Sugars and beyond. The role of sugars and the other nutrients and their potential impact on caries
The traditional concept of caries as a multifactorial transmittable and infectious disease has been challenged. Novel conceptual ideas have come to add to the complexity of this highly prevalent disease worldwide. Current etiological understanding of the disease has emphasized the pivotal role of sugars in caries. In fact, current definition points toward an ecological disease caused by the commensal microbiota that under ecological imbalances, mainly due to high and or frequent sugars consumption, creates a state of dysbiosis in the dental biofilm. This modern conceptual idea, however, tends to underrate a key issue. As humans are omnivore and consume a mix diet composed by a multitude of substances, the role of the diet in caries must not be restricted only to the presence of fermentable sugars. This review explores the contribution of other food components, ubiquitous to the diet mostly as potentially protective factors. Anticaries nutrients might determine an environmental change, affecting the ecology of the oral microbiome and partially mitigating the effect of sugars. Understanding the function of the food usually consumed by the people will contribute new knowledge on the mechanisms associated with the onset of caries, on new caries risk variables and on potential novel strategies for the prevention and treatment of the disease.
1 INTRODUCTION
There is a perception that dental caries is decreasing worldwide. This subjective feeling is certainly misleading in light of reality. As a matter of fact, untreated caries is the most prevalent condition in humans, affecting about 35% of the world population (Kassebaum et al., 2015). Remarkably, from 1990 to 2010, the burden of disease has remained basically unchanged. It is currently accepted that caries is a sugars and biofilm-dependent disease (Sheiham & James, 2015). Diet, therefore, plays a major role in caries etiopathogenesis. Yet, the effect of diet on caries has been largely focused on sugars consumption and its avoidance as a way to control the disease. While based on current knowledge to blame sugars as the sole responsible for caries causation seems correct, how other food components may become fermentable sugars modulators in caries has been substantially less investigated. This review provides an updated view of diet on caries, but including nutrients that may play an anticaries effect and the role for other commonly consumed food and drinks components. There are additional details about dental caries that you can gain on our website.
2 CURRENT CONCEPTS IN CARIES ETIOPATHOGENESIS AND THE ROLE OF THE DENTAL BIOFILM
From the early 60s, caries was understood as a multifactorial infectious and transmittable disease. The etiology was attributed to microorganisms, a susceptible tooth and fermentable substrates, all acting with the same intensity and concomitantly to induce the process (Keyes, 1960). Little variations were introduced since then. A much more detailed and complex pathogenesis has been developed over the years. Complexity was initially derived from a remarkable advancement in understanding the molecular traits of the bacterium that, for many years, was mistakenly recognized as the sole etiological agent, the Streptococcus mutans (S. mutans). Despite this progress, etiopathogenesis of the disease remained unaltered and this cariogenic bacterium kept being the focus of most research efforts to tackle the disease, even until today. Controversy still exists in that some feel that targeting the mutans streptococci would have little effect on reducing caries rates, whereas others feel that it would still make a significant impact. Indeed, it has been argued that only selectively killing S. mutans cells would restore the normal dental microbiota for a sustained caries-protective effect (Eckert, Sullivan, & Shi, 2012). The role of S. mutans in caries causation has been critically examined, and novel concepts in caries etiopathogenesis have been proposed. Although not etiological, mutans streptococci are probably the only plaque members for whom a mechanistic role for sucrose in caries has been confirmed. Currently, there is a relatively wide acceptance of the “Ecological Plaque Hypothesis” (Marsh, 2006) for caries. According to this theory, dental caries is an imbalance of the resident microflora due to an enrichment within the bacterial community of the oral pathogens caused by frequent environmental situations of low pH. Putative cariogenic bacteria are consistently isolated in health, but they are weakly competitive at neutral pH and their presence represents only a small proportion of the entire population. An even more recent perspective states that caries may be considered as an ecological sugars-dependent dysbiosis caused by pathobionts (Simón-Soro & Mira, 2015). Under a balanced and healthy diet, low in sugars, the commensal and highly abundant streptococci are capable of metabolizing carbohydrates and producing acids. Although these acids can initiate demineralization, physiological mechanisms in the mouth, that is, saliva, can restore pH, halt the onset of the lesions and remineralize the tissues at the crystal level, before cavitation. When sugars are consumed at a high frequency from the diet (Diaz-Garrido, Lozano, & Giacaman, 2016), however, an ecological imbalance of the microbiota of the mouth is created. This imbalance is called dysbiosis, whereby ubiquitous microorganisms of the dental biofilm become more virulent by bacterial competition (Kreth, Giacaman, Raghavan, & Merritt, 2016; Kuramitsu, He, Lux, Anderson, & Shi, 2007). Some of the acidogenic species that are better endowed to thrive under acidic conditions tend to prevail over their competitors, leading to a pH drop, which in turn favors demineralization of enamel and dentin (Fejerskov, 2004). Bacteria that act as commensal under healthy conditions may become pathogenic (pathobionts) when the ecological balance is broken, either by changes in gene expression or in the critical numerical threshold of the species. The steady exposure of sugars to the biofilm causes disruption of the microbial balance in the oral environment. Thus, sugars must be considered as the main, etiological factor for caries (Sheiham & James, 2015). The other variables involved in the etiopathogenesis of the disease do not really cause it, but modulate its occurrence. For example, salivary flow, fluoride exposure, plaque accumulation, tooth morphology, and structure, among several others (Bratthall & Hansel Petersson, 2005), would create more favorable or adverse conditions for the causal relation between sugars and the dental biofilm to induce carious lesions. More correctly, plaque acid is the final cause of caries and fermentable sugars are the substrate for that acid production.
Adhering to the tooth, hundreds of bacterial species colonize and co-exist forming complex associations of microorganisms known as dental biofilm or dental plaque (reviewed in Kolenbrander et al., 2002; Marsh, 2006; Takahashi & Nyvad, 2011; Nyvad, Crielaard, Mira, Takahashi, & Beighton, 2013). Besides the microorganisms, the dental biofilm is constituted by a variety of other salivary components, mainly glycoproteins, and variable amounts of bacterial extracellular polymers or extracellular polysaccharides (EPS), such as glucans and fructans, specific polymers produced from sucrose, mainly but not exclusively, by mutans streptococci (van Houte, 1980). The extracellular matrix (ECM) produced by specific microorganisms promotes microbial adhesion and cohesion and facilitates nutrient diffusion within the biofilm (Flemming & Wingender, 2010). Numerous studies have correlated caries experience with elevated numbers of the biofilm-forming microorganisms, as the quantity of S. mutans isolated from saliva or from plaque was (and for many still is) thought to play a central role in determining the risk of caries of an individual (van Houte, 1980). We have reported, nevertheless, a lack of association between caries experience and the number of isolated mutans streptococci (Giacaman, Araneda, & Padilla, 2010). Moreover, S. mutans is not isolated in all children with caries, and when found is part of a complex microbial community (Aas et al., 2008). Indeed, using a meta-transcriptomic approach by pyrosequencing the 16S rRNA gene (Simon-Soro, Guillen-Navarro, & Mira, 2014), it was reported that S. mutans only comprised between 0.02% and 0.73% of the whole bacterial community isolated from enamel and dentine carious lesions. Using RNA-sequencing technologies, specifically a long-reads, lower-coverage approach by pyrosequencing, it was reported that microbial communities are individual-specific without a common and defined fingerprinting during biofilm formation (Benitez-Paez, Belda-Ferre, Simon-Soro, & Mira, 2014). Despite the latter, recent findings suggest higher complexity within the microbial consortium through mixed-kingdom biofilms, such as the interaction between S. mutans and Candida albicans that may explain higher virulence in the dental biofilm, for example, during early childhood caries (Hwang et al., 2017). Thus, glycosyltransferases from S. mutans may bind mannans from the outer surface of C. albicans, increasing ECM formation and co-existence within biofilms. Yet, bacterial acidogenesis becomes a critical virulence factor, regardless of the bacterial composition. It is likely that the “weight” of sugars consumption overrides bacterial composition in the dental biofilm.
3 DIET AND CARIES, THE KEY STONE ROLE OF SUGARS
As early as 1922, a study conducted in preschool children attempted to prove the theory that “dental caries is initiated by acid fermentation of sticky foods, which adhere to the teeth” (Rypins, 1922). Results from this early research failed to find a direct relation, nonetheless. Research efforts continued in the same direction over time. The famous experiments by Stephan in 1940 (Stephan, 1940) led to conclude that pH drop in dental plaque was associated with the intake of starches and sugars, represented in a pH graph. From his experiments, Stephan showed that the simpler the sugars, the lower the pH and the longer the time to recover pH neutrality.
The term “total sugars” must be understood as naturally occurring and added sugars. “Added sugars” or “free sugars” are all the monosaccharides and disaccharides added to foods and beverages by the manufacturer, cook or consumer, and sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates (WHO, 2015). On the other hand, “sugar” tends to refer usually to sucrose only. It is clearly established that sugars are the main factor for the onset of caries (reviewed in (Zero, 2004)). Likewise, piling reports have demonstrated that certain species of bacteria, specifically from the mutans group (S. mutans and S. sobrinus), are endowed with efficient metabolic mechanisms to better utilize free sugars than other fermentable substrates such as starch and thus promote the formation of cariogenic biofilms. It is important to reemphasize, though, that this property is not restricted only to these bacteria, as there are many other biofilm species that can metabolize free sugars, as well. In addition to being the most efficiently metabolized carbohydrate by acidogenic bacteria to form acids (Aires et al., 2008; Ccahuana-Vasquez et al., 2007; Cury, Rebello, & Del Bel Cury, 1997), the disaccharide sucrose is also the most important source for EPS formation; the reader is referred to a comprehensive review on the topic (Klein, Hwang, Santos, Campanella, & Koo, 2015). The direct relation between sugars consumption and higher caries experience by DMFT has been recently confirmed (Bernabe, Vehkalahti, Sheiham, Lundqvist, & Suominen, 2016). The study suggested that there is a linear dose–response relationship between sugars and caries. In a recent report, the World Health Organization (WHO) has changed the recommendation for free sugars intake from the previous 10% (WHO, 2003) to 5% of daily energy intake to reduce obesity, type 2 diabetes and dental caries (Moynihan & Kelly, 2014; WHO, 2015). Another study has lately confirmed this recommendation on the grounds of a clinical study (Saido, Asakura, Masayasu, & Sasaki, 2016). The role of free sugars in caries has been highlighted and reemphasized (Sheiham & James, 2015). In the traditional view, dental caries is a disease that is considered a multifactorial condition. Although this is statement is not entirely untrue, it may be misleading. It has been usually considered that the carious process requires other fermentable carbohydrates, oral bacteria capable of fermenting the substrate and producing acids, susceptible teeth, diminished salivary flow and the absence of appropriate dose of fluoride exposure. Unfortunately, these other factors of the multifactorial process have acquired a mistaken causal role. In light of current knowledge, dental caries has a single specific cause: free sugars (Sheiham & James, 2014), which induce acid formation that is finally the cause of the lesions. This vision will force to redirect preventive efforts toward modifying dietary patterns in the high-risk population.
4 THE ROLE OF OTHER NUTRIENTS AND NON-SUGARS-CONTAINING FOODS AND DRINKS ON CARIES
As humans are omnivorous and common diets in any part of the world contain mixtures of foods and drinks and rarely comprise only free sugars, a discussion on the potential contribution of other food components on caries appears needed. Certain types of food are considered to be associated with oral health. The precise mechanisms for the putative anticaries role of some food components in caries have not been clarified. Nevertheless, a salivary flow-stimulating effect, bacterial inhibitory mechanisms, including interference with sugars fermentation, induction of bacterial competition within the dental biofilm or changes in the biofilm pH by the release of alkali, among other potential variables, might explain this protective effect. The effect of specific foods or some food components, different from free sugars, on the virulent traits of cariogenic bacteria remains minimally investigated. Exposure of the biofilm to molecules contained in certain foods may provide competitive advantages of certain species over the others.
4.1 Sucrose replacement with polyols
Polyols (sugar alcohols) are low-digestion carbohydrates. Polyols can be naturally found in fruits, vegetables and some fungi (Makinen, 2011). They are considered as “healthy” nutritional supplements (American Dietetic Association, 2004). Food additives are substances aimed at enhancing color and to sweeten or preserve foods. Currently, there are seven polyols defined as nutritive food additives, according to the European Union: sorbitol (E420), mannitol (E421), isomaltose (E953), maltitol (E965), lactitol (E966), xylitol (E967) and erythritol (E968) (European Union (2008)). Due to their slow and incomplete absorption in the gut, polyols have low nutritional value, when compared to sugars. For that reason, they are helpful in reducing caloric intake (Livesey, 2003).
Polyols have been typically considered as anticariogenic agents (Deshpande & Jadad, 2008; Runnel et al., 2013). Its use has been mostly centered in adding them to chewing gums. Its effectiveness seems to come from salivary flow stimulation through mastication, increase in saliva and biofilm pH, and early carious lesion remineralization (Burt, 2006). Among all the polyols, xylitol is by far the most widely investigated. Xylitol has been tested as caries-preventive agent in several randomized controlled trials, arguing a reduction in biofilm accumulation, in acid production (Beiswanger et al., 1998) and in the inhibition of the metabolic activity of S. mutans (Hayes, 2001). A study comparing the metabolic effect of fluorides and xylitol in vivo, nevertheless, showed that while fluoride appears to repress acid production, xylitol is not able to inhibit acidogenesis from the dental biofilm, acting as a non-fermentable sugar alcohol (Takahashi & Washio, 2011). Consumption of polyols, particularly xylitol, not only does not cause caries, but also appears to be caries inhibitory, due to futile efforts to transport it into the cell, becoming inhibitory of bacterial growth. Evidence, however, seems to be still insufficient and of low quality, as a recent systematic review seems to indicate (Riley, Moore, Ahmed, Sharif, & Worthington, 2015). Furthermore, it is relevant to discuss the need for high doses of the polyols to counteract the effect of sucrose that most likely will continue to be present in people’s dietary patterns. Also, the cost of these measures might make the intervention not suitable for most countries.
4.2 Functional foods and phenolic compounds in caries protection
Besides the case of the polyols, there exists weak evidence that some foods and nutrients may have a potential anticaries effect, reviewed in Moynihan (2007). Functional foods, nutraceutics and prebiotics from natural origin are being increasingly considered within the medical field as “healthier” approaches to treat diseases, with rapidly increasing consumption (Ozen, Pons, & Tur, 2012). There is a growing interest within the dental field for finding natural agents with anticaries properties, different to the widely used and investigated fluoridated products. Results from the scarce published studies, however, are far from being conclusive. Containing a wide range of molecules from ifferent origin and structure, an anticaries effect of various natural products has been reported (Jeon, Rosalen, Falsetta, & Koo, 2011). Among the substances with putative anticaries effect, polyphenol antioxidants contained in fruits and vegetables with a highly variable content have been claimed as potentially active against the disease (Ferrazzano et al., 2011). Phenolic compounds are substances belonging to a heterogeneous group, from simple to highly polymerized molecules, including flavonoids (quercetin and kaempferol), phenolic acids (chlorogenic acid and caffeic acid) and carotenoids (lutein and zeaxanthin). Health benefit from phenolic consumption derives not from one particular component, but from the synergistic activity of the bioactive compounds and other nutrients contained in fruits, vegetables, whole grains and other plant foods (Liu, 2013). Phenolic compounds in natural products have also been investigated as potential anticaries agents (Yoo, Murata, & Duarte, 2011). These molecules have shown to reduce caries-associated bacterial growth (Matsumoto et al., 2004) and biofilm formation (Yamanaka, Kimizuka, Kato, & Okuda, 2004). An inhibitory effect on the enzymatic activity of Gtfs has been proposed as the responsible mechanism (Nakahara et al., 1993). An investigation carried out by our group with apple concentrates showed interesting results (Giacaman, Contzen, Yuri, & Muñoz-Sandoval, 2014). S. mutans biofilms exposed to an experimental antioxidant-rich apple concentrate after a cariogenic challenge with sucrose induced lower enamel demineralization than sucrose alone, at a very low dose. Antioxidant exposure did not result in an antibacterial effect, suggesting a metabolic mechanism. Despite the promising alternative to treat caries using these natural food components, the data mostly derive from models using single-species biofilms. The results must be considered as a proof-of-principle only, as no clinical evidence has demonstrated a real benefit in decreasing caries incidence.
Most of the existent literature on the effect of other nutrients on caries has been performed using standard foods that do not discriminate food molecules and all contain high amounts of sucrose (Jensen & Schachtele, 1983). Current food patterns are increasingly incorporating processed foods, defined as any procedure that alters food from its natural state, such as freezing, drying, milling, canning, mixing or adding salt, sugar, fat, or additives. The only study that has assessed processed food in the United States has found that this type of food provides 57.3% of total energy intake (Eicher-Miller, Fulgoni, & Keast, 2012). Given the broad definition of processed food and the wide variety of feeding patterns in the population, it is difficult to estimate the role of food, in general, on the carious lesion onset. Hence, an individual outlook on the constituent nutrients results a rational first step toward understanding the participation of non-sugary food in caries. A succinct review on the contribution of some macronutrients in the caries process, different to sucrose, is presented below:
4.3 Cariogenic potential of starch
Starches are an important part of the human diet. Starch comprises a very heterogeneous group of carbohydrates. It is estimated that starches constitute almost 50% of total carbohydrate consumption in some western countries (Burt & Szpunar, 1994). Although starches are complex carbohydrates and should be more difficult to be fermented and converted into acids in the dental biofilm, they seem to preserve a cariogenic potential (Lingstrom, Holm, Birkhed, & Bjorck, 1989). A pH fall as a consequence of starch consumption was reported in a murine model (Firestone, Schmid, & Muhlemann, 1984). A distinction should be made between highly processed starch, starch-rich staple foods and unprocessed sources. Starch may be consumed raw (as in fruits and vegetables) or cooked, it may be processed to different degrees and it may have sugars added during processing. There is little evidence that less processed starches and staple starch-containing foods pose a threat to dental health (WHO, 2003). Most of the available studies have used highly processed starch (with and without sugars) in experimental studies with single-species biofilms, so this type of evidence is not considered robust enough to underpin dietary recommendations. Cariogenic potential of highly processed starches has been also documented in animal studies with selected bacterial species populating the biofilms (reviewed in Lingstrom, van Houte, & Kashket, 2000). Although an even higher cariogenic potential than sucrose of this type of complex carbohydrates has been described in rodents (Mundorff et al., 1990), studies in monkeys have evidenced a very low cariogenic potential of cooked-wheat flour (Beighton & Hayday, 1984). A study was conducted on the effect of a mix of highly processed starch and sucrose or sucrose only on demineralization of dentine slabs attached to palatal devices used for 14 days (Aires et al., 2008) in an in situ model. More demineralization was observed when specimens were extra-orally exposed to sucrose or to the combination of sucrose and starch than to only starch, without differences in demineralization between sucrose and sucrose and starch. Moreover, an in vitro study of S. mutans biofilms formed on saliva-coated hydroxyapatite disks showed that the combination of highly processed starch and sucrose produced thicker biofilms and more acidogenic than those grown in sucrose or sucrose and glucose (Duarte et al., 2008). EPS formed with starch and sucrose appeared to have higher percentage of branching when compared to those formed with sucrose alone. Combination of highly processed starch and sucrose could have an even more deleterious effect than sucrose itself. This increased cariogenic potential could be explained by the fact that when these two carbohydrates are found together in the oral environment, in the presence of salivary alpha-amylase and Gtfs, lower molecular weight sugars derived from the hydrolysis of starch are incorporated into glucose chains generating dense biofilms with stronger cohesive and adhesive properties (Bowen & Koo, 2011). The importance of the salivary alpha-amylase in the use of starch by the oral bacteria has been demonstrated. In fact, acidogenicity from cooked starch elicited by S. mutans, S. sobrinus, S. sanguinis and S. mitis was tested in the presence or absence of alpha-amylase (Aizawa et al., 2009). Only mild pH drop was detected in the absence of alpha-amylase from cooked starch. When alpha-amylase was present, however, pH dropped to a range of 3.9-4.4, with similar values among the bacterial species. Thus, cooked starch has cariogenic potential, but only in the presence of salivary alpha-amylase.
To date, there is still debate over if the combination of the sugars is more cariogenic than the two sugars separately. These inconsistencies could derive from the conditions in which the studies were conducted or to the microorganisms used, which could influence cariogenicity of the biofilms. One of the differences among the in vitro studies could be the result of the presence or absence of alpha-amylase. This enzyme is involved in the hydrolysis of highly processed starch. Therefore, a model which consistently incorporates saliva as a source of alpha-amylase during the experiment could create an environment that more closely resembles that of the mouth. Using a biofilm-caries model with the steady presence of saliva and therefore of alpha-amylase, we showed a synergistic effect in the cariogenicity of both carbohydrates, which surpasses even that of sucrose (Botelho, Villegas-Salinas, Troncoso-Gajardo, Giacaman, & Cury, 2016). The group treated with starch and sucrose caused the greatest demineralization, as well as the greatest decline in pH levels, being even greater than the positive control of sucrose alone. Highly processed starch alone induced very low cariogenicity in the model. Bacterial biomass recovered by the end of the experiments in the groups exposed to starch and sucrose was greater than that treated only with starch. Consistent with previous studies (Xiao & Koo, 2010), it was suggested that the amount of biomass is a result of the greater production of insoluble EPS in the matrix when the biofilm is exposed to this combination of sugars. A plausible explanation for this result is that it was caused by the induction of the gtfB gene, which increases its activity (i.e., production of insoluble EPS) while gtfC and gtfD genes are associated with the production of intracellular polysaccharides and soluble EPS (Duarte et al., 2008).
4.4 Oligosaccharides or glucose polymers
A large number of processed foods contain high amount of sugars in the form of glucose polymers, also referred to as starch hydrolysates (StH). Total sugars on a food label includes only mono and disaccharides and it does not include other oligosaccharides as glucose polymers (maltodextrins, glucose syrups), as these are not strictly speaking sugars, but they are fermentable and therefore, potentially cariogenic. StH derive from enzymatic or chemical partial hydrolysis of starch. Several types of StH can be distinguished, based on their molecular size, which is expressed as a value of dextrose equivalent (DE). Thus, StH are called maltodextrins or glucose syrups if they have a DE lower than 20 or higher than 20, respectively (Dongowski, 1997). Usually contained in several types of foods, including beverages, snacks, dairy products, energy drinks and sweeteners among others, StH are widely used in the food industry to provide adhesion, stabilization, and texture to processed foods. As consumption of processed foods is increasingly higher, particularly in industrialized countries, their role on caries is of high interest. Scarce research on the effect of StH on caries has been reported. Early studies in animals showed that sucrose causes higher caries incidence than StH (Grenby & Leer, 1974). A potential mechanism to explain the latter comes from a lower pH of the dental biofilm induced by sucrose than StH (Al-Khatib, Duggal, & Toumba, 2001; Moynihan, Gould, Huntley, & Thorman, 1996), but there is lack of clarity on the subject. Hence, we decided to conduct an investigation aimed to analyze the cariogenicity of S. mutans biofilms exposed to StH of different molecular size in a relevant in vitro caries model (Troncoso, Botelho, Villegas, Giacaman, & Cury, 2011). Our data showed that treatment of S. mutans biofilms with starch or StH failed to lower the pH in enamel-formed biofilms. StH failed to induce pH drop below the critical demineralization pH threshold of enamel, but when biofilms formed on dentine were exposed to StH, pH decreased below the critical pH threshold for dentine. Similar results were reported for plaque pH in one of the few in vivo studies using maltodextrins (Al-Khatib et al., 2001). A recent study showed that when a S. mutans biofilm was exposed to sucrose and a mixture of maltodextrins and sucrose, higher acidogenicity was elicited, compared to maltodextrin and glucose alone (Stegues, Arthur, & Hashizume, 2016). Importantly, glucose polymers are also used in sports drinks, bars and in energy and food supplements used during sport practice. These glucose polymers are intended to increase energy density of products without impacting on the organoleptic properties. This is interesting in older adults with root surface exposure, prone to root caries lesions, who are usually given supplements containing StH. Mild pH drops can cause dentine demineralization and subsequently root caries. Molecular size of the StH did not appear to differentially affect cariogenicity of the carbohydrates. Despite the interesting results obtained so far, this evidence is rather weak and comes only from experimental models. Given the importance of this issue, in light of the high consumption of processed foods and the scarce information available, relevant clinical research appears necessary to inform the consumers and dental professionals about the risk posited by these products in caries development.
4.5 Cariogenic potential of non-sugar sweeteners
As free sugars are detrimental for general and oral health, alternative substances have been devised to confer sweetness to food without the deleterious effect of sugar. In that context and though not WHO recommended, artificial sweeteners are becoming increasingly used to sweeten beverages, such as soda, juice, coffee, and tea. In an era where obesity and overweight have become a serious health problem with large populations affected (Caballero, 2007), sweeteners arise as a way to replace sucrose consumption to deal with this public health matter. These products have been considered as safe in a position article from the Academy of Nutrition and Dietetics (Fitch & Keim, 2012). Importantly, most of the available research on a presumptive anticariogenic or non-cariogenic effect of sweeteners comes from the pure chemical compound. Due to their very high sweet taste, commercial sweeteners are sold in combination with bulking agents, usually fermentable carbohydrates, including dextrose, maltodextrins and lactose among others. Information on the cariogenicity of the commercial products is more limited and may be of importance for enamel and dentine caries. Given the fact that evidence on the caries effect of sweeteners is still inconclusive (Stillman-Lowe, 2005), we tested the cariogenic potential on enamel and the effect on S. mutans biofilms of several commercial sweeteners in an experimental caries model (Giacaman, Campos, Muñoz-Sandoval, & Castro, 2013). All tested commercial sweeteners showed lower enamel demineralization than sucrose. Only saccharine showed less biomass and intracellular polysaccharides than the rest of the groups, suggesting a cytotoxic effect on bacteria. Stevia, sucralose, and saccharine reduced the number of viable cells when compared with sucrose. All sugar alternatives reduced EPS formation when compared with sucrose. Most commercial sweeteners appeared to be less cariogenic than sucrose, but still retaining some enamel demineralization potential. Products containing stevia, sucralose, and saccharine showed antibacterial properties and seem to interfere with bacterial metabolism. Like with antioxidants, only limited research exists to be conclusive. At this point, it is possible to conclude that a body of experimental evidence shows that highly processed starches, especially in the presence of sugars, contribute to the caries process and as such, the data suggest that foods containing both processed starches and sugars pose a risk to dental health. Highly processed starch and commercial sweeteners must be consumed and recommended cautiously, as they must not be considered caries-safe. There has been intense debate on the carcinogenic roperties of sweeteners and some of them have been banned in some countries (Carocho, Morales, & Ferreira, 2017). Finally, whether the lower cariogenicity observed for some of the products and molecules remains upon the concomitant high and or frequent consumption of sucrose or on the contrary, the effect of sucrose exceeds the benefit of consuming the other nutrients, is a matter that needs to be considered in further clinical studies.
4.6 Fatty acids as novel anticaries agents
Lipids are ubiquitous dietary nutrients in most normal diets and usually ignored in their effect on caries or in sugar-based studies. Although an anticariogenic effect of dietary fats has been claimed for a long time (Bowen, 1994; Kabara, 1986), it seems that the research line was not followed by other groups. Lipids are an extensive group of molecules that include fatty acids and their derivatives. The recommended amount of lipids to be consumed should be 15% to 30% of the energy in human diet (Irz, Shankar, & Srinivasan, 2003). Although the term lipid is used as synonym for fats, these substances are a subgroup of lipids called triglycerides. A triglyceride is an ester derived from glycerol and three fatty acids. Fatty acids are carboxylic acids with a long aliphatic tail. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28, which may be either saturated or unsaturated (Tvrzicka, Kremmyda, Stankova, & Zak, 2011). A saturated fatty acid has all the carbons of its chain bonded to hydrogen, whereas unsaturated molecules have double bonds (C=C) between carbon atoms. When fatty acids are not attached to other molecules, they are known as “free” fatty acids or “non-esterified fatty acids.”
Mechanisms associated with a putative anticaries activity of fatty acids are diverse and include the following: antimicrobial activity (Kabara, Swieczkowski, Conley, & Truant, 1972), bacteriostatic properties (Hayes, 1984; Williams, Schemehorn, McDonald, Stookey, & Katz, 1982), and the lack of metabolization of these substances by biofilm bacteria (Schuster et al., 1980). Most of the evidence on the effect of fatty acids on caries has been collected in animal studies. Compared with those treated with sucrose-rich diets, animals fed with fat-rich diets have lower caries rates (Osborn, Carey, & Fisher, 1966; Williams et al., 1982). In recent studies, an antibacterial activity against S. mutans of several free fatty acids in low concentrations, including linoleic and oleic, has been confirmed (Huang, Alimova, Myers, & Ebersole, 2011; Huang & Ebersole, 2010; Huang, George, & Ebersole, 2010). An inhibition of nutrient transport though the cell membrane and cell adhesion by fatty acids has been proposed (Williams et al., 1982). Other alternative mechanisms to explain the antibacterial activity of fatty acids include enzymatic activity, interference with oxidative phosphorylation, leakage of intracellular products, peroxidation, and auto-oxidation products of the fatty acids and cell lysis (Desbois & Smith, 2010). Yet, the exact mechanism remains unclear. Based on the scarce research in the field, we conducted a series of studies to test the potential protective effect of fatty acids on caries. Using an in vitro pH-cycling biofilm-caries model, biofilms of S. mutans were exposed to different types of free fatty acids after cariogenic challenges with sucrose (Giacaman, Jobet-Vila, & Muñoz-Sandoval, 2015). The chief finding from this study was that S. mutans biofilms exposed to sucrose first and then to unsaturated free fatty acids reduced the cariogenicity induced by sucrose. Noticeably, the effect was not only on the acidogenicity and demineralization of the enamel, but also on the biofilm properties. The demineralization inhibition induced by the presence of fatty acids observed in our study was consistent with the results from classic studies using animal models (Gustafsson, Stelling, Abramson, & Brunius, 1955; Hayes & Berkovitz, 1979). When fatty acids are included in a sugar-containing diet in rats, caries scores significantly decreased when compared with the same diet without the fatty acids (Williams et al., 1982). Likewise, although the monounsaturated (oleic, ω-6) and the polyunsaturated (linoleic) acids reduced demineralization at two of the concentrations tested, linoleic acid showed lower demineralization at a lower concentration. Polyunsaturated linoleic and monounsaturated oleic fatty acids resulted in higher antibacterial activity than the saturated stearic acid. The saturated stearic acid showed neither reduction in demineralization nor an effect on the biofilm properties. Sucrose exposure to the biofilm followed by oleic and linoleic fatty acid exposure showed less biomass than sucrose alone. As biofilm biomass comprises bacterial cells and polysaccharides (Paes Leme, Koo, Bellato, Bedi, & Cury, 2006), we speculated that these unsaturated fatty acids act on both, bacterial killing and metabolic inhibition, resulting in lower polysaccharide production. Indeed, growth inhibition or the direct killing of bacteria has been described as the major antibacterial mechanism of fatty acids (Desbois & Smith, 2010). As our experiments were conducted using a single-species biofilm model, clinical evidence was missing. To verify the anticaries activity of the free fatty acids, but now with the entire dental biofilm, an in situ study, with a crossingover, split-mouth, and double-blind design, was carried out by our research group (Giacaman, Valenzuela-Ramos, & Munoz-Sandoval, 2016). Eleven young adult healthy volunteers wore an acrylic palatal device holding enamel slabs for 15 days. The biofilm formed on each slab was submitted to a highly cariogenic challenge by exposure to 20% sucrose eight times per day for 5 min. In four of the eight challenges, sucrose exposure was immediately followed by exposure to the experimental treatment with free fatty acids for five additional minutes, including oleic, stearic, and linoleic acids, prepared at a concentration of 10 mM, to resemble concentrations previously used in vitro (Giacaman, Jobet-Vila et al., 2015). One of the study groups was exposed only to 20% sucrose and served as caries-positive control. Biofilms and enamel slabs were retrieved to assess biofilm traits and demineralization. Free fatty acid exposure to the biofilm after sucrose decreased enamel demineralization by inhibiting cariogenicity of the biofilm formed on the slabs. Poly-and monounsaturated long-chain fatty acids appeared to be effective in counteracting the deleterious effect of a highly cariogenic challenge exerted on enamel. Further clinical evidence from randomized controlled trials is needed to claim a clinical effect. Taken together, lipids, particularly unsaturated free fatty acids, may become a novel anticaries strategy. It is not possible to rule out the possibility that a potential caries inhibitory effect of increasing fat consumption derives from a concomitant decrease of carbohydrates in the diet. The use of organic, non-toxic approaches is of high interest, nevertheless. As therapeutic tools, fatty acids might be targeted as food supplements or as pharmacological agents. There may be a concern in recommending increased lipid consumption for caries control, due to potential systemic adverse effects. In a recent systematic review carried out analyzing data from 40 countries, however, results showed that about 50% of those countries had a mean consumption of fat below the recommended amount, specifically in polyunsaturated fatty acids (Harika, Eilander, Alssema, Osendarp, & Zock, 2013). Recommendations are to consume between 20% and 35% of total daily energy intake as total fat, less than 10% of the energy as saturated fatty acids and between 6% and 11% of energy as polyunsaturated fatty acids (FAO, 2010). Likewise, for almost half of the countries, the majority of the population consumed less than 6% of the daily energy as polyunsaturated fatty acids. To prevent coronary heart disease, people must meet the recommended amount of fatty acids. Because in most countries consumption of fatty acids does not meet the recommended levels to prevent chronic diseases, using these compounds in caries prevention results reasonable, as it would serve both purposes. As stated above for sugars consumption and obesity/diabetes, the dental profession may join efforts to cope with caries along with public health measures to concomitantly tackle cardiovascular diseases.
4.7 Dietary proteins and their potential role as anticariogenic agents
Along with unsaturated fatty acids from the diet, dietary proteins may act as anticaries substrate when presented from food to the dental biofilm. Higher consumption of dietary proteins has been associated with lower caries incidence and with a favorable microbiological shift (Burne & Marquis, 2000). Protein milk components, including casein, have been claimed to reduce demineralization by the formation of a thin layer that would inhibit bacterial adhesion (Papas et al., 1989). Most of the studies, however, have been performed on experimental animals with protein supplementation in a mixed diet (Dodds, 1964; Osborn et al., 1966). Those early experiments performed with rats showed that the supplementation with egg white or casein in a diet of whole wheat decreased the incidence of carious lesions. Supplementation with the amino acids lysine, methionine, and threonine, however, did not show an effect on caries. To verify the individual effect of the proteins on caries, we carried out experiments to test the hypothesis that egg ovalbumin inhibits the cariogenicity of biofilms of S. mutans under a highly cariogenic environment (Giacaman, Jobet-Vila & Muñoz-Sandoval, 2014). Using a biological caries model of S. mutans on enamel and dentine slabs, biofilms were exposed three times per day to 10% sucrose during five minutes. Immediately after sucrose, biofilms were exposed to serial dilutions of an ovalbumin solution for five additional minutes with the appropriate controls. When the biofilms and the dental slabs were analyzed, a reduction in biomass, EPS formation, but not in the number of viable cells, was observed for both dental substrates. All ovalbumin concentrations tested showed lower demineralization than sucrose alone, in a dose-dependent manner. The highest ovalbumin concentration showed an inhibitory effect on demineralization of about 30%. Aware of the limitations imposed by an in vitro approach, we aimed to confirm our findings with a relevant in situ model (Giacaman, Jara, & Valenzuela-Ramos, 2015). Cariogenicity of the whole biofilm and the multiple other oral elements present in the oral cavity could be different than a single-species model with all the variables controlled. Following the same experimental setting than that used for the in situ study with fatty acids (above), we recruited volunteers to participate in the study. Results after the experimental period showed that biofilms exposed to 20% sucrose followed by 200 μg/ml and 100 μg/ml ovalbumin showed a dose-dependent reduction in demineralization when compared with biofilms that only were exposed to sucrose. The effect of ovalbumin in the biofilm was mild, reducing only the biomass and insoluble EPS formation, but without a noticeably antibacterial activity. These effects are directed on bacterial biofilms and, as mentioned before, have obvious limitations derived from the experimental nature of them. Additional strategies to study the anticaries properties of certain proteins in the biofilm are required, even beyond the effect on the microbiome composition. For example, a metabolomic approach has been taken with interesting results (reviewed in Takahashi, 2015). Dental biofilm bacteria may locally use proteins for alkalization and acid neutralization via the arginine deiminase system (ADS), urease, or other systems to hamper pH drop. Protein effect on caries may also arise from the systemic metabolization of the nutrients in the body and not necessarily from a local effect alone. In that sense, we decided to initially explore the hypothesis that higher protein consumption was inversely associated with caries experience. We designed a cross-sectional study in schoolchildren of different socioeconomic background (Giacaman, Valenzuela, & Asbún, 2015). The aim was to explore the association between protein consumption and caries lesions. A total of one hundred twenty schoolchildren 10-11 years old were included from low and high socioeconomic status (SES). Caries status was assessed by ICDAS, and dietary nutrient intake information was obtained from a 24 hr recall and a food frequency questionnaire (FFQ). Participants were divided according to the number of lesions to assess their food/nutrient consumption patterns. We found that low-caries children consumed more protein (53.32 g/day) than participants with high number of lesions (45.56 g/day). Specifically, high-caries children from lower SES consumed significantly lower amount of proteins (20.21 g/day) than low-caries children (46.44 g/day). These results suggest that higher protein consumption could act as a protective factor for caries in children, confirming our previous results, both in vitro and in situ. These results, however, need to be controlled for level of sugars intake, to appreciate the actual magnitude of the effect. In spite of the lack of clarity on the mechanism, certain ideas may be proposed at this point. Proteins may inhibit the caries process by the metabolic use of the available peptides by oral bacteria. Several oral bacterial species, such as S. sanguinis, S. gordonii and S. salivarius, may contribute to saliva alkalization by metabolizing peptides to ammonium through the urease and the ADS enzymes (Huang, Schulte, Burne, & Nascimento, 2015; Nascimento et al., 2014). The ADS plays a key physiological function in bacteria, providing protection from the deleterious effects of low pH while generating ATP for growth and maintenance. Ammonia can keep biofilm pH more neutral even in the presence of fermentable carbohydrates. The hydrolysis of urea and the catabolism of arginine are the primary sources of bacteria-generated alkali in dental biofilms. Urea is not uncommon in the oral environment. In fact, relatively high concentrations of urea can be found in human saliva and in gingival crevicular fluid (Kopstein & Wrong, 1977), which is rapidly metabolized by bacterial ureases. Urea has been reported as a potential anticaries agent (Clancy, Pearson, Bowen, & Burne, 2000). In a similar manner, arginine has been described as an attractive anticaries molecule. Although arginine is present in high amounts in salivary peptides and proteins, it has low levels as a free amino acid in saliva. A study reported that the levels of free arginine and free lysine in the parotid saliva of caries-free adults were significantly higher than those found in the parotid saliva of individuals with a history of dental caries (Van Wuyckhuyse et al., 1995). Importantly, the metabolism of urea and ammonium through the ureases and the ADS activity can modulate the ecology of the biofilm (Huang et al., 2015). The use of arginine in the clinical setting has shown promising results in caries. Indeed, a randomized controlled trial showed that after 2 years of use of three different dentifrices, there were no statistically significant differences with respect to DMFT or DMFS between children using the dentifrices containing 1.5% arginine or 1450 ppm fluoride (Li et al., 2015). Little research has been performed using dietary peptides, such as albumins from egg, nevertheless.
4.8 Cariogenicity of complex foods, the case of milk
A lower cariogenicity of some single macronutrients has been observed. People do not consume single macronutrients, nonetheless. Hence, analyzing the role on caries of complex foods containing various nutrients is a topic of high interest. Due to their high consumption and a favorable opinion from the public about their use, milk and dairy products are of high interest in caries research. Although cariogenic per-se, the disaccharide lactose, milk’s main carbohydrate, has long been considered less harmful than sucrose in causing tooth demineralization (Rugg-Gunn, Roberts, & Wright, 1985). The cariogenicity of milk has been debatable. While some studies state that milk can cause dental caries if consumed in high frequencies (Bowen & Pearson, 1993), other investigations conclude that bovine milk associates with lower levels of caries due to a putative anticariogenic effect (Bowen & Lawrence, 2005). The caries-preventive effect of milk may derive from milk proteins with antibacterial potential, including lactoferrin, lysozyme, and peroxidase (Bowen & Pearson, 1993), resulting in cariostatic properties. Likewise, the protein casein contained in milk is capable of forming stable complexes of calcium phosphate, which prevent demineralization of the enamel or dentine (Aimutis, 2004). With that in mind, we carried out experiments to assess the cariogenic potential of milk in an experimental caries model with biofilms of S. mutans (Muñoz-Sandoval, Muñoz-Cifuentes, Giacaman, Ccahuana-Vasquez, & Cury, 2012). The biofilm formed on the tooth samples was exposed to milk and compared with a solution of pure sucrose, as a positive control. Results from those studies indicated that bovine milk appears to be less cariogenic than sucrose. Anticaries molecules present in whole milk failed to avoid the cariogenic effect of lactose on enamel and dentine demineralization. Hence, bovine milk should not be considered caries-safe, mainly for root dentine. Given the complexity of milk’s composition, it is possible to speculate that variations in some of its components could affect cariogenicity. Furthermore, many alternative milk beverages or different dairy products have become available and are becoming popular, with unknown cariogenic potential. There are several bovine milk types commercially available, including whole, skimmed, semi-skimmed, lactose-free, or sugar-containing milk. Despite the large variety of commercial milk types, little scientific evidence is available about their caries effect. How changes in milk composition can affect cariogenicity has been scarcely investigated. Based on the findings abovementioned about the potential anticaries effect of free fatty acids, it would be reasonable to think that variations in the level of fat might affect milk’s cariogenicity. We compared the cariogenicity of different commercially available bovine milks in an artificial caries model with biofilms of S. mutans (Giacaman & Muñoz-Sandoval, 2014). Our findings indicated that whole milk, containing all its fat, may be less cariogenic than a 10% sucrose solution, but not anticariogenic. Strikingly, skimmed milk resulted as cariogenic as a 10% sucrose solution, confirming the idea of a caries-protective effect of fat. The commercial lactose-free milk in reality is regular milk to which lactase has been added to lyse lactose. The individual monosaccharides glucose and galactose, however, remain and may be fermented. Hence, cariogenicity of lactose-free milk was similar to whole milk. It is common practice for many people to add sugar to milk. In our model, milk supplemented with 10% sucrose induced a cariogenicity as high as sucrose alone at the same concentration. In summary, bovine milk in most of its commercial types may be less cariogenic than sucrose, but not anticariogenic. Other milk-derived products may have implications is caries. For example, cheese consumption has been reported to be anticariogenic for a long time and experimental evidence in humans exists (Rugg-Gunn, Edgar, Geddes, & Jenkins, 1975). Although the exact mechanism has not been fully elucidated, several potential explanations have been proposed, including lowering critical pH due to calcium and phosphorus diffusion within the dental biofilm, buffering capacity from salivary stimulation from cheese, fatty acid bacterial inhibition, cheese protein adsorption on the tooth acting as a buffer (Rosen et al., 1984). Although mixed foods may contain variable quantities of anticaries components, the resulting effect on caries will depend on many variables, such as the frequency of consumption, the relative proportion of the protective nutrient/substance, or the amount of sugars contained in the food. Further clinical research appears needed to provide evidence on specific dietary recommendations on how to indicate these nutritional interventions against caries.
5 CONCLUSIONS AND IMPLICATIONS
It is clear that sugars consumption and its consequent acid production by the dental biofilm is the main causative factor for caries. Besides maintain a correct oral hygiene by toothbrushing and receiving rationalized preventive measures, such as fluoridated products, preventive efforts must focus on minimizing the frequency of sugars consumption to tackle the disease. The uncontrolled increase in diabetes and obesity worldwide shares the same causal agent than caries; sugars consumption. Joined efforts, therefore, must be taken to reduce the intake and to educate the population. Besides free sugars consumption, it is important to highlight the potential risks of consuming processed and hydrolyzed starches especially when consumed in conjunction with free sugars, as these processed carbohydrates can increase the cariogenicity of sucrose or other fermentable carbohydrates. Many complex carbohydrates of the diet, such as starch, retain a cariogenic potential, but more importantly, they can increase sucrose cariogenicity when consumed together.
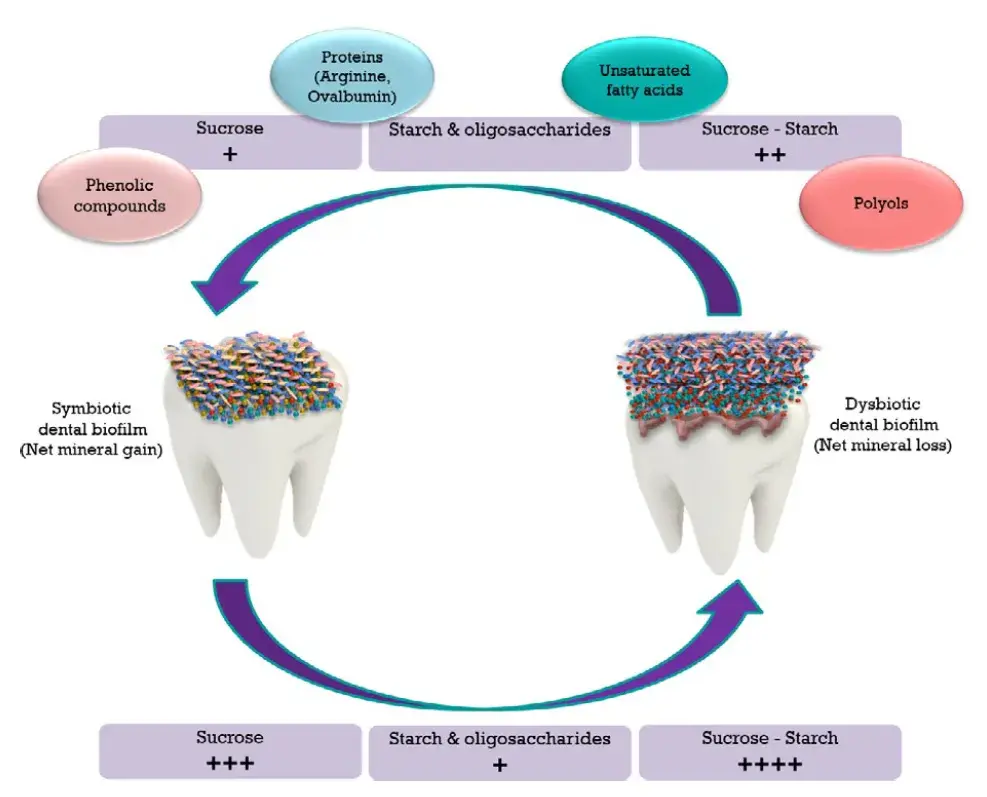 FIGURE 1. Summary of the influence of nutrients on the caries process. A healthy tooth (left) is colonized by a commensal biofilm, maintaining a state of symbiosis within the bacterial consortium. Upon frequent and sustained fermentable carbohydrate exposure (bottom), the biofilm undergoes an ecological shift leading to a state of dysbiosis (right), characterized by the predominance of acidogenic species, creating an aciduric biofilm environment. Increased acidogenicity in the biofilm will lead to a net mineral loss and the onset of lesions (right). The differential cariogenic potential of the carbohydrates is represented by “+.” Concomitant exposure of sugars with other nutrients and dietary substances may reduce sugars’ cariogenic potential (top), restoring the ecological equilibrium in the biofilm, turning the demineralization–remineralization process toward a net mineral gain (left), or at least to attenuate the deleterious effects of sugars on the dental biofilm and on the minealized tissues
FIGURE 1. Summary of the influence of nutrients on the caries process. A healthy tooth (left) is colonized by a commensal biofilm, maintaining a state of symbiosis within the bacterial consortium. Upon frequent and sustained fermentable carbohydrate exposure (bottom), the biofilm undergoes an ecological shift leading to a state of dysbiosis (right), characterized by the predominance of acidogenic species, creating an aciduric biofilm environment. Increased acidogenicity in the biofilm will lead to a net mineral loss and the onset of lesions (right). The differential cariogenic potential of the carbohydrates is represented by “+.” Concomitant exposure of sugars with other nutrients and dietary substances may reduce sugars’ cariogenic potential (top), restoring the ecological equilibrium in the biofilm, turning the demineralization–remineralization process toward a net mineral gain (left), or at least to attenuate the deleterious effects of sugars on the dental biofilm and on the minealized tissues
As normal diet comprises a wide variety of components, the effect of other nutrients on caries is of high interest. Little research has pointed toward the anticaries potential of macro-or micronutrients of the diet. In that context free fatty acids, dietary proteins, and polyphenols, among others, should be more investigated, as they appear to counteract the effect of sugars on caries (Figure 1). The role of diet on caries should not only be seen from a caries-causing standpoint, but also from a caries-protective perspective. A more holistic view of diet and nutrition should be incorporated into the curricula of the dental schools. Reducing sugars consumption may be a tough goal to achieve, so an emphasis on the other food components that can protect against caries results a novel approach to cope with the disease. Definitive answers to important questions are still unknown. For example, if specific food components must be developed as therapeutics in order to be effective against caries. Or if the form or frequency of cariogenic food consumption has to be offset with equivalency or even larger amounts of beneficial food consumption. Is compliance feasible? Further molecular and overall clinical research will unveil the mechanisms and practical effects of this alternative strategy to help caries control, to mitigate the impact of a diet containing free sugars. More dental courses are accessible for you to learn on our platform.
REFERENCES
Aas, J. A., Griffen, A. L., Dardis, S. R., Lee, A. M., Olsen, I., Dewhirst, F. E., … Paster, B. J. (2008). Bacteria of dental caries in primary and permanent teeth in children and young adults. Journal of Clinical Microbiology, 46, 1407–1417.
Aimutis, W. R. (2004). Bioactive properties of milk proteins with particular focus on anticariogenesis. Journal of Nutrition, 134, 989S–995S.
Aires, C. P., Del Bel Cury, A. A., Tenuta, L. M., Klein, M. I., Koo, H., Duarte, S., & Cury, J. A. (2008). Effect of starch and sucrose on dental biofilm formation and on root dentine demineralization. Caries Research, 42, 380–386.
Aizawa, S., Miyasawa-Hori, H., Nakajo, K., Washio, J., Mayanagi, H., Fukumoto, S., & Takahashi, N. (2009). Effects of alpha-amylase and its inhibitors on acid production from cooked starch by oral streptococci. Caries Research, 43, 17–24.
Al-Khatib, G. R., Duggal, M. S., & Toumba, K. J. (2001). An evaluation of the acidogenic potential of maltodextrins in vivo. Journal of Dentistry, 29, 409–414. American Dietetic Association (2004). Position of the American Dietetic Association: Use of nutritive and nonnutritive sweeteners. Journal of the American Dietetic Association, 104, 255–275.
Beighton, D., & Hayday, H. (1984). The establishment of the bacterium Streptococcus mutans in dental plaque and the induction of caries in macaque monkeys (Macaca fascicularis) fed a diet containing cooked-wheat flour. Archives of Oral Biology, 29, 369–372.
Beiswanger, B. B., Boneta, A. E., Mau, M. S., Katz, B. P., Proskin, H. M., & Stookey, G. K. (1998). The effect of chewing sugar-free gum after meals on clinical caries incidence. Journal of the American Dental Association, 129, 1623–1626.
Benitez-Paez, A., Belda-Ferre, P., Simon-Soro, A., & Mira, A. (2014). Microbiota diversity and gene expression dynamics in human oral biofilms. BMC Genomics, 15, 311.
Bernabe, E., Vehkalahti, M. M., Sheiham, A., Lundqvist, A., & Suominen, A. L. (2016). The shape of the dose-response relationship between sugars and caries in adults. Journal of Dental Research, 95, 167–172.
Botelho, J. N., Villegas-Salinas, M., Troncoso-Gajardo, P., Giacaman, R.A., & Cury, J. A. (2016). Enamel and dentine demineralization by a combination of starch and sucrose in a biofilm-caries model. Brazilian Oral Research, 30, e52.
Bowen, W. H. (1994). Food components and caries. Advances in Dental Research, 8, 215–220.
Bowen, W. H., & Koo, H. (2011). Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Research, 45, 69–86.
Bowen, W. H., & Lawrence, R. A. (2005). Comparison of the cariogenicity of cola, honey, cow milk, human milk, and sucrose. Pediatrics, 116, 921–926.
Bowen, W. H., & Pearson, S. K. (1993). Effect of milk on cariogenesis. Caries Research, 27, 461–466.
Bratthall, D., & Hansel Petersson, G. (2005). Cariogram–a multifactorial risk assessment model for a multifactorial disease. Community Dentistry and Oral Epidemiology, 33, 256–264.
Burne, R. A., & Marquis, R. E. (2000). Alkali production by oral bacteria and protection against dental caries. FEMS Microbiology Letters, 193, 1–6.
Burt, B. A. (2006). The use of sorbitol-and xylitol-sweetened chewing gum in caries control. Journal of the American Dental Association, 137, 190–196.
Burt, B. A., & Szpunar, S. M. (1994). The Michigan study: The relationship between sugars intake and dental caries over three years. International Dental Journal, 44, 230–240.
Caballero, B. (2007). The global epidemic of obesity: An overview. Epidemiologic Reviews, 29, 1–5.
Carocho, M., Morales, P., & Ferreira, I. (2017). Sweeteners as food additives in the XXI century: A review of what is known, and what is to come. Food and Chemical Toxicology, 107, 302–317.
Ccahuana-Vasquez, R. A., Tabchoury, C. P., Tenuta, L. M., Del Bel Cury, A. A., Vale, G. C., & Cury, J. A. (2007). Effect of frequency of sucrose exposure on dental biofilm composition and enamel demineralization in the presence of fluoride. Caries Research, 41, 9–15.
Clancy, K. A., Pearson, S., Bowen, W. H., & Burne, R. A. (2000). Characterization of recombinant, ureolytic Streptococcus mutans demonstrates an inverse relationship between dental plaque ureolytic capacity and cariogenicity. Infection and Immunity, 68, 2621–2629.
Cury, J. A., Rebello, M. A., & Del Bel Cury, A. A. (1997). In situ relationship between sucrose exposure and the composition of dental plaque. Caries Research, 31, 356–360.
Desbois, A. P., & Smith, V. J. (2010). Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Applied Microbiology and Biotechnology, 85, 1629–1642.
Deshpande, A., & Jadad, A. R. (2008). The impact of polyol-containing chewing gums on dental caries: A systematic review of original randomized controlled trials and observational studies. Journal of the American Dental Association, 139, 1602–1614.
Diaz-Garrido, N., Lozano, C., & Giacaman, R. A. (2016). Frequency of sucrose exposure on the cariogenicity of a biofilm-caries model. European Journal of Dentistry, 10, 345–350.
Dodds, M. L. (1964). Protein and lysine as factors in the cariogenicity of a cereal diet. Journal of Nutrition, 82, 217–223.
Dongowski, G. (1997), R. L. Whistler and J. N. BeMiller: Carbohydrate Chemistry for Food Scientists. 241 pages. St. Paul, MN, USA, Eagan Press, 41: 318.
Duarte, S., Klein, M. I., Aires, C. P., Cury, J. A., Bowen, W. H., & Koo, H. (2008). Influences of starch and sucrose on Streptococcus mutans biofilms. Oral Microbiology and Immunology, 23, 206–212.
Eckert, R., Sullivan, R., & Shi, W. (2012). Targeted antimicrobial treatment to re-establish a healthy microbial flora for long-term protection. Advances in Dental Research, 24, 94–97.
Eicher-Miller, H. A., Fulgoni, V. L. 3rd, & Keast, D. R. (2012). Contributions of processed foods to dietary intake in the US from 2003-2008: A report of the food and nutrition science solutions joint task force of the academy of nutrition and dietetics, American Society for Nutrition, institute of food technologists, and international food information council. Journal of Nutrition, 142, 2065s–2072s.
European Union (2008). Regulation (EC) No 1333/2008 of The European Parliament and of the Council of 16 December 2008 On Food Additives.
FAO (2010). Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food and Nutrition Paper, 91, 1–166.
Fejerskov, O. (2004). Changing paradigms in concepts on dental caries: Consequences for oral health care. Caries Research, 38, 182–191.
Ferrazzano, G. F., Amato, I., Ingenito, A., Zarrelli, A., Pinto, G., & Pollio, A. (2011). Plant polyphenols and their anti-cariogenic properties: A review. Molecules, 16, 1486–1507.
Firestone, A. R., Schmid, R., & Muhlemann, H. R. (1984). Effect of the length and number of intervals between meals on caries in rats. Caries Research, 18, 128–133.
Fitch, C., & Keim, K. S. (2012). Position of the academy of nutrition and dietetics: Use of nutritive and nonnutritive sweeteners. Journal of the Academy of Nutrition and Dietetics, 112, 739–758.
Flemming, H. C., & Wingender, J. (2010). The biofilm matrix. Nature Reviews Microbiology, 8, 623–633.
Giacaman, R. A., Araneda, E., & Padilla, C. (2010). Association between biofilm-forming isolates of mutans streptococci and caries experience in adults. Archives of Oral Biology, 55, 550–554.
Giacaman, R. A., Campos, P., Muñoz-Sandoval, C., & Castro, R. J. (2013). Cariogenic potential of commercial sweeteners in an experimental biofilm caries model on enamel. Archives of Oral Biology, 58, 1116–1122.
Giacaman, R. A., Contzen, M. P., Yuri, J. A., & Muñoz-Sandoval, C. (2014). Anticaries effect of an antioxidant-rich apple concentrate on enamel in an experimental biofilm-demineralization model. Journal of Applied Microbiology, 117, 846–853.
Giacaman, R., Jara, C., & Valenzuela-Ramos, R. (2015). Anticariogenic activity of egg ovalbumin after sucrose exposure to the oral biofilm on enamel, in situ. 62th ORCA Congress July 1–4, Brussels, Belgium Abstracts Index N 22. Caries Research, 2015(49), 1–72.
Giacaman, R. A., Jobet-Vila, P., & Muñoz-Sandoval, C. (2014). Anti-caries activity of egg ovalbumin in an experimental caries biofilm model on enamel and dentin. 61th ORCA Congress July 2–5, Greifswald, Germany. Abstracts. Index N 9. Caries Research, 2014(48), 384–450.
Giacaman, R. A., Jobet-Vila, P., & Muñoz-Sandoval, C. (2015). Fatty acid effect on sucrose-induced enamel demineralization and cariogenicity of an experimental biofilm-caries model. Odontology, 103, 169–176.
Giacaman, R. A., & Muñoz-Sandoval, C. (2014). Cariogenicity of different commercially available bovine milk types in a biofilm caries model. Pediatric Dentistry, 36, 1–6.
Giacaman, R., Valenzuela, V., & Asbún, K. (2015). Dietary Protein Consumption and Caries Prevalence by ICDAS in Schoolchildren. International Association for Dental Research (IADR) 93rd General Session and Exhibition, Boston, MA, EE.UU. March 12, 2015. Abstract.
Giacaman, R. A., Valenzuela-Ramos, R., & Munoz-Sandoval, C. (2016). In situ anticariogenic activity of free fatty acids after sucrose exposure to oral biofilms formed on enamel. American Journal of Dentistry, 29, 81–86.
Grenby, T. H., & Leer, C. J. (1974). Reduction in ‘smooth-surface’ caries and fat accumulation in rats when sucrose in the drinking-water is replaced by glucose syrup. Caries Research, 8, 368–372.
Gustafsson, G., Stelling, E., Abramson, E., & Brunius, E. (1955). Experiments with various fats in a cariogenic diet. IV. Experimental dental caries in golden hamsters. Acta Odontologica Scandinavica, 13, 75–84.
Harika, R. K., Eilander, A., Alssema, M., Osendarp, S. J., & Zock, P. L. (2013). Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: A systematic review of data from 40 countries. Annals of Nutrition & Metabolism, 63, 229–238.
Hayes, M. L. (1984). The effects of fatty acids and their monoesters on the metabolic activity of dental plaque. Journal of Dental Research, 63, 2–5.
Hayes, C. (2001). The effect of non-cariogenic sweeteners on the prevention of dental caries: A review of the evidence. Journal of Dental Education, 65, 1106–1109.
Hayes, M. L., & Berkovitz, B. K. (1979). The reduction of fissure caries in Wistar rats by a soluble salt of nonanoic acid. Archives of Oral Biology, 24, 663–666. van Houte, J. (1980). Bacterial specificity in the etiology of dental caries. International Dental Journal, 30, 305–326.
Huang, C. B., Alimova, Y., Myers, T. M., & Ebersole, J. L. (2011). Short-and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Archives of Oral Biology, 56, 650–654.
Huang, C. B., & Ebersole, J. L. (2010). A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Molecular Oral Microbiology, 25, 75–80.
Huang, C. B., George, B., & Ebersole, J. L. (2010). Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Archives of Oral Biology, 55, 555–560.
Huang, X., Schulte, R. M., Burne, R. A., & Nascimento, M. M. (2015). Characterization of the arginolytic microflora provides insights into pH homeostasis in human oral biofilms. Caries Research, 49, 165–176.
Hwang, G., Liu, Y., Kim, D., Li, Y., Krysan, D. J., & Koo, H. (2017). Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathogens, 13, e1006407.
Irz, X., Shankar, B., & Srinivasan, C. S. (2003). Dietary Recommendations in the Report of a Joint WHO/FAO. Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases (WHO Technical Report Series).
Jensen, M. E., & Schachtele, C. F. (1983). Plaque pH measurements by different methods on the buccal and approximal surfaces of human teeth after a sucrose rinse. Journal of Dental Research, 62, 1058–1061.
Jeon, J. G., Rosalen, P. L., Falsetta, M. L., & Koo, H. (2011). Natural products in caries research: Current (limited) knowledge, challenges and future perspective. Caries Research, 45, 243–263.
Kabara, J. J. (1986). Dietary lipids as anticariogenic agents. Journal of Environmental Pathology, Toxicology and Oncology, 6, 87–113.
Kabara, J. J., Swieczkowski, D. M., Conley, A. J., & Truant, J. P. (1972). Fatty acids and derivatives as antimicrobial agents. Antimicrobial Agents and Chemotherapy, 2, 23–28.
Kassebaum, N. J., Bernabé, E., Dahiya, M., Bhandari, B., Murray, C. J., & Marcenes, W. (2015). Global burden of untreated caries: A systematic review and metaregression. Journal of Dental Research, 94, 650–658.
Klein, M. I., Hwang, G., Santos, P. H., Campanella, O. H., & Koo, H. (2015). Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Frontiers in Cellular and Infection Microbiology, 5, 10.
Kolenbrander, P. E., Andersen, R. N., Blehert, D. S., Egland, P. G., Foster, J. S., & Palmer, R. J. Jr. (2002). Communication among oral bacteria. Microbiology and Molecular Biology Reviews, 66, 486–505, table of contents.
Kopstein, J., & Wrong, O. M. (1977). The origin and fate of salivary urea and ammonia in man. Clinical Science and Molecular Medicine, 52, 9–17.
Kreth, J., Giacaman, R. A., Raghavan, R., & Merritt, J. (2016). The road less traveled -defining molecular commensalism with Streptococcus sanguinis. Molecular Oral Microbiology, 32, 181–196.
Kuramitsu, H. K., He, X., Lux, R., Anderson, M. H., & Shi, W. (2007).Interspecies interactions within oral microbial communities. Microbiology and Molecular Biology Reviews, 71, 653–670.
Li, X., Zhong, Y., Jiang, X., Hu, D., Mateo, L. R., Morrison, B. M. Jr, & Zhang, Y. P. (2015). Randomized clinical trial of the efficacy of dentifrices containing 1.5% arginine, an insoluble calcium compound and 1450 ppm fluoride over two years. The Journal of Clinical Dentistry, 26, 7–12.
Lingstrom, P., Holm, J., Birkhed, D., & Bjorck, I. (1989). Effects of variously processed starch on pH of human dental plaque. Scandinavian Journal of Dental Research, 97, 392–400.
Lingstrom, P., van Houte, J., & Kashket, S. (2000). Food starches and dental caries. Critical Reviews in Oral Biology and Medicine, 11, 366–380.
Liu, R. H. (2013). Health-promoting components of fruits and vegetables in the diet. Advances In Nutrition, 4, 384S–392S.
Livesey, G. (2003). Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutrition Research Reviews, 16, 163–191.
Makinen, K. K. (2011). Sugar alcohol sweeteners as alternatives to sugar with special consideration of xylitol. Medical Principles and Practice, 20, 303–320.
Marsh, P. D. (2006). Dental plaque as a biofilm and a microbial community -implications for health and disease. BMC Oral Health, 6(Suppl 1), S14.
Matsumoto, M., Tsuji, M., Okuda, J., Sasaki, H., Nakano, K., Osawa, K., … Ooshima, T. (2004). Inhibitory effects of cacao bean husk extract on plaque formation in vitro and in vivo. European Journal of Oral Sciences, 112, 249–252.
Moynihan, P. (2007). Foods and dietary factors that prevent dental caries. Quintessence International, 38, 320–324.
Moynihan, P. J., Gould, M. E., Huntley, N., & Thorman, S. (1996). Effect of glucose polymers in water, milk and a milk substitute on plaque pH in vitro. International Journal of Paediatric Dentistry, 6, 19–24.
Moynihan, P. J., & Kelly, S. A. (2014). Effect on caries of restricting sugars intake: Systematic review to inform WHO guidelines. Journal of Dental Research, 93, 8–18.
Mundorff, S. A., Featherstone, J. D., Bibby, B. G., Curzon, M. E., Eisenberg, A. D., & Espeland, M. A. (1990). Cariogenic potential of foods. I. Caries in the rat model. Caries Research, 24, 344–355.
Muñoz-Sandoval, C., Muñoz-Cifuentes, M. J., Giacaman, R. A., Ccahuana-Vasquez, R. A., & Cury, J. A. (2012). Effect of bovine milk on streptococcus mutans Biofilm cariogenic properties and enamel and dentin demineralization. Pediatric Dentistry, 34, 197–201.
Nakahara, K., Kawabata, S., Ono, H., Ogura, K., Tanaka, T., Ooshima, T., & Hamada, S. (1993). Inhibitory effect of oolong tea polyphenols on glycosyltransferases of mutans Streptococci. Applied and Environment Microbiology, 59, 968–973.
Nascimento, M. M., Browngardt, C., Xiaohui, X., Klepac-Ceraj, V., Paster, B. J., & Burne, R. A. (2014). The effect of arginine on oral biofilm communities. Molecular Oral Microbiology, 29, 45–54.
Nyvad, B., Crielaard, W., Mira, A., Takahashi, N., & Beighton, D. (2013). Dental caries from a molecular microbiological perspective. Caries Research, 47, 89–102.
Osborn, M. O., Carey, J. F., & Fisher, A. K. (1966). Effect of dietary protein and fat on dental caries in the rat. Journal of Dental Research, 45, 1564.
Ozen, A. E., Pons, A., & Tur, J. A. (2012). Worldwide consumption of functional foods: A systematic review. Nutrition Reviews, 70, 472–481.
Paes Leme, A. F., Koo, H., Bellato, C. M., Bedi, G., & Cury, J. A. (2006). The role of sucrose in cariogenic dental biofilm formation–new insight. Journal of Dental Research, 85, 878–887.
Papas, A. S., Palmer, C. A., Rounds, M. C., Herman, J., McGandy, R. B., Hartz, S. C., … DePaola, P. (1989). Longitudinal relationships between nutrition and oral health. Annals of the New York Academy of Sciences, 561, 124–142.
Riley, P., Moore, D., Ahmed, F., Sharif, M. O., & Worthington, H. V. (2015). Xylitol-containing products for preventing dental caries in children and adults. Cochrane Database Systematic Review, CD010743.
Rosen, S., Min, D. B., Harper, D. S., Harper, W. J., Beck, E. X., & Beck, F. M. (1984). Effect of cheese, with and without sucrose, on dental caries and recovery of Streptococcus mutans in rats. Journal of Dental Research, 63, 894–896.
Rugg-Gunn, A. J., Edgar, W. M., Geddes, D. A., & Jenkins, G. N. (1975). The effect of different meal patterns upon plaque pH in human subjects. British Dental Journal, 139, 351–356.
Rugg-Gunn, A. J., Roberts, G. J., & Wright, W. G. (1985). Effect of human milk on plaque pH in situ and enamel dissolution in vitro compared with bovine milk, lactose, and sucrose. Caries Research, 19, 327–334.
Runnel, R., Makinen, K. K., Honkala, S., Olak, J., Makinen, P. L., Nommela, R., … Saag, M. (2013). Effect of three-year consumption of erythritol, xylitol and sorbitol candies on various plaque and salivary caries-related variables. Journal of Dentistry, 41, 1236–1244.
Rypins, R. (1922). Studies on the relations of types of diet to dental caries: A statistical survey of children under school age relating to two dietetic theories on the etiology of dental caries. Journal of Dental Research, 4, 405–433.
Saido, M., Asakura, K., Masayasu, S., & Sasaki, S. (2016). Relationship between dietary sugar intake and dental caries among Japanese preschool children with relatively low sugar intake (Japan Nursery School SHOKUIKU Study): A nationwide cross-sectional study. Maternal and Child Health Journal, 20, 556–566.
Schuster, G. S., Dirksen, T. R., Ciarlone, A. E., Burnett, G. W., Reynolds, M. T., & Lankford, M. T. (1980). Anticaries and antiplaque potential of free-fatty acids in vitro and in vivo. Pharmacology and Therapeutics in Dentistry, 5, 25–33.
Sheiham, A., & James, W. P. (2014). A reappraisal of the quantitative relationship between sugar intake and dental caries: The need for new criteria for developing goals for sugar intake. BMC Public Health, 14, 863.
Sheiham, A., & James, W. P. (2015). Diet and dental caries: The pivotal role of free sugars reemphasized. Journal of Dental Research, 94, 1341–1347.
Simon-Soro, A., Guillen-Navarro, M., & Mira, A. (2014). Metatranscriptomics reveals overall active bacterial composition in caries lesions. Journal of Oral Microbiology, 6, 25443.
Simón-Soro, A., & Mira, A. (2015). Solving the etiology of dental caries. Trends in Microbiology, 23, 76–82.
Stegues, C. G., Arthur, R. A., & Hashizume, L. N. (2016). Effect of the association of maltodextrin and sucrose on the acidogenicity and adherence of cariogenic bacteria. Archives of Oral Biology, 65, 72–76.
Stephan, R. M. (1940). Two factors of possible importance in relation to the etiology and treatment of dental caries and other dental diseases. Science, 92, 578–579.
Stillman-Lowe, C. (2005). Dietary factors and dental caries. Evidence Based Dentistry, 6, 7–8.
Takahashi, N. (2015). Oral microbiome metabolism: From “Who Are They?” to “What Are They Doing?”. Journal of Dental Research, 94, 1628–1637.
Takahashi, N., & Nyvad, B. (2011). The role of bacteria in the caries process: Ecological perspectives. Journal of Dental Research, 90, 294–303.
Takahashi, N., & Washio, J. (2011). Metabolomic effects of xylitol and fluoride on plaque biofilm in vivo. Journal of Dental Research, 90, 1463–1468.
Troncoso, P., Botelho, J., Villegas, M., Giacaman, R., & Cury, J. (2011). Cariogenic potential of starch hydrolysates in enamel and dentin.: IV Latin American Region Meeting and XXIV Annual Meeting of the IADR Chilean Division. October 3, Santiago, Chile.
Tvrzicka, E., Kremmyda, L. S., Stankova, B., & Zak, A. (2011). Fatty acids as biocompounds: Their role in human metabolism, health and disease–a review. Part 1: Classification, dietary sources and biological functions. Biomedical papers of the Medical Faculty of the University Palacký, Olomouc, Czechoslovakia, 155, 117–130.
