Root dentinal microcracks: a post-extraction experimental phenomenon?
We appreciate the opportunity to comment on the letter from Zaslansky and colleagues on our recently published study regarding the nonexistence of root dentinal microcracks in a fresh cadaver-based experimental model (De-Deus et al. 2019). Our point-by-point responses to each comment raised in the letter are as follows:
- ‘The study was performed on young adult cadavers (mean age 31). This fact should be explicitly mentioned in the abstract and conclusions. It is the older teeth that typically exhibit cracks and these are the teeth often treated. The authors acknowledge that the sampling used in their study is limited, but only later in the discussion. Clearly the inclusion of older individuals could radically change the results of the study’.
Identifying the limitations of a study is an essential component of any research report as it informs the design and implementation of future studies, thus providing the opportunity for researchers to consider more innovative and improved ways to conduct new experiments. In addition, self-identification of the limitations or shortcomings of a study confirms that their potential impact on the findings was considered carefully. Guidelines for scientific writing suggest that the limitations of a study should be addressed in the Discussion section (Gastel & Day 2017). As a consequence of the letter, we reviewed the last 40 studies published on dentinal microcracks in peer-reviewed journals and none stated their limitations in the Abstract and/or Conclusions as implied in the letter.
In their letter, Zaslansky and colleagues stated that ‘Clearly the inclusion of older individuals could radically change the results of the study’. The use of the word ‘Clearly’ does not appear to be appropriate as it is not supported by the current best available evidence on this issue. In other words, there is no clear evidence suggesting that age is a critical factor for either the presence or creation of new root dentine microcracks. Considering the current body of evidence on this complex issue, we do not believe that the use of the cadavers of young adult undermines the overall conclusion of the study.
In summary, the Discussion section in our paper includes a reference to the potential impact of the age of the cadavers; indeed, the text reads: However, it is necessary to emphasize that the sampling used in the current study has one limitation, the age range of the cadavers which was between 19 and 44 years old (mean age, 31 years). Therefore, future work should focus on assessing the presence of dentinal defects in older cadavers. Thus, we believe the issue of the age of the cadavers was addressed directly and was acknowledged as a potential limitation in an appropriate position within the manuscript. Moreover, we hope that our study inspires other experienced research groups worldwide to plan and assess the status of dentine in teeth from older fresh cadavers through the gold standard non-destructive analytical method, that is micro-CT.
2. ‘The authors loosely refer to the term “high resolution”. However, the pixel size used was 13 microns, suggesting that the resolution is in the order of 25 microns or worse and that only gaps larger than this are detectable. Consequently, any cracks where the edges are closer than this are invisible in this study. Although this resolution may be considered “high” by some, much higher resolutions are available nowadays and are probably needed for this purpose (Moinzadeh et al. 2016)’.
It is curious that Zaslansky and colleagues have reduced the complex concept of ‘high resolution’ into a simple reference to ‘pixel size’. By definition, the analytical tool used in this study, micro-CT, is also termed high resolution X-ray CT (Stock 2009). Technically, the term ‘high resolution’ is not directly correlated to pixel size, but to a combination of the spatial resolution of the device and contrast resolution of the object (density and thickness) and device (energy, current and exposition time). Contrast resolution is a measure of how well a feature can be distinguished from the neighbouring background, whilst spatial resolution describes how well small details can be imaged or small features can be located with respect to a reference point. Therefore, the interplay of contrast sensitivity and spatial resolution defines what can be achieved with a CT scan (Stock 2009). Obviously, because the actual resolution needed for a particular application depends on the microstructural features of interest and their shapes, there are several devices in the market with different features aiming to cover various applications. In our study, a SkyScan 1173 micro-CT device was used. This equipment reaches a spatial resolution of 8 lm corresponding to an approx. 5 9 10—7 cubic mm voxel size (Fitri et al. 2016); however, according to the manufacturer, the 3D spatial resolution detectability of a SkyScan 1173 is still high (4–5 lm in high contrast resolution).
In summary, (i) it is a misconception to believe that only the pixel size determines what is identifiable in an output image from a micro-CT scan. Consequently, the term ‘high resolution’ was not referred to ‘loosely’, but applied properly; (ii) Zaslansky and colleagues are correct that we scanned with 13 microns, which means that the pixel size is in the order of 25 microns and that only defects larger than this are observable. However, since 2016 the resolution parameters used were validated by our group in terms of their ability to detect root dentinal microcracks. It has been demonstrated experimentally that all micro-cracks observable through direct optical microscopy are also observed on micro-CT images scanned with a pixel size of 14.16 lm (De-Deus et al. 2016) (Fig. 1). It is of note that one of the authors of the letter is a co-author of a recent publication on dentinal microcracks using micro-CT technology in which the pixel size was 17.18 lm (PradeepKumar et al. 2019).
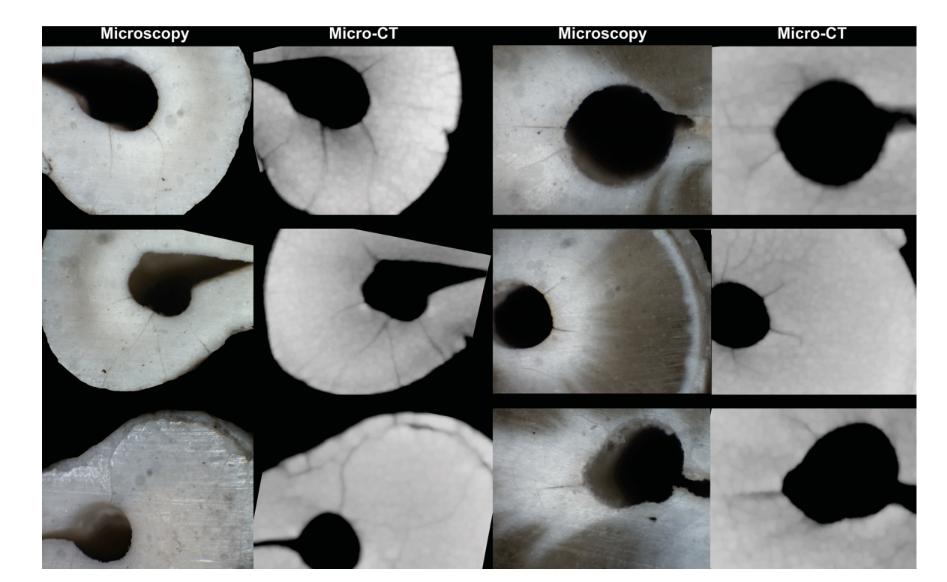
3. ‘The abstract claims that in more than 65000 cross sectional images from 178 teeth, no dentinal cracks were detected. This calls for some attention to the concept of contrast. Specifically, it has been shown by Rödig et al. (2018) that cracks observed in dry roots become invisible in hydrated specimens’.
There are two factors able to explain the term contrast:
- The first factor deals with a key point: Is it possible to ‘see’ root dentinal microcracks in micro-CT images? For that, we developed a validation experiment (a pilot study to confirm the experimental method was sound), which consisted of the experimental induction of dentinal microcracks in a molar tooth removed atraumatically from the alveolar bone of a cadaveric model. After several weeks of induced dehydration, the molar tooth was replaced inside the socket in the alveolar bone and rescanned using the same parameters. The artificially induced microcracks were clearly observed in the cross-sectional micro-CT images.
- although the findings on moisture content of dentine by Rödig et al. (2018) are interesting, their experimental conditions varied substantially from ours. They investigated the impact of wet storage conditions using a moist foam in an uncontrolled relative humidity, which is quite different from the relative humidity in cadaveric or in vivo environments. Moreover, Rödig and co-workers demonstrated that the drier the conditions, the greater the ability of micro-CT scanning to detect microcracks, to quote the authors: ‘Significantly more microcracks were identified after 24 h than after 2-h dry time’. In contrast, it is interesting to note that dentinal microcracks were not observable during the validation experiment in our study even after 10 weeks of a slow dehydration process. Therefore, we are convinced that the validation experiments performed prior to our main study confirmed that all microcracks are able to be observed under the current experimental conditions, that is, a tooth inside a bone block with the scanning parameters used, and that the visualization of microcracks was not affected by the contrast issues related to the relative humidity of the specimens.
4. ‘This is because lab micro-CT has strong limitations in contrast, as shown previously (Zaslansky et al. 2011)’.
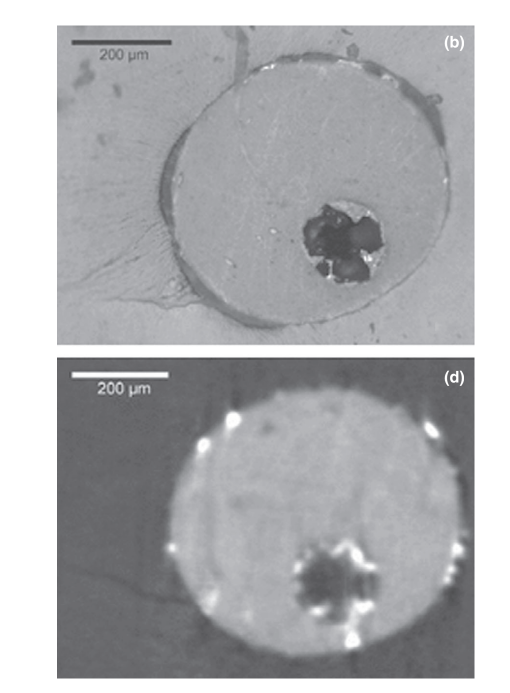
Zaslansky et al. (2011) used micro-CT to evaluate the interfaces within root canal walls and filling materials, which is a completely different and technically more challenging than dentinal microcracks, because of the presence of dense materials within the root canal space. Even using a device (SkyScan 1072) which allows a higher spatial resolution (5 lm corresponding to an approx. 1 x 10-7 cubic mm voxel size) than the SkyScan 1173 we used, the output quality of the image presented by the authors (Fig. 2) is clearly poorer than ours and may be as a consequence of the parameters chosen for scanning and reconstruction. On the other hand, we agree that the contrast resolution of micro-CT devices is limited when compared with synchrotron-based tomography. However, these limitations cannot be considered ‘strong’ as stated by Zaslansky and colleagues since unexpectedly fine-grade data from CT images have been extracted despite these limitations (Johns et al. 1993). It is already known that, because of the inherent resolution limitations of X-ray CT, all material boundaries are blurred to some extent, and thus, the material in any one voxel can affect CT values of adjacent voxels (Ketcham & Carlson 2001). This is termed the partial-volume effect. Partial volume effects were used by Johns et al. (1993) to measure crack sizes in crystalline rocks down to a scale that is considerably finer than even the pixel dimensions. Ketcham & Carlson (2001) also demonstrated that individual fractures that appear on the scan (100-lm slice) through a fractured limestone had widths that were significantly smaller (up to 5 lm) than the pixel dimensions (42 lm) (see fig. 6 in their study). Thus, the methodology proposed and used by our group is scientifically based and also validated in other fields of science to evaluate a phenomenon with similar dimensions and features to root dentine microcracks.
5. ‘The abstract conclusion is misleading: “This in situ cadaveric model revealed the lack of pre-existing dentinal microcracks in non-endodontically treated teeth. Thus, the finding of dentinal microcracks observed in previous cross-sectional images of stored extracted teeth is unsound and not valid”. The fact that the authors did not find cracks in their sample is not equivalent to the claim that previous results are “not valid”. This appears to be an “argumentum ad ignorantiam”: absence of evidence is not evidence of absence’.
We disagree that there is ‘absence of evidence’ to conclude that the inferences from root sectioning studies on root dentinal microcracks are unsound and not valid. In the framework of this subject, the phenomenon of microcracks with root sectioning method fits the thoughts evoked by H. L. Mencken (1917): ‘For every complex problem there is an answer that is simple, clear and invariably wrong’. In fact, a rule of thumb in science dictates that the more complex and sophisticate the (research) method is, the better its reliability is. Our group have published several studies both on stored extracted teeth and on teeth from cadavers using nondestructive micro-CT evaluation, and all of them convey the same conclusion: no new microcracks were induced by root canal instrumentation or canal filling (De-Deus et al. 2014, 2015, 2016, 2017a,b, Zuolo et al. 2017). Moreover, there are other studies using micro-CT confirming that root dentinal microcracks are not related to mechanical shaping of the canal (Bayram et al. 2017a,b, Oliveira et al. 2017, Rödig et al. 2019, Uğur Aydın et al. 2019). Taken together, these reproducible micro-CT outcomes largely contrast with the results of previous cross-sectioning studies and therefore provide strong evidence to support the rationale of our conclusion. Moreover, logical reasoning dictates that the main outcome of both methodologies (micro-CT vs. root sectioning) cannot coexist and be both accepted by the scientific community; one methodology makes the other naturally invalid. Since micro-CT is a reliable and experimentally sound analytical tool widely used and accepted in several fields, it is necessary to discuss and emphasize the limitations of destructive and simplistic research designs used to study a multifaceted and complex phenomenon such as root dentinal microcracks. However, the burden to demonstrate the effectiveness of cross-sectional methods does not rest on our shoulders. Zaslansky and colleagues appear to be arguing that the results from root sectioning studies are somehow valid, which gives the impression of a ‘reductio ad absurdum’ argument by them implying: ‘Accepting micro-CT outcome does not allow cross-sectional results to be refuted’. However, with this claim Zaslansky and colleagues forget that both methods cannot coexist as both cannot be valid scientifically.
6. ‘The authors further conclude that “it should be assumed that microcracks observed in stored extracted teeth subjected to root canal treatments are a result of the extraction process.. .” which is not supported by their own results. While teeth may crack or fracture during extraction, this is not necessarily the case, as demonstrated by these authors where teeth scanned both in bone and after extraction showed no cracks’.
First, it is important to emphasize that the expression we used, ‘it should be assumed’, was adopted to confirm that our conclusions are an extrapolation of what was observed experimentally. In addition, this assumption was raised previously in a study that Zaslansky and colleagues used to support some of the arguments in their letter (Arias et al. 2014). Finally, selecting a part of a sentence and ignoring the remainder can lead to a misinterpretation of the entire sentence and the meaning it was trying to convey. The complete sentence reads ‘In the meantime, until proven otherwise, it should be assumed that dentinal microcracks observed in stored extracted teeth subjected to root canal procedures are in fact a result of the extraction process and/or the post-extraction storage conditions’.
7. ‘While we agree with the authors that post-extraction storage conditions need to be considered carefully, it is unrealistic to expect all future endodontic research on this subject to be performed on fresh cadavers especially when all bureaucratic and ethical aspects are considered’
We are glad that Zaslansky and colleagues agree that time-consuming and challenging experimental steps such as the post-extraction storage conditions need to be considered carefully. It is important to underline that we strongly believe that the use of fresh cadavers should be regarded as a real possibility for future research for several aspects of Endodontics. One of the critical problems in the study of root dentinal microcracks was created by the use and trust in an overly simplistic experimental model. It is fair to say that complex and more sophisticated methods are a natural consequence of scientific evolution and better-quality translational research. Hence, it is timely to quote the Editorial entitled ‘Improving the design, execution, reporting and clinical translation of laboratory-based studies in Endodontology’ recently published in the IEJ by Nagendrababu et al. (2019): ‘Our professional research duty is to stop ignoring poor design, faulty execution, imprecise reporting and unclear clinical translation that laboratory-based studies have with endodontic clinical practice’. That said, we hope that the experimental model developed in our study plus the thought-provoking character of the results encourage the use of better-quality sampling [e.g. teeth still inside bone blocks of fresh cadavers], at least by the most experienced and consolidated research groups worldwide. This is indeed expected in a growing biomedical area such as Endodontics and proven by several studies using cadavers already published in the field.
8. ‘In fact, evidence suggest that cadavers of old individuals exhibit large number of preoperative cracks (Arias et al. 2014)’.
We do not agree that evidence suggests that cadavers of old individuals have larger numbers of preoperative cracks. In the study of Arias et al. (2014), quoted by Zaslansky and colleagues, preoperative microcracks were counted after root sectioning and observed under direct optical microscopy, a destructive method with pitfalls that led to flawed conclusions, as demonstrated experimentally by Stringheta et al. (2017). It is of note that Arias et al. (2014) was a pilot study with only six teeth that focussed on the challenge of using cadavers to achieve a better experimental model, which encouraged us to use better-quality specimens and a refined experimental model. As commented previously, the limitation regarding the average age of the cadavers used in our study was clearly addressed in the Discussion section of our manuscript.
9. ‘Considering the information shown in their own paper, we put forward that the abstract should reflect the uncertainty in the data as to not mislead the uninformed reader’.
Our conclusions were not based ‘only’ on the results of our paper alone, but on the robust data on this topic reported in numerous articles published in the last 6 years by our group (De-Deus et al. 2014, 2015, 2016, 2017a,b, Zuolo et al. 2017). The Discussion section considered and examined in depth all the various issues that could have impacted on the results giving the so-called ‘uninformed reader’ all the relevant information in this part of the article. Interestingly, the findings of our study were validated recently by the publication of PradeepKumar et al. (2019), in which one author of this letter collaborated.
We would like to end our response by focusing on what really matters: the interplay between the use of prime-quality sampling (fresh cadavers) and a gold standard analytical tool (micro-CT) in a complex but also a close-to-ideal experimental model to assess the status of dentine. We believe it is timely to shift the burden of proof on this topic. The burden of proof usually occurs when a phenomenon is assumed to be true because it has not yet been proven to be false. Up to now, the results from a close-to-ideal method have not identified any dentinal microcracks; furthermore, there is no proof of the existence of root dentinal microcracks in the clinical setting. In other words, thus far, root dentinal microcracks are a phenomenon only and uniquely observable under laboratory experimental conditions, which questions their existence in real life. Hence, we wish to shift the burden of proof and suggest the need to draw fundamental attention to the as yet unproven clinical occurrence of root dentinal microcracks.
In the meantime, we restate that such phenomenon observed in stored extracted teeth should be referred to as experimental root dentinal microcracks.
Authors: G. De-Deus, D. M. Cavalcante, F. G. Belladonna, J. Carvalhal, E. M. Souza, R. T. Lopes, M. A. Versiani, E. J. N. L. Silva, P. M. H. Dummer
References:
- Arias A, Lee YH, Peters CI, Gluskin AH, Peters OA (2014) Comparison of 2 canal preparation techniques in the induction of microcracks: a pilot study with cadaver mandibles. Journal of Endodontics 40, 982–5.
- Bayram HM, Bayram E, Ocak M, Uygun AD, Celik HH (2017a) Effect of ProTaper gold, self-adjusting file, and XP-endo shaper instruments on dentinal microcrack formation: a micro-computed tomographic study. Journal of Endodontics 43, 1166–9.
- Bayram HM, Bayram E, Ocak M, Uzuner MB, Geneci F, Celik HH (2017b) Micro-computed Tomographic Evaluation of Dentinal Microcrack Formation after Using New Heat-treated Nickel-titanium Systems. Journal of Endodontics 43, 1736–9.
- De-Deus G, Silva EJ, Marins J et al. (2014) Lack of causal relationship between dentinal microcracks and root canal preparation with reciprocation systems. Journal of Endodontics 40, 1447–50.
- De-Deus G, Belladonna FG, Souza EM et al. (2015) Micro-computed tomographic assessment on the effect of ProTaper Next and Twisted File Adaptive systems on dentinal cracks. Journal of Endodontics 41, 1116–9.
- De-Deus G, Belladonna FG, Marins JR et al. (2016) On the causality between dentinal defects and root canal preparation: a micro-CT assessment. Brazilian Dental Journal 27, 664–9.
- De-Deus G, Belladonna FG, Silva EJNL et al. (2017a) Micro-CT assessment of dentinal micro-cracks after root canal filling procedures. International Endodontic Journal 50, 895–901.
- De-Deus G, Carvalhal JCA, Belladonna FG et al. (2017b) Dentinal microcrack development after canal preparation: a longitudinal in situ micro-computed tomography study using a cadaver model. Journal of Endodontics 43, 1553–8.
- De-Deus G, Cavalcante DM, Belladonna FG et al. (2019) Root dentinal microcracks: a post-extraction experimental phenomenon? International Endodontic Journal 52, 857–65.
- Fitri LA, Asyana V, Ridwan T et al. (2016) Dual energy micro CT SkyScan 1173 for the characterization of urinary stone. Journal of Physics: Conference Series 694, 012053.
- Gastel B, Day RA (2017) How to Write and Publish a Scientific Paper, 8th edn. Cambridge: Cambridge University Press, p 344.
- Johns RA, Steude JS, Castanier LM, Roberts PV (1993) Non-destructive measurements of fracture aperture in crystalline rock cores using X-ray computed tomography. Journal of Geophysical Research 98, 1889–900.
- Ketcham RA, Carlson WD (2001) Acquisition, optimization and interpretation of X-ray computed tomographic imagery: applications to the geosciences. Computers G Geo-sciences 27, 381–400.
- Mencken HL (1917) The Divine Afflatus. New York: Evening Mail.
- Moinzadeh AT, Farack L, Wilde F, Shemesh H, Zaslansky P (2016) Synchrotron-based Phase Contrast-enhanced Micro-Computed Tomography Reveals Delaminations and Material Tearing in Waterexpandable Root Fillings Ex Vivo. Journal of Endodontics 42, 776–81.
- Nagendrababu V, Murray PE, Ordinola-Zapata R et al. (2019) Improving the design, execution, reporting and clinical translation of laboratory-based studies in Endodontology. International Endodontic Journal 52, 1089.
- Oliveira BP, Câmara AC, Duarte DA, Heck RJ, Antonino ACD, Aguiar CM (2017) Micro-computed tomographic analysis of apical microcracks before and after root canal preparation by hand, rotary, and reciprocating instruments at different working lengths. Journal of Endodontics 43, 1143–7.
- PradeepKumar AR, Shemesh H, Archana D et al. (2019) Root canal preparation does not induce dentinal microcracks in vivo. Journal of Endodontics. [Epub ahead of print].
- Rödig T, Müller C, Hoch M et al. (2018) Moisture content of
- root canal dentine affects detection of microcracks using micro-computed tomography. International Endodontic Journal 51, 357–63.
- Rödig T, Krämer J, Müller C, Wiegand A, Haupt F, Rizk M (2019) Incidence of microcracks after preparation of straight and curved root canals with three different NiTi instrumentation techniques assessed by micro-CT. Australian Endodontic Journal doi: 10.1111/aej.12339.
- Stock SR (2009) Microcomputed Tomography: Methodology and Applications. Boca Raton: CRC Press.
- Stringheta CP, Pelegrine RA, Kato AS et al. (2017) Micro-computed tomography versus the cross-sectioning method to evaluate dentin defects induced by different mechanized instrumentation techniques. Journal of Endodontics 43, 2102–7.
- Uğur Aydın Z, Keskin NB, Özyürek T (2019) Effect of Reciproc blue, XP-endo shaper, and WaveOne gold instruments on dentinal microcrack formation: A micro-computed tomographic evaluation. Microscopy Research and Technique 82, 856–60.
- Zaslansky P, Fratzl P, Rack A, Wu MK, Wesselink PR, Shemesh H (2011) Identification of root filling interfaces by microscopy and tomography methods. International Endodontic Journal 44, 395–401.
- Zuolo ML, De-Deus G, Belladonna FG et al. (2017) Micro-computed tomography assessment of dentinal microcracks after root canal preparation with TRUShape and Selfadjusting file systems. Journal of Endodontics 43, 619–22.
