Dentinal Microcrack Development after Canal Preparation: A Longitudinal in Situ Micro–computed Tomography Study Using a Cadaver Model
Abstract
Introduction: The purpose of this study was to evaluate the development of dentinal microcracks after root canal preparation with Reciproc and ProTaper Universal systems using an in situ cadaver model by means of a micro–computed tomography (micro-CT) imaging system. Methods: At autopsy, 8 maxillary bone blocks having at least the first and second premolar teeth (n = 16) were excised, scanned at a resolution of 13.18 mm, and randomly distributed into 2 groups (n = 8) according to the preparation protocol: Reciproc and ProTaper Universal systems. Root canals were prepared up to R25 and F2 instruments in the Reciproc and ProTaper Universal groups, respectively. After the preparation procedures, the specimens were scanned again, and the registered preoperative and postoperative cross-section images of the roots (n = 19,060) were screened to identify the presence of dentinal defects. Results: In the Reciproc group, 9176 cross-section images were analyzed, and no crack was observed. In the ProTaper Universal group, 244 of 9884 cross-section slices (2.46%) had dentinal defects; however, all defects were already present in the corresponding preoperative images, indicating that no new microcrack was created after canal preparation. Conclusions: In situ root canal preparation of maxillary premolars with Reciproc and ProTaper Universal systems did not induce the formation of dentinal microcracks in a cadaver model as observed by micro–CT. (J Endod 2017;43:1553–1558)
In recent years, the occurrence of root fracture in either sound or endodontically treated/restored teeth has become amajor concern in endodontics. Root fracture has been defined as a devastating clinical event, and it is currently one of the leading causes of tooth loss. Throughout the years, several hypothetical etiologies for root fracture were suggested including hypotheses that the root fracture would start from dentinal micro- cracks caused by dentin dehydration, post placement and corrosion, spreader design, or excessive forces during filling procedures. Some years later, Bier et al and Shemesh et al also correlated dentinal microcrack formation to root canal preparation performed by motor-driven nickel-titanium (NiTi) instruments. Because the mechanical preparation of the root canal has become the mainstream for root canal shaping, it is not a surprise that this phenomenon has increasingly gained importance in the endodontic research field Overall, the methodology used in most of the ex vivo studies on dentinal micro-crack formation includes the sectioning of the sample, followed by postoperative observation of the exposed dentinal surface by using optical microscopic devices. However, this experimental model has some critical limitations that reduce its overall reliability, such as the destructive nature of the method, the two-dimensional observation, the absence of a full-tooth range inspection, and the lack of longitudinal follow-up, because it does not allow for the screening of the non-prepared sample. In this way, it is unlikely that the results reported in most of these studies, in which cracks were observed in more than 40% of the samples, would reflect the clinical reality. Scientific logic behind this inconclusive scenario would dictate that limitations of the conventional methods are indeed prone to systematic analysis errors and, consequently, far from an ideal experimental model.
Recent technological advances in the field of imaginology, such as the introduction of micro–computed tomography (micro-CT) in dental research, have led to a more comprehensive understanding regarding dentinal microcrack formation. Micro-CT is a highly accurate and nondestructive technology that allows the longitudinal assessment of the specimens throughout the experimental procedures; consequently, each tooth serves as its own control, hundreds of slices can be evaluated per specimen, and all extension of the defects can be tracked. By using this method, De Deus et al showed a clear lack of causal relationship between dentinal microcrack development and canal preparation with rotary and reciprocating systems. This conclusion was later confirmed by other studies using the same methodology. However, authors reported a significant number of preexistent defects on the roots probably caused by excessive extraction forces and/or storage condition of the teeth. Consequently, these conditions also do not stand for a close-to-ideal experimental model. Thus, even with a considerable body of evidence accumulated during the last 30 years, several aspects regarding crack formation and endodontic procedures remain inconclusive, and critical questions are still open. Recently, a cadaveric model was suggested as an ideal methodological approach for a comprehensive evaluation of dentinal microcrack formation because the viscoelastic properties of the attachment apparatus would absorb the forces applied to the dental tissues during root canal preparation procedures.
To the best of the authors’ knowledge, the current scientific literature lacks a nondestructive in situ longitudinal experimental report on this issue. Therefore, this study was designed to investigate the potential cause-effect relationship between root canal preparation performed by 2 motor-driven NiTi systems (Reciproc; VDW, Munich, Germany and ProTaper Universal; Dentsply Maillefer, Ballaigues, Switzerland) and dentinal microcrack formation in a cadaver model by using micro-CT technology.
Materials and Methods
Sample Size Calculation
The ideal sample size for this cadaver model on microcrack formation was calculated on the basis of the study of Arias et al. The estimated 3.125 effect size was input together with an alpha-type error of 0.05 and a power-beta of 0.95 into a t test for independent means statistical family (G*Power 3.1 for MacIntosh). The results pointed to a minimum total sample size of 8 teeth to observe differences in microcracks between the groups.
Sample Selection
Eight dentoalveolar maxillary bone blocks containing 3–5 adjacent teeth were collected from autopsy of different adult donors after family member’s informed consent obtained under a research protocol approved by the local Forensic Department and the National Committee on Health Research Ethics (protocol #931.732). The age of the donors ranged from 19 to 30 years (mean age, 23 years). Inclusion criteria were the presence of non-carious maxillary first and second premolars surrounded by alveolar bone and periodontal ligament. Bone blocks were kept stored in —20◦C and submitted to the experimental procedures within 1 month from their collection.
Before the scanning procedures, frozen bone blocks were removed from the freezer and put into a refrigerator at a constant temperature of 8◦C for a slow defrost. After 3–4 hours, each bone block was scanned in a micro-CT device (SkyScan 1173; Bruker-microCT, Kontich, Belgium) by using an isotropic resolution of 13.18 mm at 90 kV and 88 mA through 360◦ rotation around the vertical axis, with a rotation step of 0.5◦, camera exposure time of 1000 milliseconds, and frame averaging of 5. The x-rays were filtered with a 1-mm-thick aluminum filter. The acquired images were reconstructed into cross-sectional slices with NRecon v.1.6.10 software (Bruker-microCT) by using standardized parameters for beam hardening (15%), ring artifact correction of 5, and contrast limits (0.0095–0.03), resulting in the acquisition of 1100–1300 transverse cross sections per bone block.
Root Canal Preparation
After the scanning and reconstruction procedures, the first and second maxillary premolars from each bone block were selected for the experimental procedures (n = 16). The first premolars had 2 canals, whereas the second premolars had only 1 root canal. After conventional access cavity preparation, the working length (WL) was established 1 mm from the apical foramen by using a size 10 K-file (Dentsply Maillefer) with the aid of an apex locator (Root ZX; J Morita USA Inc, Irvine, CA) and confirmed by digital radiograph. After that, glide path was established by scouting a stainless steel size 15 K-file (Dentsply Maillefer) up to the WL. Then, teeth were randomly assigned to 2 experimental groups (n = 8). In the Reciproc group, R25 instrument (25/0.08) was activated in reciprocating motion (VDW Silver; VDW) and moved in the apical direction with light apical pressure by using a slow in-and-out pecking motion of about 3 mm in amplitude. After 3 pecking motions, the instrument was removed from the canal and cleaned. The WL was reached in the third wave of instrumentation for all teeth. In the ProTaper Universal group, SX instrument was used to one half of the WL, followed by S1, S2, F1, and F2 instruments to the full WL, with a gentle in- and-out motion (VDW Silver), according to the manufacturer’s instructions (SX, S1, and S2, 300 rpm and 3 Ncm; F1 and F2, 300 rpm and 2 Ncm).
Each set of instruments was used to enlarge 2 teeth, and an experienced operator performed all experimental procedures after substantial training with the systems. During preparation, a total of 30 mL 2.5% sodium hypochlorite was delivered in each root canal by using a 31-gauge NaviTip double sideport needle (Ultradent Products Inc, South Jordan, UT). A final irrigation with 5 mL 17% EDTA and 5 mL bidistilled water, followed by drying with absorbent paper points (Dentsply Maillefer), was performed. Then, the bone blocks were submitted to a new scan and reconstruction applying the initial parameter settings.
Image Analysis
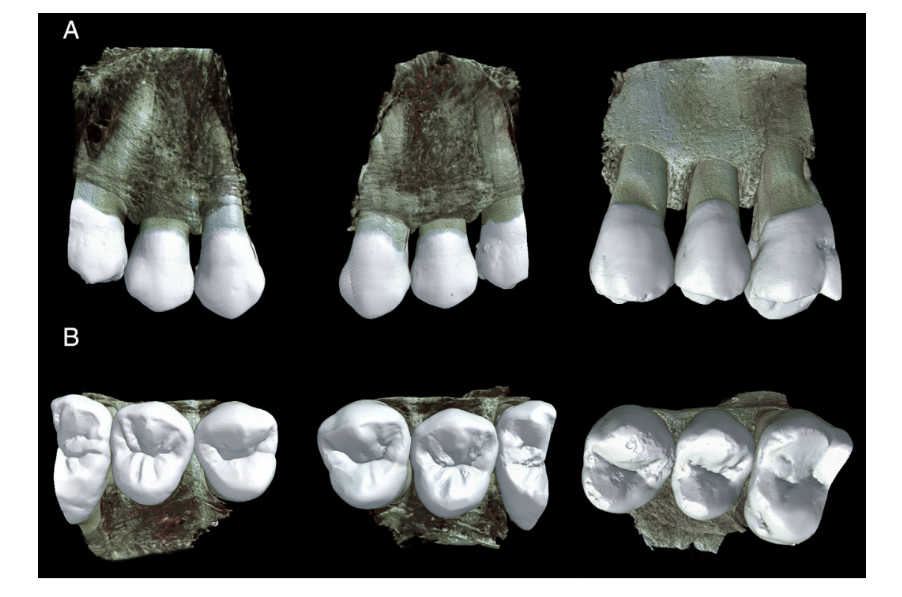
The reconstructed image stacks of the bone blocks before and after canal preparation were co-registered by using the affine algorithm of the 3D Slicer v.4.6.2 software (available from http://www.slicer.org). CTVol v.2.3 (Bruker-microCT) was used for the three-dimensional visualization and qualitative analysis of the bone blocks (Fig. 1). Then, all cross-section images of the premolar teeth (n = 19,060) were screened from the cementoenamel junction to the apex by 3 previously calibrated examiners who were blinded to the experimental groups, aiming to identify the presence of dentinal defects. To validate the screening process, image analyses were repeated twice at 2-week intervals; in case of divergence, images were examined together until an agreement was reached.
Results
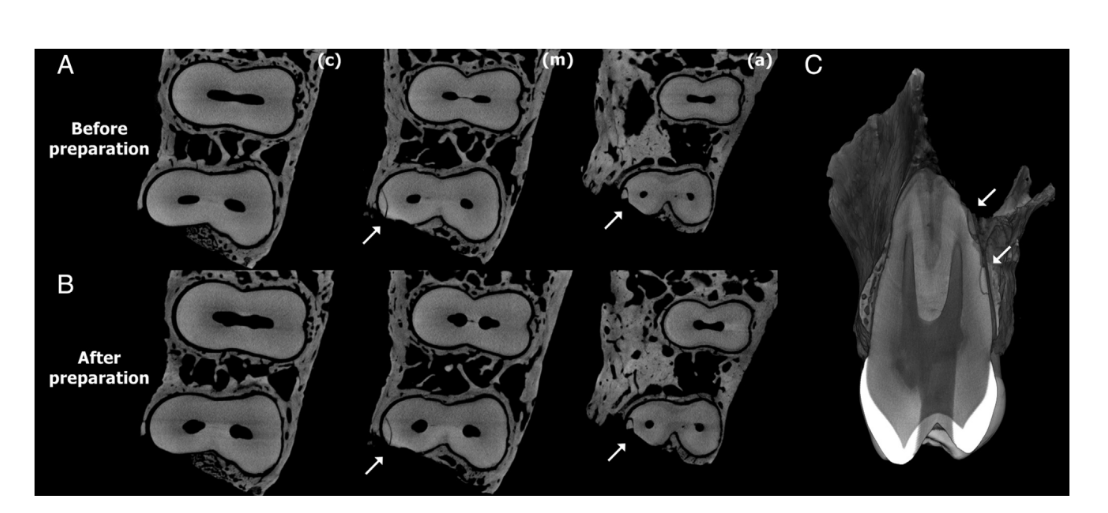
Figures 2 and 3 show representative images from coronal, middle, and apical thirds of the roots of the premolar teeth before and after preparation with Reciproc and ProTaper Universal systems, respectively. In the Reciproc group, 9176 cross-section images were analyzed, and no crack was observed. In the ProTaper Universal group, 244 of 9884 cross-section slices (2.46%) had dentinal defects. These defects were observed in only 1 tooth and were already present in the corresponding preoperative images (Fig. 4).
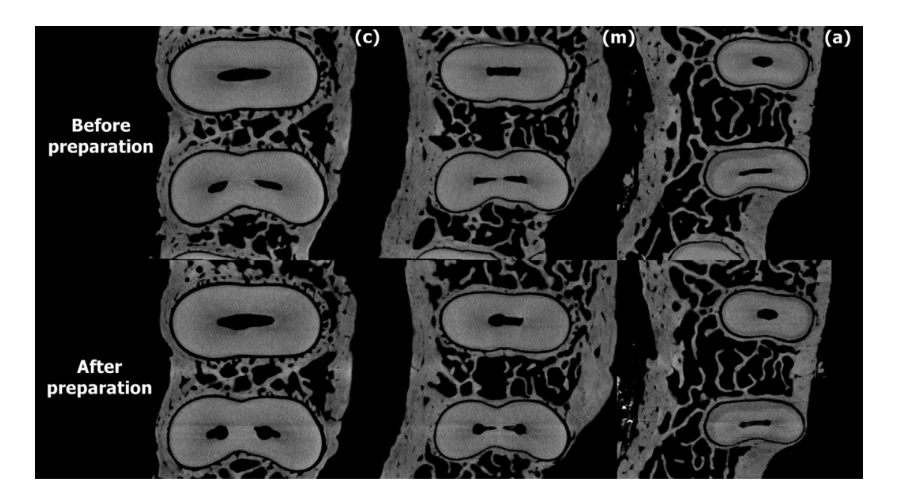
with Reciproc system.
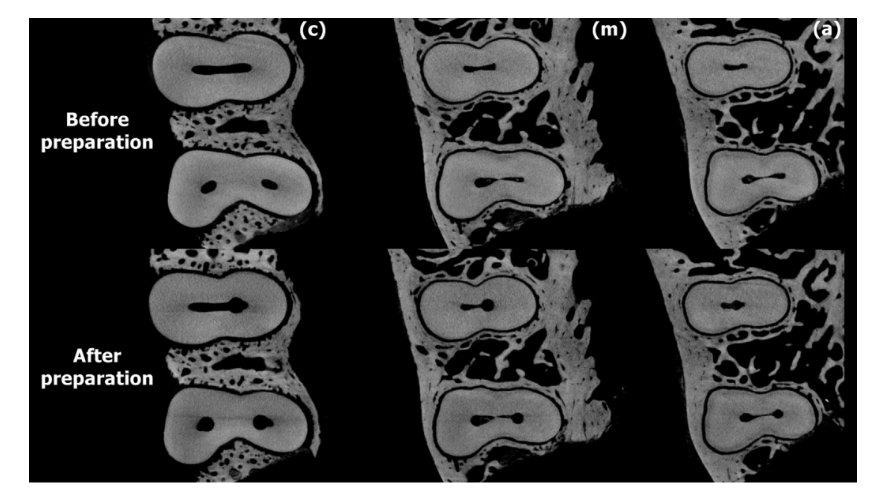
with ProTaper Universal system.
Discussion
In the current study, an in situ cadaver model was used to study the effect of root canal preparation with 2 well-established NiTi systems (Reciproc and ProTaper Universal) on the development of dentinal defects through micro-CT imaging. The dentinal defects observed after preparation procedures were already present in the corresponding preoperative images, indicating that no new micro- crack was created by the tested systems. In fact, the defects were located at the border of just 1 tooth (Fig. 4), and these cracks were probably caused during the procedures to remove the bone block from the jaw. Therefore, the mechanical enlargement of the root canal system of maxillary premolars in this study could not be associated with microcrack formation. The present results markedly contrast with several previous publications that have demonstrated a clear correlation between root canal preparations and the initiation and/or propagation of dentinal cracks.
Nowadays, most of the studies correlating mechanical preparation and the development of dentinal defects are based on root sectioning methods and direct observation by optical microscopy. ProTaper Universal and Reciproc instruments used in rotation and reciprocation, respectively, were assessed herein because of the contrasting reports on their impact on root dentin resulting in dentinal defects. In these studies, the incidence of dentinal microcracks after preparation with ProTaper Universal system up to the F2 instrument ranged from 50% to 80%, whereas preparation with
Reciproc R25 instruments caused cracks in 5% to 65% of the sample. This high incidence of cracks observed after canal preparation with these instruments is far from the reality of the clinical setting, suggesting a major drawback in this destructive experimental model. Therefore, this discrepancy of results can be fairly explained by meaningful differences between the experimental models.
Although the use of non-prepared teeth as controls appears to validate the main conclusions of the root-sectioning studies, these groups were only able to control the mechanical stresses caused by the mechanical NiTi preparation system per se, leaving aside the interplay as well as the accumulative effect of all sources of stresses in which the experimental groups were submitted, such as the chemical attack of sodium hypochlorite–based irrigation and the sectioning procedure. Interestingly, dentinal defects have been also reported in the untreated control teeth in 2 root-sectioning studies, and authors explained their presence was because of forces induced during extraction, excessive loads caused by occlusal dysfunction before extraction, previous trauma, and/or the cutting procedures. It is important to consider that because conventional sectioning techniques allow the evaluation of only a few slices per tooth, there is a real chance of missing existing defects along the root, which means that control groups in these studies were likely to underestimate the presence of pretreatment microcracks. On the other hand, a markedly different outcome was reported by a previous study using micro-CT technology, in which 9016 slices with dentinal defects were observed in the control group, demonstrating the reliability of this method.
An additional concern regarding studies on crack generation is related to the conditions of the sample storage, because the biomechanical response of root dentin to root canal instrumentation was recently demonstrated to be influenced by dentin hydration. In hydrated roots, instrumentation with hand, reciprocating, or rotary NiTi instruments did not result in residual microstrain concentrations. Considering that crack propagation may continue in root slices even after 1 month of storage with no further stress over dentin, the baseline status of the sample is crucial for the reliability of the laboratory studies on dentinal microcrack development. The necessary temperature of storage is not exactly defined by scientific data, and the effect of different storage temperatures on the biomechanical and the biological behavior is discussed controversially. There is no international agreement, general regulations, or standards of tissue banking on a specific storage temperature for teeth. Recent statements of the American Association of Tissue Banks recommend a storage temperature of —20◦C for up to 6 months of storage and —40◦C for longer periods of deep frozen preservation. Actually, the influences of duration of storage and freezing temperature on biomechanical properties of teeth are not entirely understood and are still to be determined. In this study, storage temperature of —20◦C, as recommended by American Association of Tissue Banks, and slow defrost for scanning and preparation procedures did not affect the structure of the bone or teeth.
The main motivation for this study stems from the outcome of 2 in vivo studies and 2 studies using fresh human cadaver models, in which no crack was observed after different procedures when the tooth was kept in situ. According to the authors, the viscoelastic properties of the attachment apparatus would absorb the forces applied to the dental tissues, preventing microcrack formation after different endodontic procedures, which supports the current results. In this study, the use of an in situ fresh cadaver model in which the bone and periodontal ligament remained intact, associated with a highly accurate and nondestructive methodology in the assessment of the specimens, is supported by a previous study in which these methodological approaches were suggested as a more reproducible method to test microcracks. In addition, a step further was taken during sample selection and distribution to reduce the influence of other confounding factors that have been correlated to cracked teeth, such as the storage mean and time, group of teeth, the root and root canal anatomy, the age and sex of the patients, root and canal morphologies, as well as masticatory function and the presence of excursive interferences or any parafunctions that teeth could be subjected to during the patient’s lift. It is expected that the thought-provoking character of the current results may lead other research groups to follow and improve this in situ longitudinal micro-CT methodology, bringing the possibility to better understand the complexity of dentinal microcrack development in teeth.
Therefore, under the conditions of this in situ cadaver model study, it can be concluded that root canal preparation of maxillary pre- molars with Reciproc and ProTaper Universal systems did not induce the formation of dentinal microcracks, as observed by micro-CT imaging.
Authors: Gustavo De-Deus, DDS, MSc, PhD, Júlio César de Azevedo Carvalhal, Felipe Gonçalves Belladonna, MSc,Emmanuel João Nogueira Leal Silva, Ricardo Tadeu Lopes, Renato Evando Moreira Filho, Erick Miranda Souza, José Claudio Provenzano, Marco Aurélio Versiani
References:
- Llena-Puy MC, Forner-Navarro L, Barbero-Navarro I. Vertical root fracture in endodontically treated teeth: a review of 25 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;92:553–5.
- Touré B, Faye B, Kane AW, et al. Analysis of reasons for extraction of endodontically treated teeth: a prospective study. J Endod 2011;37:1512–5.
- Yoshino K, Ito K, Kuroda M, Sugihara N. Prevalence of vertical root fracture as the reason for tooth extraction in dental clinics. Clin Oral Investig 2015;19:1405–9.
- Walton RE, Michelich RJ, Smith GN. The histopathogenesis of vertical root fractures. J Endod 1984;10:48–56.
- Zadik Y, Sandler V, Bechor R, Salehrabi R. Analysis of factors related to extraction of endodontically treated teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:e31–5.
- Petersen KB. Longitudinal root fracture due to corrosion of an endodontic post. J Can Dent Assoc 1971;37:66–8.
- Pitts DL, Matheny HE, Nicholls JI. An in vitro study of spreader loads required to cause vertical root fracture during lateral condensation. J Endod 1983;9:544–50.
- Obermayr G, Walton RE, Leary JM, Krell KV. Vertical root fracture and relative defor-mation during obturation and post cementation. J Prosthet Dent 1991;66:181–7.
- Bier CAS, Shemesh H, Tanomaru-Filho M, et al. The ability of different nickel-titanium rotary instruments to induce dentinal damage during canal preparation. J Endod 2009;35:236–8.
- Shemesh H, Bier CAS, Wu MK, et al. The effects of canal preparation and filling on the incidence of dentinal defects. Int Endod J 2009;42:208–13.
- Bürklein S, Tsotsis P, Schäfer E. Incidence of dentinal defects after root canal preparation: reciprocating versus rotary instrumentation. J Endod 2013;39:501–4.
- Hin ES, Wu M-K, Wesselink PR, Shemesh H. Effects of Self-Adjusting File, Mtwo, and ProTaper on the root canal wall. J Endod 2013;39:262–4.
- Liu R, Hou BX, Wesselink PR, et al. The incidence of root microcracks caused by 3 different single-file systems versus the ProTaper system. J Endod 2013;39:1054–6.
- Arias A, Lee YH, Peters CI, et al. Comparison of 2 canal preparation techniques in the induction of microcracks: a pilot study with cadaver mandibles. J Endod 2014;40: 982–5.
- Arslan H, Karatas¸ E, Çapar ID, et al. Effect of ProTaper Universal, Endoflare, Revo-S, HyFlex coronal flaring instruments, and Gates Glidden drills on crack formation. J Endod 2014;40:1681–3.
- Versiani MA, Souza E, De-Deus G. Critical appraisal of studies on dentinal radicular microcracks in endodontics: methodological issues, contemporary concepts, and future perspectives. Endod Topics 2015;33:87–156.
- De-Deus G, Silva EJNL, Marins J, et al. Lack of causal relationship between dentinal microcracks and root canal preparation with reciprocation systems. J Endod 2014; 40:1447–50.
- De-Deus G, Belladonna FG, Souza EM, et al. Micro-computed tomographic assessment on the effect of ProTaper Next and Twisted File Adaptive systems on dentinal cracks. J Endod 2015;41:1116–9.
- De-Deus G, Belladonna FG, Marins JR, et al. On the causality between dentinal defects and root canal preparation: a micro-CT assessment. Braz Dent J 2016;27: 664–9.
- De-Deus G, Belladonna FG, Silva EJ, et al. Micro-CT assessment of dentinal micro-cracks after root canal filling procedures. Int Endod J 2016 Sep 30; http:// dx.doi.org/10.1111/iej.12706 [Epub ahead of print].
- Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 2012;30: 1323–41.
- Çapar ID, Arslan H, Akcay M, Uysal B. Effects of ProTaper Universal, ProTaper Next, and HyFlex instruments on crack formation in dentin. J Endod 2014;40:1482–4.
- Yoldas O, Yilmaz S, Atakan G, et al. Dentinal microcrack formation during root canal preparations by different NiTi rotary instruments and the Self-Adjusting File. J Endod 2012;38:232–5.
- Ashwinkumar V, Krithikadatta J, Surendran S, Velmurugan N. Effect of reciprocating file motion on microcrack formation in root canals: an SEM study. Int Endod J 2014; 47:622–7.
- Abou El Nasr HM, Abd El Kader KG. Dentinal damage and fracture resistance of oval roots prepared with single-file systems using different kinematics. J Endod 2014;40: 849–51.
- Priya NT, Chandrasekhar V, Anita S, et al. Dentinal microcracks after root canal preparation: a comparative evaluation with hand, rotary and reciprocating instrumentation. J Clin Diagn Res 2014;8:70–2.
- Barreto MS, Moraes Rdo A, Rosa RA, et al. Vertical root fractures and dentin defects: effects of root canal preparation, filling, and mechanical cycling. J Endod 2012;38: 1135–9.
- Lim H, Li FC, Friedman S, Kishen A. Residual microstrain in root dentin after canal instrumentation measured with digital Moir´e interferometry. J Endod 2016;42: 1397–402.
- Adorno CG, Yoshioka T, Jindan P, et al. The effect of endodontic procedures on apical crack initiation and propagation ex vivo. Int Endod J 2013;46:763–8.
- American Association of Tissue Banks. AATB Standards for Tissue Banking (Section E: E4.120 Frozen and Cryopreserved Tissue), 12th ed. McLean, VA: AATB; 2008.
- Calzonetti KJ, Iwanowski T, Komorowski R, Friedman S. Ultrasonic root end cavity preparation assessed by an in situ impression technique. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:210–5.
- Rose E, Svec T. An evaluation of apical cracks in teeth undergoing orthograde root canal instrumentation. J Endod 2015;41:2021–4.
- De Bruyne MA, De Moor RJ. SEM analysis of the integrity of resected root apices of cadaver and extracted teeth after ultrasonic root-end preparation at different intensities. Int Endod J 2005;38:310–9.
