193–109.
26. Madruga FC, Ogliari FA, Ramos TS, Bueno M, Moraes RR. Calcium hydroxide, pH-neutralization and formulation of model self-adhesive resin cements. Dent Mater 2013;29:413–418.
27. Marghalani HY. Sorption and solubility characteristics of self-adhesive resin cements. Dent Mater 2012;28:187–198.
28. Mendes LC MI, Miranda MS, Benzi MR. Dual-curing, self-adhesive resin cement: influence of the polymerization modes on the degree conversion and microhardness. Mater Res 2010;13:171–176.
29. Moraes RR, Boscato N, Jardim PS, Schneider LF. Dual and self-curing potential of self-adhesive resin cements as thin films. Oper Dent 2011; 36:635–642.
30. Osorio R, Pisani-Proenca J, Erhardt MCG. Resistance of ten contemporary adhesives to resin-dentine bond degradation. J Esthet Restor Dent 2009; 21:352–354.
31. Pameijer CH. A review of luting agents. Int J Dent 2012;2012:752861.
32. Papia E, Larsson C, du Toit M, Vult von Steyern P. Bonding between oxide ceramics and adhesive cement systems: a systematic review. J Biomed Mater Res Part B Appl Biomater 2014;102:395–413.
33. Pashley DH, Tayb FR, Breschic L, Tjäderhanee L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dent Mater 2011;27:1–16.
34. Radovic I, Monticelli F, Goraccic C, Vulicevicd ZR, Ferrari M. Self-adhesive resin cements: A literature review. J Adhes Dent 2008;10:251–258.
35. Sideridou I TV, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002;23:1819–1829.
36. Takahashi H. Effect of calcium salt of 10-methacryloyloxydecyl dihydrogen phosphate produced on the bond durability of one-step self-etch adhesive. J Dent Mater 2014;33:394–401.
37. Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dent Mater 2011;27:17–28.
38. Vrochari AD, Eliades G, Hellwig E, Wrbas KT. Curing efficiency of four selfetching, self-adhesive resin cements. Dent Mater 2009;25:1104–1108.
38. Yang B, Barloi A, Kern M. Influence of air-abrasion on zirconia ceramic bonding using an adhesive composite resin. Dent Mater 2010;26:44–50.
40. Zorzin J, Petschelt A, Ebert J, Lohbauer U. pH neutralization and influence on mechanical strength in self-adhesive resin luting agents. Dent Mat 2012;28:672–679.

