Use of Silver Diamine Fluoride for Dental Caries Management in Children and Adolescents, Including Those with Special Health Care Needs
Abstract
Background: This manuscript presents evidence-based guidance on the use of 38 percent silver diamine fluoride (SDF) for dental caries management in children and adolescents, including those with special health care needs. A guideline workgroup formed by the American Academy of Pediatric Dentistry developed guidance and an evidence-based recommendation regarding the application of 38 percent SDF to arrest cavitated caries lesions in primary teeth.
Types of studies reviewed: The basis of the guideline’s recommendation is evidence from an existing systematic review "Clinical trials of silver diamine fluoride in arresting caries among children: A systematic review." (JDR Clin Transl Res 2016;1[3]:201-10). A systematic search was conducted in PubMed®/MEDLINE, Embase®, Cochrane Central Register of Controlled Trials, and gray literature databases to identify randomized controlled trials and systematic reviews reporting on the effect of silver diamine fluoride and address peripheral issues such as adverse effects and cost. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the quality of the evidence and the evidence-to-decision framework was employed to formulate a recommendation.
Results: The panel made a conditional recommendation regarding the use of 38 percent SDF for the arrest of cavitated caries lesions in primary teeth as part of a comprehensive caries management program. After taking into consideration the low cost of the treatment and the disease burden of caries, panel members were confident that the benefits of SDF application in the target populations outweigh its possible undesirable effects. Per GRADE, this is a conditional recommendation based on low-quality evidence.
Conclusions and practical implications: The guideline intends to inform the clinical practices involving the application of 38 percent SDF to enhance dental caries management outcomes in children and adolescents, including those with special health care needs. These recommended practices are based upon the best available evidence to-date.
Scope and purpose
The guideline intends to inform the clinical practices involving the application of silver diamine fluoride (SDF) to enhance dental caries management outcomes in children and adolescents, ncluding those with special health care needs. Silver diamine fluoride in this guideline’s recommendation refers to 38 percent SDF, the only formula available in the United States. These recommended practices are based upon the best available evidence to-date. However, the ultimate decisions regarding disease management and specific treatment modalities are to be made by the dental professional and the patient or his/her representative, acknowledging individuals’ differences in disease propensity, lifestyle, and environment.
The guideline provides practitioners with easy to understand evidence-based recommendations. The American Academy of Pediatric Dentistry's (AAPD) evidence-based guidelines are being Academy of Medicine (formerly known as the Institute of Medicine) and mandated by the National Guideline Clearinghouse™ (NGC), a database of evidence-based clinical practice guidelines and related documents maintained as a public resource by the Agency for Healthcare Research and Quality (AHRQ) of the U.S. Department of Health and Human Services (USDHHS). Further details about children's appointment organization are accessible for you to learn in our course "Psychology and management of children's behavior".
Health intents and expected benefits or outcomes. The guideline is based on analysis of data included in a recent systematic review and meta-analysis and summarizes evidence of the benefits and safety of SDF application in the context of dental caries management, mainly its effectiveness in arresting cavitated caries lesions† in the primary dentition. Its intent is to provide the best available information for practitioners and patients or their representatives to determine the risks, benefits, and alternatives of SDF application as part of a caries management program. Prevention of new caries lesion development and outcomes in permanent teeth, such as root caries lesion arrest, were not the focus of this guideline; however, because they are of interest and relevant to caries management within the scope of pediatric dentistry, they are mentioned and will be included in future iterations of the guideline as the supporting evidence base increases.
Clinical questions addressed. The panel members used the Population, Intervention, Control, and Outcome (PICO) formulation to develop the clinical questions that will aid practitioners in the use of SDF in primary teeth with caries lesions. Does the application of SDF arrest cavitated caries lesions as effectively as other treatment modalities in primary teeth?
Methods
This guideline adheres to the National Academy of Medicine's guideline standards4 and the recommendations of the Appraisal of Guidelines Research and Evaluation (AGREE) instrument. The guidance presented is based on an evaluation of the evidence presented in a 2016 systematic review published by Gao and colleagues.
Search strategy. Literature searches were used to identify systematic reviews that would serve as the basis of the guideline. Secondly, the results of the searches served as sources of evidence or information on issues related to, but outside the context of, the PICO, such as cost, adverse effects, and patient preferences.
Literature searches were conducted in PubMed®/MEDLINE, Embase®, Cochrane Central Register of Controlled Trials, gray literature, and trial databases to identify systematic reviews and randomized controlled trials of SDF. Search results were reviewed in duplicate at both the title and abstract and the full-text level when warranted. Disagreements were resolved by consensus; if agreement could not be reached, the AAPD Evidence-Based Dentistry Committee (EBDC) overseeing the workgroup was consulted to settle the question. A detailed description of the search strategies is presented in Appendix I.
Inclusion and exclusion criteria. The criteria used to identify publications for use in the guideline were determined by the clinical PICO question. See Appendix I for search strategies. Publications which addressed the use of SDF to arrest caries lesions in primary teeth, regardless of language, merited full-text review; in vitro studies and studies of the use of SDF outside of the guideline’s stated outcomes were excluded. No new randomized controlled trials were identified that warranted updating the meta-analysis found in the systematic review selected as the basis for this guideline.
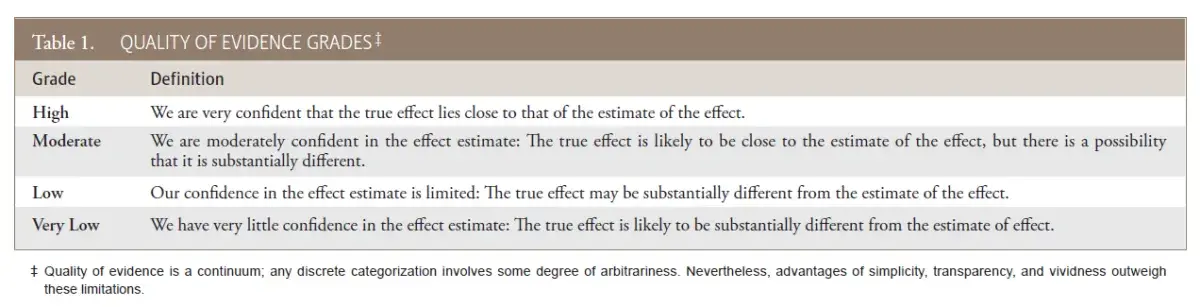
Assessment of the evidence. The main strength of this guideline is that it is based on a systematic review of prospective randomized and controlled trials of SDF1. Evidence was assessed via the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach6, a widely adopted and peer reviewed system of evaluating study quality (Table 1). The guideline recommendation is based on the meta-analysis of four controlled trials (three randomized), extracted in duplicate, from a systematic review of SDF1. Randomized (RCTs) and controlled clinical trials (CCTs) offer the highest level of clinical evidence; therefore, a recommendation based on a systematic review and meta-analysis of graded RCTs/CCTs provides more reliable and accurate conclusions that can be applied towards patient care.
Table 1. Quality of evidence grades‡
 This guideline is limited by the small number of RCTs evaluating SDF, the heterogeneity of the included trials, and selection bias that may have been introduced by possibly poor sequence generation7,8 and selective reporting by one study.
This guideline is limited by the small number of RCTs evaluating SDF, the heterogeneity of the included trials, and selection bias that may have been introduced by possibly poor sequence generation7,8 and selective reporting by one study.
Weaknesses of this guideline are inherent to the limitations found in the systematic review upon which this guideline is based. Major limitations of the supporting literature include lack of calibration and/or evidence of agreement for examiners assessing clinical outcomes and unclear definitions or inconsistent criteria for caries lesion activity. Arguably, without a valid and reliable method to determine lesion activity at baseline and follow-up, misclassification bias is possible, especially because clinicians cannot be blinded with regard to SDF application (due to the dark staining). The absence of rigorous caries detection and activity measurement criteria in the reviewed literature can decrease the validity of the reported results. Other reviewers of the systematic review1 noted similar and additional limitations.
Formulation of the recommendations. The panel formulated this guideline collectively via surveys, teleconferences, and electronic communications from January 2017–August 2017. The panel used the evidence-to-decision framework in an iterative manner to formulate the recommendations. Specifically, the main methods used were discussion, debate, and consensus seeking. To reach consensus, the panel voted anonymously on all contentious issues and on the final recommendation. GRADE was used to determine the strength of the evidence.
Understanding the recommendations. GRADE rates the strength of a recommendation as either strong or conditional. A strong recommendation “is one for which guideline panel is confident that the desirable effects of an intervention outweigh its undesirable effects (strong recommendation for an intervention) or that the undesirable effects of an intervention outweigh its desirable effects (strong recommendation against an intervention).” A strong recommendation implies most patients would benefit from the suggested course of action (i.e., either for or against the intervention). A conditional recommendation “is one for which the desirable effects probably outweigh the undesirable effects (conditional recommendation for an intervention) or undesirable effects probably outweigh the desirableeffects (conditional recommendation against an intervention), but appreciable uncertainty exists.” A conditional recommendation implies that not all patients would benefit from the intervention. The individual patient’s circumstances, preferences, and values need to be assessed more than usual. Practitioners need to allocate more time for consultation along with explanation of the potential benefits and harms to the patients and their caregivers when recommendations are rated as conditional. Practitioners’ expertise and judgment as well as patients’ and their caregivers’ needs and preferences establish the suitability of the recommendation to individual patients. The strength of a recommendation presents different implications for patients, clinicians, and policy makers (Table 2).
Table 2. Implications of strong and conditional recommendations for different users of guidelines
 Recommendations
Recommendations
The SDF panel supports the use of 38 percent SDF for the arrest of cavitated caries lesions in primary teeth as part of a comprehensive caries management program. (Conditional recommendation, low-quality evidence)
Summary of findings
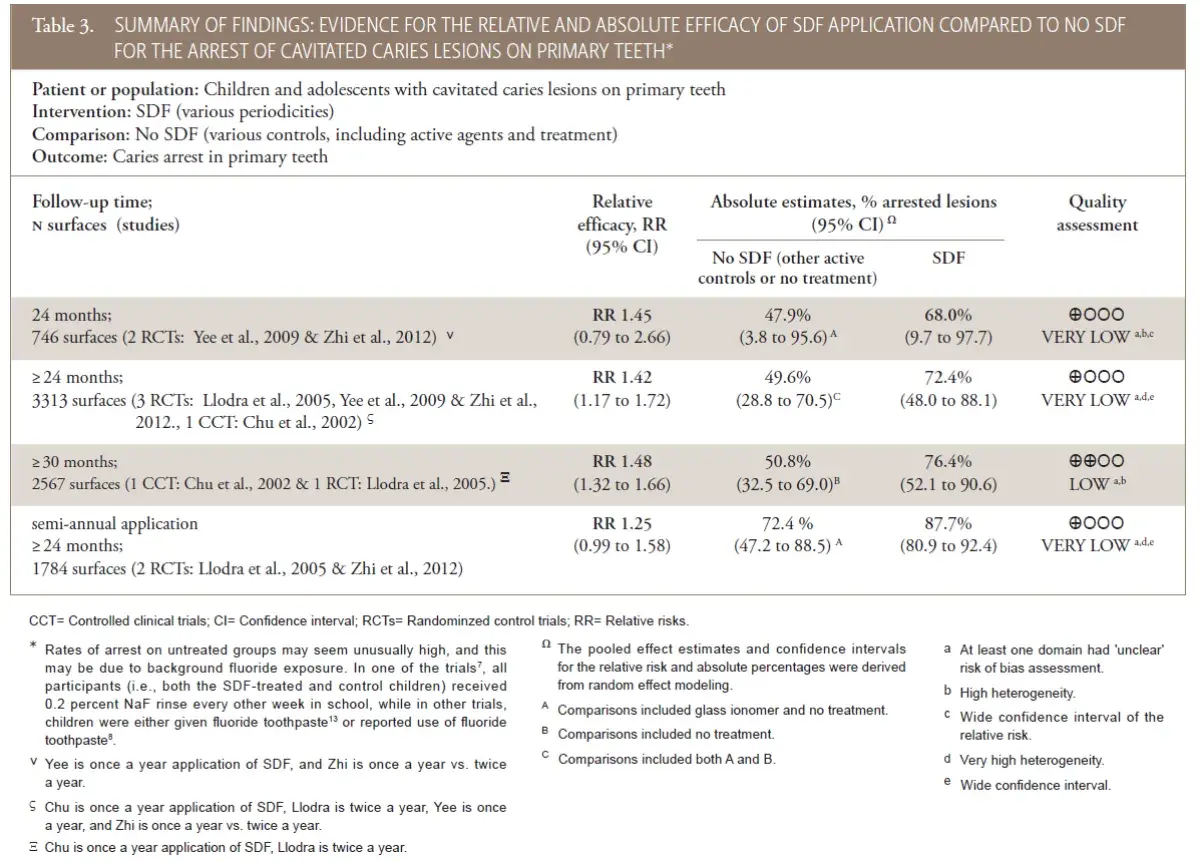
The recommendation is based on data from a meta-analysis of data extracted from RCTs and CCTs of SDF efficacy with various follow-up times and controls (Table 3). Based on the pooled estimates of SDF group, approximately 68 percent (95 percent confidence interval [95% CI]=9.7 to 97.7) of cavitated caries lesions in primary teeth would be expected to be arrested two years after SDF application (with once or twice a year application). Using data with longest follow-up time (at least 30 months follow-up; n=2,567 surfaces from one RCT7 and one CCT8), SDF had 48 percent higher (95% CI=32 to 66) success rate in caries lesion arrest compared to the controls (76 percent versus 51 percent arrested lesions, in absolute terms). In other words, 248 more cavitated caries lesions would be expected to arrest by treatment with SDF compared to control treatments, per 1000 surfaces after at least 30 months followup. Considering the stratum with most data (n=3,313 surfaces from three RCTs and one CCT, with follow-up of 24 months or more), similar estimates of relative and absolute efficacy were produced (i.e., RR 1.42 [95% CI=1.17 to 1.72]) and 72 percent versus 50 percent arrested lesions, in absolute terms. Other follow-up and application frequency strata are listed in the summary of findings (Table 3). The range of estimates of SDF efficacy between the included trials was categorically wide. Rates of arrest on untreated groups may seem unusually high, and this may be due to background fluoride exposure. In one of the trials, all participants (i.e., both the SDF-treated and control children) received 0.2 percent sodium fluoride (NaF) rinse every other week in school, while in other trials, children were either given fluoride toothpaste or reported use of fluoride toothpaste. The panel determined the overall quality of the evidence for this comparison was low or very low, owing to serious issues of risk of bias (unclear method for randomization, selective reporting, and high heterogeneity) in the included studies. No studies were identified regarding the arresting effect of SDF on cavitated caries lesions in adult patients. The panel suggests that similar treatment effects may be expected for other age groups, but the lack of evidence informing this recommendation restrained the panel from providing an evidence-based recommendation.
Table 3. Summary of findings: evidence for the relative and absolute efficacy of sdf application compared to no SDF for the arrest of cavitated caries lesions on primary teeth*
 The panel made a conditional recommendation regarding the use of SDF for the arrest of cavitated caries lesions in primary teeth as part of a comprehensive caries management program. After taking in consideration the low cost of the treatment and the disease burden of caries, panel members were confident that the benefits of SDF application in the target populations outweigh its possible undesirable effects. Specifically:
The panel made a conditional recommendation regarding the use of SDF for the arrest of cavitated caries lesions in primary teeth as part of a comprehensive caries management program. After taking in consideration the low cost of the treatment and the disease burden of caries, panel members were confident that the benefits of SDF application in the target populations outweigh its possible undesirable effects. Specifically:
Untreated decay in young children remains a challenge, from clinical and public health standpoints, in the U.S. and worldwide. It confers significant health and quality of life impacts to children and their families, and it is marked by pronounced disparities.
Surgical-restorative work in young children and those with special management considerations (e.g., individuals with special health care needs) often requires advanced pharmacologic behavior guidance modalities (e.g., sedation, general anesthesia). These pathways of care have additional health risks and limitations (e.g., possible effects on brain development in young children, mortality risks), and often are not accessible, at all or in a timely manner. The U.S. Food and Drug Administration has issued a warning “that repeated or lengthy use of general anesthetic and sedation drugs during surgeries or procedures inchildren younger than three may affect the development of children’s brains.”
The cost of managing severe early childhood caries is disproportionally high, especially when hospitalization is necessary. The need to treat children in a hospital setting with general anesthesia is a common scenario in the U.S. and other countries. Studies report that children from the less-affluent regions have higher dental surgery rates than those from more-affluent communities (25.7 vs. 6.9 per 1,000), which results in an economic burden for communities already impacted by the effects of poverty-related health problems.
With caries lesion arrest rates upwards of 70 percent (i.e., higher than other comparable interventions), SDF presents as an advantageous modality. Besides its efficacy, SDF is favored by its less invasive (clinically and in terms of behavior guidance requirements) nature and its inexpensiveness.
The undesirable effects of SDF (mainly esthetic concerns due to dark discoloration of carious SDF-treated dentin) are outweighed by its desirable properties in most cases, while no toxicity or adverse events associated with its use have been reported.
In sum, the panel felt confident that a conditional recommendation was merited because, although a majority of patients would benefit from the intervention, individual circumstances, preferences, and values need to be assessed by the practitioner after explanation and consultation with the caregiver.
Research considerations. Research is needed on the use of SDF to arrest caries lesions in both primary and permanent teeth. The panel urges researchers to conduct well-designed randomized clinical trials comparing the outcomes of SDF to other treatments for the arrest of caries lesions in primary and permanent teeth.
Potential adverse effects. Silver diamine fluoride contains approximately 24-28 percent (weight/volume) silver and 5-6 percent fluoride (weight/volume).23 Exposure to one drop of SDF orally would result in less fluoride ion content than is present in a 0.25 mL topical treatment of fluoride varnish. The exact amount of silver and fluoride present in one drop of SDF is determined by the specific gravity of the liquid and the dropper used. More studies are required to determine that amount, given the stability of the product manufactured and packaged in the U.S.
In published clinical trials encompassing over 4,000 young children worldwide, exposure to manufacturer’s recommended amounts of SDF has not resulted in any reported deaths or systemic adverse effects.
Oral absorption can include absorption in mucous membranes in the mouth and the nasal cavity. The short-term health effects in humans as a result of exposure to water or food containing specific levels of silver are unknown. The Environmental Protection Agency (EPA) suggests levels of silver in drinking water not to exceed 1.142 mg/L (1.42 ppm). Silver diamine fluoride should not be used in patients with an allergy to silver compounds.
The main disadvantage of SDF is its esthetic result (i.e., permanently blackens enamel and dentinal caries lesions and creates a temporary henna-appearing tattoo if allowed to come in contact with skin). Skin pigmentation is temporary since the silver does not penetrate the dermis. Desquamation of the skin with pigmentation occurs when keratinocytes are shed over a period of 14 days. Silver diamine fluoride also permanently stains most surfaces (e.g., counters, clothing) with which it comes into contact.
Guideline implementation. This guideline will be published in the AAPD’s Reference Manual and the journal, Pediatric Dentistry. Social media, news items, and presentations will be used to notify AAPD members about the new guideline.
This guideline will be available as an open access publication on the AAPD’s website. Patient education materials are being developed and will be offered in the AAPD’s online bookstore. See Appendix II for practical SDF guidance and the Resource Section of the AAPD Reference Manual for a SDF chairside guide.
Cost considerations. Silver diamine fluoride is an effective and inexpensive means of arresting cavitated caries lesions inprimary teeth.27 It is inexpensive due to the low cost of materials and supllies and relatively short chair time required for application. Nevertheless, an empirical cost analysis discussion for SDF would need to address the several additional considerations and parameters. First, given the wide array of surgical and non-surgical management approaches for cavitated caries lesions in the primary dentition, agreement on consensus endpoints and, therefore, total cost is challenging and controversial. Second, cost should include patient/family and practitioner time, health care services utilized, and cost of non-health impacts, if any. Third, SDF economic analyses are likely best approached via a cost-utility framework, wherein expenditures are juxtaposed to quality-adjusted or disease-free years. To illustrate the importance of defining a consensus treatment endpoint, in this scenario disease-free years can be interpreted as caries inactive, no surgical intervention needed, or pain-free years. Finally, the economic benefits of SDF application must be considered in the context of pathways of clinical care (i.e., disease management) and account, among other factors, for the risks and costs associated with advanced behavior management techniques (e.g., indicated surgical-restorative work may require sedation or general anesthesia in some cases), families’ preferences, and opportunity costs (e.g., time investment beyond the direct costs).
Recommendation adherence criteria
Guidelines are used by insurers, patients, and health care practitioners to determine quality of care. In principle, following best practices and guidelines is believed to improve outcomes and reduce inappropriate care.28 Therefore, measuring adherence to oral health-related guidelines is key and can serve as manifestation of the dental community’s role as a “responsible steward of oral health.”29 Though measurement of oral health outcomes is in its early days at both system and practice levels, system-level performance measures for some oral health areas have been developed by the Dental Quality Alliance of the American Dental Association in partnership with the AAPD and other dental organizations. The goals of professional accountability, transparency, and oral health care quality can be furthered through these measures.
Workgroup. In December 2016, the AAPD’s Board of Trustees approved a panel nominated by the EBDC to develop a new evidence-based clinical practice guideline on SDF. The panel consisted of general and pediatric dentists in public and private practice involved in research and education; the stakeholders consisted of representatives from general dentistry, dental hygiene, governmental and non-governmental agencies, and international and specialty dental organizations.
Stakeholders and external review. This guideline was reviewed by external and internal stakeholders continuously from the beginning of the process until the formulation of the guideline. Stakeholders were invited to take part in anonymous surveys to determine the scope and outcomes of the guideline, bringing in points of view from different geographical regions, dental specialties, and patient advocates. Comments also were sought on the draft of the guideline. All stakeholder comments were taken into consideration, addressed, and acted upon as appropriate per group deliberation. Additional feedback from stakeholders is expected after publication and dissemination of the guideline.
Intended users. The target audience for this guideline is general dentists, pediatric dentists, pediatricians, and family practice physicians. Public and private payors will benefit from reviewing the evidence for coverage decisions regarding SDF use, and patients and patient advocates may find it useful as a reference for current available treatments for caries management. The target populations include children and adolescents, including those with special health care needs.
Guideline updating process. The AAPD’s EBDC will monitor the biomedical literature to identify new evidence that may impact the current recommendations. These recommendations will be updated five years from the time the last systematic search, unless the EBDC determines that an earlier revision or update is warranted.
Funding. The preparation of this guideline was funded by the American Academy of Pediatric Dentistry, a dental specialty organization with over 10,000 members.
Author contributions. All authors contributed to the formation and drafting of the guideline recommendations and the manuscript. Dr. Crystal served as chair of the workgroup and provided expert oversight. Dr. Marghalani provided statistical support and created the GRADE tables. Ms. Graham provided methodical support for the development of the guideline, including search strategy development. All authors contributed to the critical revision of the manuscript and approved the guideline.
Declaration of interest. Dr. Crystal is a member of the AAPD Editorial Board. Dr. Divaris is a member of the AAPD Editorial Board. Dr. Marghalani is an ad hoc reviewer for Pediatric Dentistry. Dr. Sulyanto is an ad hoc reviewer for Pediatric Dentistry. Dr. Wright is a member of the AAPD Editorial Board. No other conflicts of interested were reported.
Appendices
Appendix I—Search strategies
PubMed® (MEDLINE)– no date limit
Search #1. 145 results
cariestop OR "silver diamine fluoride"[Supplementary Concept] OR "silver diamine" OR "silver diammine" OR “diamine fluoride” OR “diammine fluoride” OR saforide OR “Riva star”
Search #2. 6589771 results (randomized controlled trial[pt] OR controlled clinical trial [pt] OR randomi*[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR "clinical trial"[tw] OR "clinical trials"[tw] OR "evaluation studies"[Publication Type] OR "evaluation studies as topic"[MeSH Terms] OR "evaluation study"[tw] OR evaluation studies[tw] OR "intervention studies"[MeSH Terms] OR "intervention study"[tw] OR "intervention studies"[tw] OR "cohort studies"[MeSH Terms] OR cohort[tw] OR "longitudinal studies"[MeSH Terms] OR "longitudinal"[tw] OR longitudinally[tw] OR "prospective"[tw] OR prospectively[tw] OR "follow up"[tw] OR "comparative study"[Publication Type] OR "comparative study"[tw] OR systematic[subset] OR "meta-analysis"[Publication Type] OR "meta-analysis as topic" [MeSH Terms] OR "meta-analysis"[tw] OR "meta-analyses" [tw]) NOT (animals [mh] NOT humans [mh])
Search #3. 14 results
#1 and #2
Search #4. 410530 results
(systematic[sb] OR meta-analysis[pt] OR meta-analysis as topic[mh] OR meta-analysis[mh] OR meta analy*[tw] OR metanaly*[tw] OR metaanaly*[tw] OR met analy*[tw] OR research overview*[tiab] OR collaborative review*[tiab] OR collaborative overview*[tiab] OR systematic review*[tiab] OR comparative efficacy[tiab] OR comparative effectiveness[tiab] OR outcomes research[tiab] OR systematic overview*[tiab] OR methodological overview*[tiab] OR methodologic overview* [tiab] OR methodological review*[tiab] OR methodologic review*[tiab] OR quantitative review*[tiab] OR quantitative overview*[tiab] OR quantitative synthes*[tiab] OR pooled analy*[tiab] OR Cochrane[tiab] OR Medline[tiab] OR Pubmed [tiab] OR Medlars[tiab] OR handsearch*[tiab] OR hand search* [tiab] OR meta-regression*[tiab] OR metaregression*[tiab] OR data synthes*[tiab] OR data extraction[tiab] OR data abstraction*[tiab] OR mantel haenszel[tiab] OR peto[tiab] OR dersimonian[tiab] OR dersimonian[tiab] OR fixed effect* [tiab] OR "Cochrane Database Syst Rev"[Journal])
Search #5. 14 results
#1 and #4*
Search #6. 890576 results
("Economics"[Mesh] OR "Cost of Illness"[Mesh] OR "Cost Savings"[Mesh] OR "Cost Control"[Mesh] OR "Cost-Benefit Analysis"[Mesh] OR "Health Care Costs"[Mesh] OR "Direct Service Costs"[Mesh] OR "economics"[Subheading] OR cost))
Search #7. 8 results
#1 AND #6
Appendix II — Practical guidance *
* Silver diamine fluoride in this guideline’s recommendation refers to 38 percent SDF, the only formula available in the United States.
Setting
Practitioners must first consider the current standard of care of the setting where SDF therapy is intended for use. Silver diamine fluoride is optimally utilized in the context of a chronic disease management protocol, one that allows for the monitoring of the clinical effectiveness of SDF treatment, disease control, and risk assessment.
Practical recommendation: Know the setting where SDF is to be used to be consistent with goals of patient-centered care.
Indications and usage
The following scenarios may be well-suited for the use SDF:
High caries-risk patients with anterior or posterior active cavitated lesions.
Cavitated caries lesions in individuals presenting with behavioral or medical management challenges.
Patients with multiple cavitated caries lesions that may not all be treated in one visit.
Difficult to treat cavitated dental caries lesions.
Patients without access to or with difficulty accessing dental care.
Active cavitated caries lesions with no clinical signs of pulp involvement.
Practical recommendation: SDF is a valuable caries lesion–arresting tool that can be used in the context of caries management. Evaluate carefully which patients/teeth will benefit from SDF application.
Preparation of patients and practitioners
Informed consent, particularly highlighting expected staining of treated lesions, potential staining of skin and clothes, and need for reapplication for disease control, is recommended.
The following practices are presented to support patient safety and effectively use SDF:
Universal precautions.
No operative intervention (e.g., affected or infected dentin removal) is necessary to achieve caries arrest.
Protect patient with plastic-lined bib and glasses.
Cotton roll or other isolation as appropriate.
Use a plastic dappen dish as SDF corrodes glass and metal.
Carefully dispose of gloves, cotton rolls, and micro brush into plastic waste bag.
Application
Carious dentin excavation prior to SDF application is not necessary. Caries dentin excavation may reduce proportion of arrested caries lesions that become black, and may be considered for esthetic purposes.30 Functional indicator of effectiveness (i.e., caries arrest) is when staining on dentinal carious surfaces is visible.
The following steps may vary depending on differing practices, settings, and patients:
Remove gross debris from cavitation to allow better SDF contact with denatured dentin.
Minimize contact with gingiva and mucous membranes to avoid potential pigmentation or irritation; consider applying cocoa butter or use cotton rolls to protect surrounding gingival tissues, with care to not inadvertently coat the surfaces of the carious lesion.
Dry with a gentle flow of compressed air (or use cotton rolls/gauze to dry) affected tooth surfaces.
Bend micro sponge brush, dip and dab on the side of the dappen dish to remove excess liquid before application; apply SDF directly to only the affected tooth surface.
Dry with a gentle flow of compressed air for at least one minute.
Remove excess SDF with gauze, cotton roll, or cotton pellet to minimize systemic absorption. Continue to isolate site for up to three minutes when possible.
Practical recommendation: No need for surgical intervention (e.g., dentin excavation). SDF application is minimally invasive and easy for the patient and the practitioner. It may be desirable for the caries lesion to be free of gross debris for SDF to have maximum contact with the affected dentin surface.
Application time
An application time of one minute, drying with a gentle flow of compressed air, is recommended. Clinical studies that report application times range from 10 seconds to three minutes. A current review states that application time in clinical studies does not correlate to outcome. More studies are needed to confirm an ideal protocol.
Practical recommendation: Ideal time of application should be one minute, using a gentle flow of compressed air until liquid is dry. When using shorter application periods, monitor carefully at post-op and re-care to evaluate arrest and consider re-application.
Post-operative instructions
No postoperative limitations are listed by the manufacturer. Eating and drinking immediately following application is acceptable. Patients may brush with fluoridated toothpaste as per regular routine following SDF application. Several SDF clinical trials recommended no eating or drinking for 30 minutes – one hour. As patients are used to these recommendations for in-office topical fluoride applications, the recommendation may not be unreasonable to patients, and it may allow for better arrest results. More clinical studies are needed to establish best practices.
Application frequency
The effectiveness of one-time SDF application in arresting dental caries lesions ranges from 47 percent to 90 percent, depending on the lesion size and the location of the tooth and the lesion. One study showed that anterior teeth had higher rates of caries lesion arrest than posterior teeth. The effectiveness of caries lesion arrest, however, decreases over time. After a single application of 38 percent SDF, 50 percent of the arrested surfaces at six months had reverted to active lesions at 24 months. Reapplication may be necessary to sustain arrest. Annual application of SDF is more effective in arresting caries lesions than application of five percent sodium fluoride varnish four times per year. Increasing frequency of application can increase caries arrest rate. Biannual application of SDF increase the rate of caries lesion arrest compared to annual application. Studies that had three times per year applications showed higher arrest rates. Frequency of application after baseline has been suggested at three month follow up, and then semiannual recall visits over two years. One option is to place SDF on active lesions in conjunction with fluoride varnish (FV) on the rest of the dentition, or alternate SDF on caries lesions and FV on the rest of the dentition at three months interval to achieve arrest and prevention in high risk individuals. Another study recommends one month post operative evaluation of treated lesions with optional reapplication as required to achieve arrest of all targeted lesions. Individuals with high plaque index and lesions with plaque present display lower rates of arrest. Addressing other risk factors like presence of plaque may increase the rate of successful treatment outcomes.
Practical recommendation: If the setting allows, monitor caries lesion arrest after 2-4 week period and consider reapplication as necessary to achieve arrest of all targeted lesions. Provide re-care monitoring based on patient’s disease activity and caries risk level (every three, four, or six months). Careful monitoring and behavioral intervention to reduce individual risk factors should be part of a comprehensive caries management program that aims not only to sustain arrest of existing caries lesions, but also to prevent new caries lesion development.
Adverse reactions
No severe pulpal damage or reaction to SDF has been reported. However, SDF should not be placed on exposed pulps. Teeth with deep caries lesions should be closely monitored clinically and radiographically.
Serum concentration of fluoride following SDF application per manufacturer recommendations posed little toxicity risk and was below EPA oral reference dose in adults. The following adverse effects have been noted in the literature:
Metallic/bitter taste.
Temporary staining to skin which resolves in 2-14 days.
Mucosal irritation/lesions resulting from inadvertent contact with SDF, resolved within 48 hours.
Esthetics
The hallmark of SDF is a visible dark staining that is a sign of caries arrest on treated dentin lesions. This dark discoloration is permanent unless restored. A recent study that assessed parental perceptions and acceptance of SDF based on the staining found that staining on posterior teeth was more acceptable than on anterior teeth. Although staining on anterior teeth was perceived as undesirable, most parents preferred this option to avoid the use of advanced behavioral guidance techniques such as sedation or general anesthesia to deliver traditional restorative care. It was also found that about one-third of parents found SDF treatment unacceptable under any circumstance due to esthetic concerns. To identify those patients, a thorough informed consent, preferably with photographs that show typical staining, is imperative. To improve esthetics, once the disease is controlled and patient’s circumstances allow, treated and now-arrested cavitated caries lesions can be restored.
There are additional details about primary teeth treatment that you can obtain on our website.
Other considerations
Coding – D1354; Reimbursement for this procedure varies among states and carriers. Third-party payors’ coverage is not consistent on the use of this code per tooth or per visit. Practitioners are cautioned to check insurance coverage for this code as it is transitioning in most areas.
Caries arrest is more likely on the maxillary anterior teeth and buccal/lingual smooth surfaces
Pretreatment of dentin with SDF does not adversely affect bond strength of resin composite to dentin.
References
1. Gao S, Zhao I, Hiraishi N, et al. Clinical trials of silver diamine fluoride in arresting caries among children: A systematic review. JDR Clin Transl Res 2016;1(3):201-10.
2. Longbottom C, Huysmans M-C, Pitts N, Fontana M. Glossary of key terms. In: Detection, Assessment, Diagnosis and Monitoring of Caries. Vol 21. Karger. Basel, N.Y.; 2009:209-16. Cited by: Fontana M, Young DA, Wolff MS, Pitts NB, Longbottom C. Defining dental caries for 2010 and beyond. Dent Clin North Am 2010;54(3):423-40.
3. Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: A key to evidence-based decisions. ACP J Club 1995;123(3):A12-13.
4. Institute of Medicine. Clinical Practice Guidelines We Can Trust. 2011.
5. Brouwers MC, Kerkvliet K, Spithoff K. The AGREE Reporting Checklist: A tool to improve reporting of clinical practice guidelines. BMJ 2016;352:i1152.
6. Schünemann H, Brożek J, Guyatt G, Oxman A. Quality of evidence. GRADE Handbook: Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Update Oct. 2013. The GRADE Working Group.
7. Llodra JC, Rodriguez A, Ferrer B, Menardia V, Ramos T, Morato M. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res 2005; 84(8):721-4.
8. Chu CH, Lo ECM, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arrestingdentin caries in Chinese pre-school children. J Dent Res 2002;81(11):767-70.
9. Cheng, Linda L. Limited evidence suggesting silver diamine fluoride may arrest dental caries in children. Br Dent J 2017;222(7):516.
10. Gold J. Limited evidence links silver diamine fluoride and caries arrest in children. J Evid Based Dent Pract 2017;17 (3):265-7.
11. Alonso-Coello P, Oxman AD, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ 2016;353: i2089.
12. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328 (7454):1490.
13. Yee R, Holmgren C, Mulder J, Lama D, Walker D, van Palenstein Helderman W. Efficacy of silver diamine fluoride for Arresting Caries Treatment. J Dent Res 2009;88(7): 644-7.
14. Listl S, Galloway J, Mossey P, Marcenes W. Global economic impact of dental diseases. J Dent Res 2015;94(10): 1355-61.
15. Chaffee BW, Rodrigues PH, Kramer PF, Vítolo MR, Feldens CA. Oral health-related quality-of-life scores differ by socioeconomic status and caries experience. Community Dent Oral Epidemiol 2017;45(3):216-24.
16. Owings L. Toothache Leads to Boy’s Death. ABC News. March 5, 2007.
17. Schroth RJ, Quiñonez C, Shwart L, Wagar B. Treating early childhood caries under general anesthesia: A national review of Canadian data. J Can Dent Assoc 2016;82(g20): 1488-2159.
18. Griffin SO, Gooch BF, Beltrán E, Sutherland JN, Barsley R. Dental services, costs, and factors associated with hospitalization for Medicaid-eligible children, Louisiana 1996–97. J Public Health Dent 2000;60(1):21-7.
19. Nagarkar SR, Kumar JV, Moss ME. Early childhood caries–related visits to emergency departments and ambulatory surgery facilities and associated charges in New York state. J Am Dent Assoc 2012;143(1):59-65.
20. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. December 14, 2016.
21. Hicks CG, Jones JE, Saxen MA, et al. Demand in pediatric dentistry for sedation and general anesthesia by dentist anesthesiologists: A survey of directors of dentist anesthesiologist nd pediatric dentistry residencies. Anesth Prog 2012; 59(1):3-11.
22. Liu J, Probst JC, Martin AB, Wang J-Y, Salinas CF. Disparities in dental insurance coverage and dental care among US children: the National Survey of Children’s Health. Pediatrics 2007;119(Supplement 1):S12-S21.
23. Mei ML, Chu CH, Lo ECM, Samaranayake LP. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int J Paediatr Dent 2013;23(4): 279-85.
24. Horst JA, Ellenikiotis H, Milgrom PL. UCSF protocol for caries arrest using silver diamine fluoride: Rationale, indications and consent. J Calif Dent Assoc 2016;44(1): 16-28.
25. Jackson SM, Williams ML, Feingold KR, Elias PM. Pathobiology of the stratum corneum. West J Med 1993;158 (3):279.
26. American Academy of Pediatric Dentistry. Chairside guide: Silver diamine fluoride in the management of dental caries lesions. Pediatr Dent 2017;39(6):478-9.
27. Alliance for Cavity Free Future. Silver fluoride and silver diamine fluoride.
28. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: Advancing guideline development, reporting and evaluation in health care. Can Med Assoc J 2010;182(18): E839-E842.
29. Dental Quality Alliance. Quality measurement in dentistry: A guidebook. June 2016.
30. Lo EC, Chu CH, Lin HC. A community-based caries control program for pre-school children using topical fluorides: 18-month results. J Dent Res 2001;80(12):2071-4.
31. Zhi QH, Lo ECM, Lin HC. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent 2012;40(11):962-7.
32. Dos Santos VEJ, de Vasconcelos FMN, Ribeiro AG, Rosenblatt A. Paradigm shift in the effective treatment of caries in schoolchildren at risk. Int Dent J 2012;62(1):47-51.
33. Fung M, Duangthip D, Wong M, Lo E, Chu C. Arresting dentine caries with different concentration and periodicity of silver diamine fluoride. JDR Clin Transl Res 2016;1 (2):143-52.
34. Duangthip D, Chu CH, Lo ECM. A randomized clinical trial on arresting dentine caries in preschool children by topical fluorides–18 month results. J Dent 2016;44: 57-63.
35. Crystal YO, Niederman R. Silver diamine fluoride treatment considerations in children’s caries management. Pediatr Dent 2016;38(7):466-71.
36. Nishino M, Yoshida S, Sobue S, Kato J, Nishida M. Effect of topically applied ammoniacal silver fluoride on dental caries in children. J Osaka Univ Dent Sch 1969;9:149-55.
37. Okuyama T. [On the penetration of diammine silver fluoride into the carious dentin of deciduous teeth (author’s transl)]. Shigaku Odontol J Nihon Dent Coll 1974;61(6):1048-71.
38. Gotjamanos T. Pulp response in primary teeth with deep residual caries treated with silver fluoride and glass ionomer cement ('atraumatic' technique). Aust Dent J 1996;41(5): 328-34.
39. Vasquez E, Zegarra G, Chirinos E, et al. Short term serum pharmacokinetics of diammine silver fluoride after oral application. BMC Oral Health 2012;12:60.
40. Crystal YO, Janal MN, Hamilton DS, Niederman R. Parental perceptions and acceptance of silver diamine fluoride staining. J Am Dent Assoc 2017; 148(7):510-8.
41. Quock RL, Barros JA, Yang SW, Patel SA. Effect of silver diamine fluoride on microtensile bond strength to dentin. Oper Dent 2012;37(6):610-6.
42. Selvaraj K, Sampath V, Sujatha V, Mahalaxmi S. Evaluation of microshear bond strength and nanoleakage of etch-andrinse and self-etch adhesives to dentin pretreated with silver diamine fluoride/potassium iodide: An in vitro study. Indian J Dent Res 2016;27(4):421-5.
