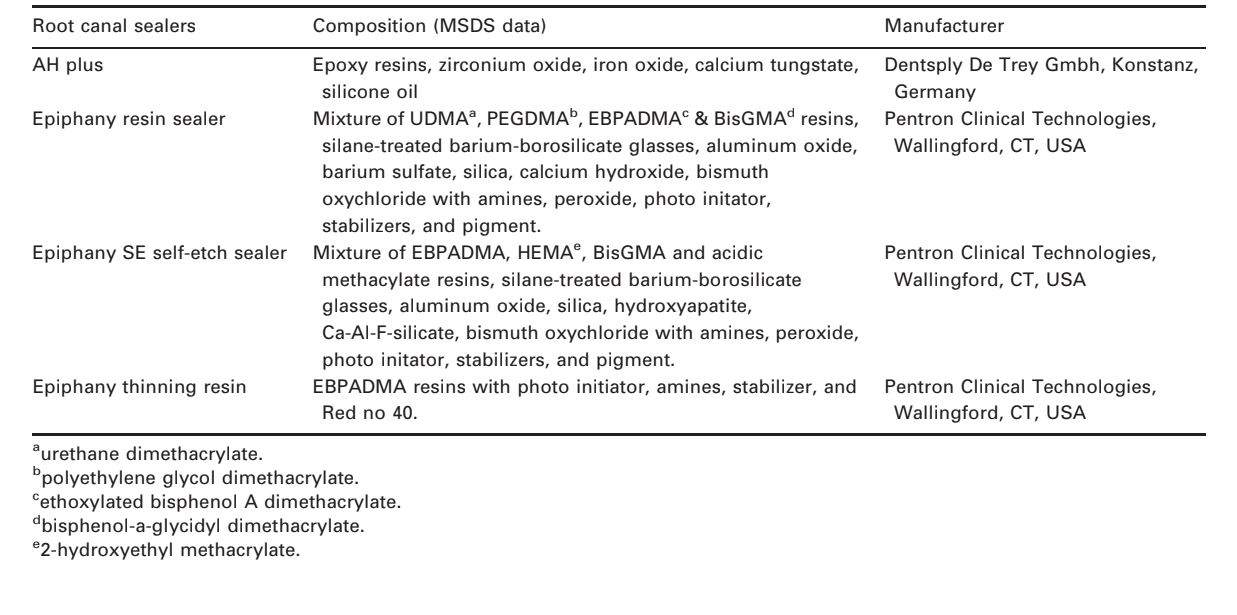
A comparative study of physicochemical properties of AH Plus, Epiphany, and Epiphany SE root canal sealers
Abstract
Aim: To assess the physicochemical properties and the surface morphology of AH Plus, Epiphany, and Epiphany SE root canal sealers.
Methodology: Five samples of each material were employed for each test according to ANSI/ADA specification 57. The samples were assigned to four groups: (i) AH Plus; (ii) Epiphany; (iii) Epiphany + Thinning Resin; (iv) Epiphany SE. The distilled water used during the solubility test was submitted to spectrometry to verify the release of calcium ions. The morphologies of the external surface and the cross-section of the samples were analysed by means of a scanning electron microscope (SEM). Statistical analysis was performed by using One-Way anova and post hoc Tukey–Kramer tests with the null hypothesis set as 5%.
Results: Setting time, flow and radiopacity results were in accordance with ANSI/ADA requirements whereas the dimensional change of all sealers and solubility of Epiphany did not fulfil ANSI/ADA protocols. AH Plus and Epiphany SE were similar in terms of flow, radiopacity, solubility and dimensional change. The spectrometry test revealed significant calcium ion release from Epiphany with and without the thinning resin. SEM analysis revealed essentially a homogeneous surface with compact layer and some rough areas.
Conclusions: Setting time, flow, and radiopacity tests conformed to ANSI/ADA standardization. The dimensional change in all groups and the solubility of Epiphany were greater than values considered acceptable, with higher amounts of calcium ion release. Epiphany SE revealed more organized, compacted, and homogeneous polymers in a reduced resin matrix when compared with the other groups.
Introduction
Complete filling of the prepared root canal system is an important component of successful root canal treatment (Suebnukarn et al. 2008). The function of root canal fillings is to seal the root canal system to prevent microorganisms and/or their toxic products reaching the periodontal tissues (Sundqvist et al. 1998).
Synthetic resins have been used as root filling materials for many decades. The most successful resin-based sealers are the AH series, which was developed more than 50 years ago. AH Plus (Dentsply De Trey Gmbh, Konstanz, Germany), a mixture of epoxy-amines, is the result of this product development and is frequently used as a control material in research (Ørstavik 2005).
The recent introduction of Resilon (Resilon Research LLC, Madison, CT, USA) as an alternative root filling material offers the promise of adhesion to dentine. The first generation of this filling system consisted of a thermoplastic synthetic polymer-based root filling material (Resilon), a dual-curable resin-based composite (Epiphany), a self-etching Primer, and the Thinning Resin that may be used to adjust the viscosity of the sealer (Shipper et al. 2005, Merdad et al. 2007). The second generation replaced the original sealer and primer with Epiphany SE Self-Etch Sealer (Pentron Clinical Technologies, Wallingford, CT, USA), a self-etch dual-cure, hydrophilic resin sealer that potentially bonds to both Resilon and to dentine in the canal, without a separate priming step (Pentron 2007).
The purpose of this laboratory study was to assess the setting time, flow, radiopacity, solubility, and dimensional changes of Epiphany SE, in comparison with the original sealer, combined or not with the Thinning Resin, and the well-established epoxy-amine resin sealer AH Plus, according to ANSI/ADA (2000) standards. In addition, the morphologies of the external surface and the cross-section of all sealers were analysed using scanning electron microscopy (SEM).
Materials and methods
Setting time, flow, radiopacity, solubility, and dimensional changes after setting for AH Plus (group I), Epiphany (group II), Epiphany plus Thinning Resin (group III), and Epiphany SE (group IV) root canal sealers were measured according to ANSI/ADA (2000) standards for root canal sealing materials. Analyses were performed by a single examiner who was blind to the materials identification.
All materials (Table 1) were manipulated according to the manufacturer’s instructions. To standardize and homogenize the amount of material, 0.3 g of sealer was dispensed onto a mixing pad and spatulated for 15 s for each tested sample. As the sealers in groups II, III, and IV were dual-cure resins, they were mixed and handled in a darkroom using a low wattage red safelight bulb (15 W). In group III, for each 0.3 g of freshly manipulated sealant one drop of the Thinning Resin (0.04 g) was dropped onto it. For physicochemical tests, the arithmetic mean of five replicates for each sealer was recorded and considered as the result of the test.
Setting time
Five plaster of Paris cast rings, having an internal diameter of 10 mm and a thickness of 2 mm were prepared. The external borders of the moulds were fixed with wax on a glass plate (75 x 25 x 1 mm). The moulds were then filled with the material and transferred to a chamber with 95% relative humidity (RH) and a temperature of 37°C. In group I (AH Plus), 150 ± 10 s from the start of mixing the sealer, a Gilmore-type needle with a mass of 100 ± 0.5 g having a flat end of 2.0 ± 0.1 mm in diameter was carefully lowered vertically onto the horizontal surface of each sample. The needle tip was cleaned and the probing was repeated until indentations ceased to be visible. If the results differed by more than ±5%, the test was repeated. In groups II, III and IV (Epiphany, Epiphany plus Thinning Resin and Epiphany SE, respectively), after the 150 ± 10 s from the start of mixing the sealer, the specimens were light-cured for 40 s (Ultralux Dabi Atlante, Ribeirão Preto, São Paulo, Brazil) and the setting time was measured as described above.
Radiopacity test
Five acrylic plates (2.2 cm x 4.5 cm x 1 mm), containing four wells measuring 1 mm in depth and 5 mm in diameter, were prepared and placed over a glass plate covered by cellophane sheet. Each well was filled with one of the sealers, following a sequence according to the setting time of the material, from the longest to the shortest, so that the samples would be ready for radiographic evaluation after the final setting of all materials. In order to avoid the formation of bubbles, in group I, the freshly mixed sealer was introduced into the wells using a syringe whilst in groups II, III and IV, the respective material applicators were used. Another glass plate covered with cellophane was placed on top until complete setting (chemically or light-cured), then any excess sealer was removed. Each plate was kept in an incubator (37°C, 95% RH) for a period corresponding to three times the setting time.
Each of the acrylic plates containing the sealers was positioned, at the time of the radiographic exposure, alongside another acrylic plate (1.3 cm x 4.5 cm x 1 mm), containing an aluminium stepwedge, made of 1100 alloy, with the thickness varying from 1 to 10 mm, in uniform steps of 1 mm each (ANSI/ADA 2000). This set of acrylic plates was placed in front of this phosphor plate, next to the aluminium step wedge, and a digital radiograph was taken (Digora™ system; Soredex Orion Corporation, Helsinki, Finland). Radio-graphic images were obtained using the Spectro 70 X-ray machine (Dabi Atlante, Ribeirão Preto, São Paulo, Brazil), at 70 kVp and 8 mA. The object-to-focus distance was 30 cm (ANSI/ADA 2000) and the exposure time was 0.2 s. Exposed imaging plates of the test samples were scanned immediately after exposure (Digora™ Scanner) and analysed using Digora™ for Windows 5.1 software.
Flow test
A total of 0.5 mL of sealer was placed on a glass plate (10 x 10 x 3 mm) using a graduated disposable 3 mL syringe. At 180 ± 5 s after the onset of mixing, another plate with a mass of 20 ± 2 g and a load of 100 N plus was applied centrally on top of the material. Ten minutes after the commencement of mixing, the load was removed and the average of the major and minor diameters of the compressed discs were measured using a digital calliper with a resolution of 0.01 mm (Mitutoyo MTI Corporation, Tokyo, Japan). If both measurements were consistent to within 1 mm, the results were recorded. If the major and minor diameter discs were not uniformly circular or did not match within 1 mm, the test was repeated.
Solubility
A 1.5-mm-thick cylindrical Teflon® (Polytetrafluroethylene; DuPont, HABIA, Knivsta, Sweden) mould measuring 7.75 mm in inner diameter was filled with freshly mixed sealer. The mould was supported by a larger glass plate and covered with a cellophane sheet. An impermeable nylon thread was placed inside the material and another glass plate, also covered with cellophane film, was positioned on the mould and pressed manually in such a way that the plates touched the entire mould in a uniform manner. The assembly was placed in an incubator (37 °C, 95% RH) and left to stand for a period corresponding to three times the setting time. As soon as the samples were removed from the mould, they were weighed three times each with a degree of accuracy of 0.0001 g (HM-200; A & D Engineering, Inc., Bradford, MA, USA), and the mean reading recorded. The samples were suspended by nylon thread and placed two-by-two inside a plastic vessel with a wide opening containing 7.5 mL of deionised distilled water, taking care to avoid any contact between them and the inner surface of the container. The containers were sealed and left for 7 days in an incubator (37°C, 95% RH). After this period, the samples were removed from the containers, rinsed with deionised distilled water, blotted dry with absorbent paper, and placed in a dehumidifier for 24 h. Afterwards, they were weighed again. The weight loss of each sample (initial mass minus final mass), expressed as percentage of the original mass (m% = mi–mf), was taken as the solubility of the sealer.
A volume of 7.5 mL of distilled water from each sample was poured into a cleaned and dried porcelain crucible. Each crucible was put into a muffle and burned at 550°C. Ash was dissolved in 10 mL of a concentrated nitric acid using a glass stick. Following this, the samples were put into 50 mL volumetric flasks and the volume made up with ultrapure deionised water (MilliQ; Millipore, Billerica, MA, USA). The solutions attained were sprayed into the atomic absorption spectrophotometer (Perkin Elmer, Überlingen, Germany) to verify the presence of calcium ions. The arithmetic mean of three replicates for each specimen was recorded and considered as the result, expressed as μg mL–1.
Dimensional change
Five Teflon® moulds, prepared for the production of 3.58-mm high cylindrical specimen measuring 3 mm in diameter, were placed on a glass plate wrapped with a fine cellophane sheet. The moulds were filled with a slight excess of freshly mixed sealers and a microscope slide, also wrapped in cellophane, was pressed onto the upper surface of the mould. The assembly was held in a C-shaped clamp and transferred to an incubator (37 °C, 95% RH) and left to stand for a period corresponding to three times the setting time. After this period, the flat ends of the moulds, containing the samples, were ground with 600 grit wet sandpaper. The samples were removed from the mould, measured with a digital calliper, stored in a 50-mL vessel containing 2.24 mL of deionised distilled, and kept in an incubator (37°C, 95% RH) for 30 days. The sample was then removed from the container, blotted dry on absorbent paper, and measured again for length. The percentage of the dimensional alterations was calculated using the formula:
((L30 — L)/L) × 100
where L30 is the length of the sample after 30 days of storage and L is the initial length of the sample.
SEM examination
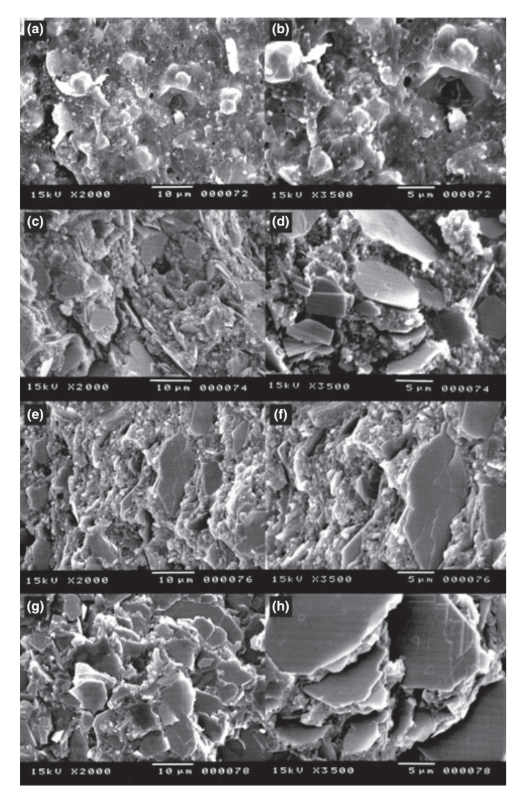
For SEM examination, cylindrical Teflon® moulds (3 x 4 mm) were filled with freshly mixed sealers. The moulds were supported by a glass plate covered with a cellophane sheet and placed in a chamber (37°C, 95% RH) for a period corresponding to three times the setting time. After that, the samples were sectioned with a size 15 disposable surgical scalpel blade, fixed on a metallic stub (10 x 5 mm), and sputter-coated with gold-palladium (Bal-Tec AG, Balzers, Germany) at 20 mA. The morphologies of the external surface and the cross-section of the samples were qualitatively analysed under a field emission SEM (Jeol JSM 5410; Jeol Technic Co., Tokyo, Japan) at an accelerating voltage of 15 kV, a working distance from 6 to 10 mm, and at different magnifications.
Statistical analysis
Five specimens from each group were tested and the means were compared statistically. The Kolmogorov– Smirnov revealed that the results were consistent with a normal distribution curve (GMC 8.1; USP, Ribeirão Preto, SP, Brazil) thus, parametric statistical analysis was possible (One-Way anova and post-hoc Tukey– Kramer test), and the null hypothesis was set as 5% (GraphPad InStat; GraphPad Software Inc., CA, USA).
Results
Setting time
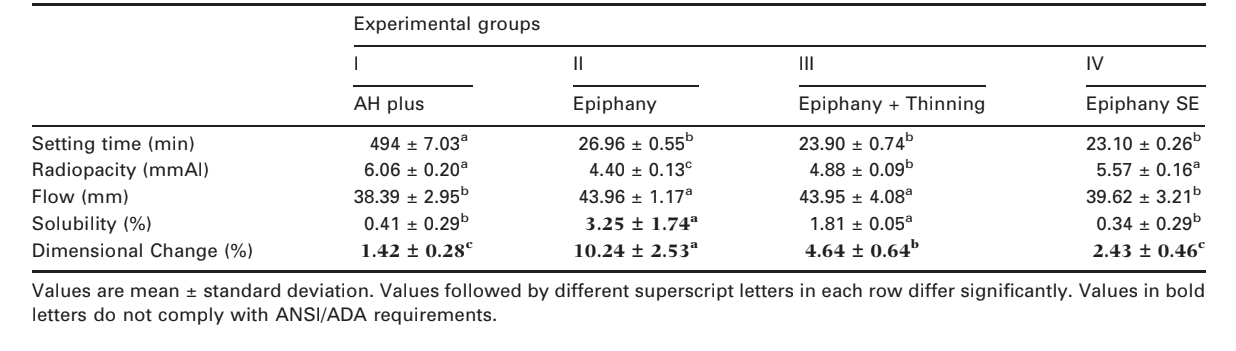
The ANSI/ADA (2000) requires that the setting time of a sealer shall be within 10% of that stated by the manufacturers. Thus, AH Plus and Epiphany have a stated setting time of 8 h (480 min) and 25 min of setting time, respectively. Hence, the mean values obtained were in agreement with the ANSI/ADA standard. Statistical analysis demonstrated that the setting time of group I (494.0 ± 7.03 min) was significantly higher than groups I–IV (P < 0.05) (Table 2).
Radiopacity test
All materials demonstrated radiopacity above the 3 mm of aluminium recommended by ANSI/ADA (2000) specification 57. Statistical analysis demonstrated similar radiopacity among groups I (6.06 ± 0.20 mmAl) and IV (5.57 ± 0.16 mmAl), which were significantly higher than groups III (4.88 ± 0.09 mmAl) and II (4.40 ± 0.13 mmAl), respectively (P < 0.05) (Table 2).
Flow test
The ANSI/ADA (2000) requires that a sealer shall have a diameter of no less than 20 mm and all groups conformed to the standards. Statistical analysis revealed that the results of groups II (43.96 ± 1.17 mm) and III (43.95 ± 4.08 mm) were significantly higher than groups I (38.39 ± 2.95 mm) and IV (39.62 ± 3.21 mm) (P < 0.05) (Table 2).
Solubility
A root canal sealer should not exceed 3% by mass when the solubility of the set material is tested (ANSI/ ADA 2000). In contrast to Groups I, III and IV, the solubility for group II (3.25 ± 1.74%) did not conform to ANSI/ADA standard (P < 0.05) (Table 2). The deionised distilled water used for the solubility test and submitted to atomic absorption spectrometry revealed a significant level of Ca+2 release in groups II (356.86 ± 6.10 μg mL–1) and III (465.74 ± 7.12 μg mL–1) compared with groups I (2.27 ± 1.01 μg mL–1) and IV (3.09 ± 1.02 μg mL–1) (P < 0.05).
Dimensional change
Neither sealer conformed to the ANSI/ADA standardisation, which states that the mean linear shrinkage of the sealer shall not exceed 1% or 0.1% in expansion. Statistical analysis demonstrated similar results among groups I (1.42 ± 0.28%) and IV (2.43 ± 0.46%) which were significantly lower than groups III (4.64 ± 0.64%) and II (10.24 ± 2.53%), respectively (P < 0.05) (Table 2).
SEM examination
The scanning electron microscopy of the cross-section of the specimens revealed the presence of sphere-shaped and plate-shaped polymers of different sizes that were nonhomogeneously dispersed within groups I and II, respectively. In contrast, groups III and IV had a more uniform and compact layer comprised of plate-shaped polymers. In group IV, more uniform and organized layer with higher amount of polymers in a reduced resin matrix was observed (Fig. 1).
Discussion
Root filling materials must have several properties, ranging from biocompatibility to mechanical sealing ability (Ørstavik 2005). Technological tests have been systematized by standards organizations, as American National Standards Institute/American Dental Association (ANSI/ADA 2000), to assess the physical and technological properties of endodontic filling materials. Although ANSI/ADA requirements have no specification regarding the use of digital radiography, it was used in this research in view of its widespread availability and advantages (Carvalho-Junior et al. 2007a). In this study, AH Plus, a two-component paste root canal sealer, based on polymerisation reaction of epoxy resin amines was tested for comparison, as it has been continuously used in comparative studies of physicochemical, biological and antimicrobial properties of root canal sealers (Sousa et al. 2006, Versiani et al. 2006, de Campos-Pinto et al. 2008). The introduction of Resilon maybe a viable alternative to Gutta-percha in clinical practice (Cotton et al. 2008), as it is made out of a soft resin, it can potentially bond to the Epiphany, a metacrylate resin-based sealer (Shipper et al. 2005, Pawinska et al. 2006). The new Epiphany SE is an evolution of Epiphany and differs from the latter due the substitution of urethane dimethacrylate monomer (UDMA), a more flexible aliphatic base monomer that has some hydrophilic properties (Skrtic & Antonucci 2007), by 2-hydroxyethyl methacrylate (HEMA), a highly hydrophilic monomer (Table 1).
The setting time is primarily a control test on the stable behaviour of a product and is dependent on the constituent components, their particle size, the ambient temperature, and relative humidity (Ørstavik 1983, Ørstavik et al. 2001, Ørstavik 2005). There is no stipulated standard setting time for sealers, but clinical convenience demands that it must be long enough to allow placement and adjustment of root filling if necessary. In the present study, AH Plus had a setting time almost 20 times higher than the other sealers due it is a two-component paste based on a slow polymerisation reaction of epoxy resin amines, where the conversion of monomers into polymers occurs gradually (Lin-Gibson et al. 2006). Epiphany and Epiphany SE are dual-curable resin composites containing a new redox catalyst (Pawinska et al. 2006). With the aim of creating an immediate coronal seal, the manufacturer states that 40 s of light is required to cure the coronal surface of the material, whereas the entire filling will self-cure in approximately 15–30 min (Nagas et al. 2008).
In this study, even though Epiphany and Epiphany SE were mixed and manipulated in a darkroom during the experimental procedures, yet when the specimens were exposed to a light cure source, a thin superficial noncured layer was always observed after the required setting time. The polymerisation of the sealer/dentine interface could possibly be affected by oxygen molecules present in dentine tubules. According to Franco et al. (2002), the oxygen inhibits vinyl polymerisation in composite resins and 40–60% of the carbon bonds remained unsatured. The lack of photoactivation throughout the entire specimen contributes to its incomplete polymerisation, leaving residual monomers in the sealer at the deepest regions of the specimen (Rached-Junior et al. 2009).
A degree of radiopacity is essential for the control of root filling placement. While the standards require only a lower limit to this property, it should be realized that extreme contrast in a material may lead to a false impression of a dense and homogenous fill (Ørstavik 2005). In the present work all sealers fulfilled ANSI/ ADA (2000) recommendations. Epiphany SE and AH Plus had significantly higher values and were statistically similar (P > 0.05). An analysis of the composition of the materials revealed they all have radiopacifier agents (Table 1); AH Plus contains zirconium oxide, iron oxide and calcium tugstate (Tanomaru-Filho et al. 2007) whilst Epiphany and Epiphany SE contain silane-treated barium-borosilicate glass in addition to barium sulphate, bismuth and silica (Epiphany SE MSDS Data, Taşdemir et al. 2008). Despite having almost the same composition, Epiphany SE was more radiopaque than Epiphany due the additional presence of Ca-Al-F-silicate (Epiphany SE, MSDS Data). On the other hand, Epiphany Thinning Resin has no filler and is used to adjust the viscosity of the Epiphany sealer (Merdad et al. 2007); consequently, it had no influence on radiopacity.
The ability of the sealer to flow is an important feature (Alicia Karr et al. 2007, Almeida et al. 2007) that depends on particle size, rate of shear, temperature, time, the internal diameter of the canals and the rate of insertion (Ørstavik 2005). Even though Epiphany and Epiphany plus Thinning were significantly higher than AH Plus and Epiphany SE (P < 0.05), all sealers were consistent with ANSI/ADA (2000) standards.
In order to measure the stability of sealers, their solubility and dimensional changes were evaluated. Solubility simply means the loss of mass during a period of immersion in water (Carvalho-Junior et al. 2007b), whereas dimensional change demonstrates, in percentage terms, the shrinkage or expansion of the material following setting (Ørstavik 2005). In the present study, despite ANSI/ADA recommendations, a modification previously proposed for both tests (Carvalho-Junior et al. 2007b) was used, which achieved similar results with a decrease in the material volume necessary for the production of the test samples. The volume reduction of filling material required to produce test samples can contribute to rational use of endodontic materials in the laboratory studies.
Solubility results of AH Plus, Epiphany plus Thinning and Epiphany SE were within ANSI/ADA (2000) standards, although Epiphany and Epiphany plus Thinning were similar and showed higher values. Epiphany (3.25 ± 1.74%) did not conform to the ANSI/ADA (2000) in absolute values; however, the lower limit of the range of values was within the recommendations. Atomic absorption spectrometry analysis conducted to determine the components released during the solubility test revealed extensive calcium release for Epiphany and Epiphany plus Thinning, which was consistent with previous findings (Versiani et al. 2006). Ethoxylated bisphenol A dimethacrylate (EBPADMA), a component of Epiphany sealer, is a low viscosity base monomer used to adjust the viscosity of resins systems (Merdad et al. 2007, Skrtic & Antonucci 2007). According to Skrtic & Antonucci (2007), EBPADMA forms a more open network structure with lower cross-linking density, which enhances the diffusion of ions into the storage media. This factor possesses influences in the substantial release of calcium ions found with Epiphany and Epiphany plus Thinning. As calcium ion release has been shown to favour a more alkaline pH environment (Leonardo et al. 2006), this result may explain the reduced inflammatory response observed in in vivo studies (Shipper et al. 2005, Sousa et al. 2006, de Campos-Pinto et al. 2008).
Notwithstanding the presence of EBPADMA in Epiphany SE (Table 1), its solubility and release of calcium ions were dissimilar in comparison with Epiphany and Epiphany plus Thinning. All three Epiphany’s sealers contain EBPADMA, however, in Epiphany SE, UDMA is replaced by HEMA, which is a low-weight hydrophilic monomer (Nakabayashi & Pashley 1998). Both Bis-GMA- and EBPADMA-based resins attained a higher methacrylate conversion when the hydrophilic monofunctional HEMA was included as a co-monomer in the resin (Skrtic & Antonucci 2007). Higher degrees of conversion for resins with a relatively greater content of HEMA could be attributed to its high diffusivity and monofunctionality (Regnault et al. 2008). Furthermore, it should be noted that dual cure cements are characterized by a slower chemical polymerisation where the light does not reach (Braga and Ferracane 2004). Thus, the better mechanical and chemical properties of Epiphany SE could be explained by the low level of calcium ions release observed in this study. It should be also highlighted that the manufacturer does not indicate the amount of EBPADMA monomer that was removed from the constituents of Epiphany.
In this study, the greatest dimensional changes occurred with Epiphany and Epiphany plus Thinning and this may be ascribed to the open cross-linked matrix formed in these materials, which produces a weaker structure, favouring calcium ions release. This fragile structure leads to water sorption and, consequently, higher expansion, as observed in this study (Table 2).
The lack of information on new generation of resin-based endodontic sealers hinders reliable comparisons. Further studies should aim for a better understanding of their physical, mechanical and chemical properties and how best to use them in specific clinical procedures.
Conclusions
Overall, setting time, flow, and radiopacity tests of AH Plus, Epiphany, Epiphany plus Thinning, and Epiphany SE conformed to ANSI/ADA standards. The dimensional change in all groups and the solubility of Epiphany were greater than values considered acceptable, with higher amount of calcium ion release. Epiphany mixed with Thinning Resin and Epiphany SE had lower solubility values than Epiphany. SEM analysis revealed that Epiphany SE had more organized, compact, and homogeneous polymers in a reduced resin matrix than AH Plus and Epiphany.
Authors: L. M. Resende, F. J. A. Rached-Junior, M. A. Versiani, A. E. Souza-Gabriel, C. E. S. Miranda, Y. T. C. Silva-Sousa, M. D. Sousa Neto
References:
- Alicia Karr N, Baumgartner JC, Marshall JG (2007) A comparison of gutta-percha and Resilon in the obturation of lateral grooves and depressions. Journal of Endodontics 33, 749–52.
- Almeida JF, Gomes BP, Ferraz CC, Souza-Filho FJ, Zaia AA (2007) Filling of artificial lateral canals and microleakage and flow of five endodontic sealers. International Endodontic Journal 40, 692–9.
- ANSI/ADA (2000) Specification No. 57 Endodontic Sealing Material. Chicago, USA: ANSI/ADA.
- Braga RR, Ferracane JL (2004) Alternatives in polymerization contraction stress management. Critical Reviews in Oral Biology and Medicine 15, 176–84.
- Carvalho-Junior JR, Correr-Sobrinho L, Correr AB, Sinhoreti MA, Consani S, Sousa-Neto MD (2007a) Radiopacity of root filling materials using digital radiography. International Endodontic Journal 40, 514–20.
- Carvalho-Junior JR, Correr-Sobrinho L, Correr AB, Sinhoreti MA, Consani S, Sousa-Neto MD (2007b) Solubility and dimensional change after setting of root canal sealers: a proposal for smaller dimensions of test samples. Journal of Endodontics 33, 1110–6.
- Cotton TP, Schindler WG, Schwartz SA, Watson WR, Hargreaves KM (2008) A retrospective study comparing clinical outcomes after obturation with Resilon/Epiphany or Gutta-Percha/Kerr sealer. Journal of Endodontics 34, 789–97.
- de Campos-Pinto MM, de Oliveira DA, Versiani MA, Silva- Sousa YT, de Sousa-Neto MD, da Cruz Perez DE (2008) Assessment of the biocompatibility of Epiphany root canal sealer in rat subcutaneous tissues. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 105, e77–81.
- Franco EB, Lopes LG, D’Alpino PH, Pereira JC, Mondelli RF, Navarro MF (2002) Evaluation of compatibility between different types of adhesives and dual-cured resin cement. The Journal of Adhesive Dentistry 4, 271–5.
- Leonardo MR, Hernandez ME, Silva LA, Tanomaru-Filho M (2006) Effect of a calcium hydroxide-based root canal dressing on periapical repair in dogs: a histological study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 102, 680–5.
- Lin-Gibson S, Landis FA, Drzal PL (2006) Combinatorial investigation of the structure-properties characterization of photopolymerized dimethacrylate networks. Biomaterials 27, 1711–7.
- Merdad K, Pascon AE, Kulkarni G, Santerre P, Friedman S (2007) Short-term cytotoxicity assessment of components of the epiphany resin-percha obturating system by indirect and direct contact millipore filter assays. Journal of Endodontics 33, 24–7.
- Nagas E, Uyanik MO, Sahin C, Durmaz V, Cehreli ZC (2008) Effects of different light-curing units and obturation techniques on the seal of the Resilon/Epiphany system. Journal of Endodontics 34, 1230–2.
- Nakabayashi N, Pashley DH (1998) Hybridization of dental hard tissues, 1st edn. Chicago: Quintessence Pub. Co.
- Ørstavik D (1983) Physical properties of root canal sealers: measurement of flow, working time, and compressive strength. International Endodontic Journal 16, 99–107.
- Ørstavik D (2005) Materials used for root canal obturation: technical, biological and clinical testing. Endodontic Topics 12, 25–38.
- Ørstavik D, Nordahl I, Tibballs JE (2001) Dimensional change following setting of root canal sealer materials. Dental Materials 17, 512–9.
- Pawinska M, Kierklo A, Marczuk-Kolada G (2006) New technology in endodontics – the Resilon-Epiphany system for obturation of root canals. Advances in Medical Science 51(Suppl 1), 154–7.
- Pentron (2007) Epiphany® Soft Resin Endodontic Obturation System. Wallingford, CT, USA: Pentron Clinical Technologies, LLC.
- Rached-Junior FJ, Souza-Gabriel AE, Alfredo E, Miranda CE, Silva-Sousa YT, Sousa-Neto MD (2009) Bond strength of epiphany sealer prepared with resinous solvent. Journal of Endodontics 35, 251–5.
- Regnault WF, Icenogle TB, Antonucci JM, Skrtic D (2008) Amorphous calcium phosphate/urethane methacrylate resin composites. I. Physicochemical characterization. Journal of Materials Science: Materials in Medicine 19, 507–15.
- Shipper G, Teixeira FB, Arnold RR, Trope M (2005) Periapical inflammation after coronal microbial inoculation of dog roots filled with gutta-percha or resilon. Journal of Endodontics 31, 91–6.
- Skrtic D, Antonucci JM (2007) Dental composites based on amorphous calcium phosphate – resin composition/physicochemical properties study. Journal of Biomaterials Applications 21, 375–93.
- Sousa CJ, Montes CR, Pascon EA, Loyola AM, Versiani MA (2006) Comparison of the intraosseous biocompatibility of AH Plus, EndoREZ, and Epiphany root canal sealers. Journal of Endodontics 32, 656–62.
- Suebnukarn S, Rungcharoenporn N, Sangsuratham S (2008) A Bayesian decision support model for assessment of endodontic treatment outcome. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 106, e48–58.
- Sundqvist G, Figdor D, Persson S, Sjögren U (1998) Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 85, 86–93.
- Tanomaru-Filho M, Jorge EG, Guerreiro Tanomaru JM, Goncalves M (2007) Radiopacity evaluation of new root canal filling materials by digitalization of images. Journal of Endodontics 33, 249–51.
- Taşdemir T, Yesilyurt C, Yildirim T, Er K (2008) Evaluation of the radiopacity of new root canal paste/sealers by digital radiography. Journal of Endodontics 34, 1388–90.
- Versiani MA, Carvalho-Junior JR, Padilha MI, Lacey S, Pascon EA, Sousa-Neto MD (2006) A comparative study of physicochemical properties of AH Plus and Epiphany root canal sealants. International Endodontic Journal 39, 464–71.

/social-network-service/media/default/6758/89a8282e.png)
