Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments
ABSTRACT
Objective: To determine whether leukocyte-platelet-rich fibrin (L-PRF) and advanced platelet-rich fibrin (A-PRF+) differ in their in vitro capacity to induce proliferation and migration of periodontal fibroblasts.
Background: L-PRF and A-PRF + are autologous materials used in periodontal regenerative surgery. They derive from blood from patients, but have different characteristics. The literature is controversial regarding the effects of the two PRF preparations on periodontal tissue fibroblasts.
Materials and methods: L-PRF and A-PRF + membranes were prepared from eight patients and incubated in 3 mL of culture medium for 2 days. Gingival fibroblasts (G-F) and periodontal ligament fibroblast (PDL-F) primary cells were retrieved from 7 donors. These cells were pre-cultured for 1 day in wound healing experiment plates leaving a gap of 500 ± 50 μm in a concentration of 3.3 x 105 cells/mL. 70 μL of the cell suspension was placed in each half of the well. Thereafter, the pre-cultured L-PRF and A-PRF + supernatants were added to the experimental plates, and the fibroblasts were incubated for another 24 h. Medium alone (NEG) and fibroblast growth factor II (FGF) were used as controls. Subsequently, cell migration was registered for 24 h with live cell imaging in a time frame microscope at 5% CO2 in air at 37°C. Images were analyzed using ImageJ. Cell proliferation and cell viability were measured.
Results: L-PRF and A-PRF + induced higher cell proliferation than FGF and NEG. Both A-PRF + and L-PRF induced significant faster artificial wound closure than controls. Both PRF conditioned media induced faster cell migration in the initial phase (P < .01), but in the stoppage phase, the induced migration was higher for the A-PRF+, compared with L-PRF (P < .01).
Conclusion: L-PRF and A-PRF + have a stimulatory effect on migration and proliferation of periodontal fibroblasts, and artificial wound closure was longer sustained by A-PRF + than L-PRF.
There are additional details about PRF application that you can obtain on our website.
1 INTRODUCTION
Periodontitis is a common chronic inflammatory disease of the tooth-supporting tissues that results in connective tissue attachment loss, alveolar bone degradation, and eventually tooth loss. The aim of periodontal therapy was to reduce the inflammatory burden and establish a healthy microbial environment. In presence of remaining pockets, surgical resective approaches are widely used, but the efficacy is based on the sacrifice of hard and soft periodontal tissues. A possible alternative to resective periodontal surgery is the regeneration of the aforementioned tissues. The aim of regenerative processes is to create a new periodontal ligament on the root surface, which consists of connective tissue fibers directly inserted on the tooth root itself and which is produced by periodontal ligament fibroblast cells (PDL-F). The regenerative potential of PDL-F makes them key players for the success of periodontal regenerative therapy. Gingival fibroblasts (G-F) are equally important in the reconstruction of proper gingival soft tissues. However, to express their regenerative potential, these cells must somehow be guided. A variety of materials and techniques have been investigated for periodontal regeneration procedures, including the “natural bone regeneration” approach which was introduced in the early 2000 by Choukroun and colleagues, on the path of the studies on platelet-rich plasma capabilities and growth factor properties performed by RE Marx. The terminology originates from the origin of the material, for which the patient's own blood is used and processed without addition of anticoagulants.
The most recent platelet-rich fibrin (PRF) concentrates are able to form a tridimensional fibrin network, containing living cells, that can act as a scaffold in the early phases of wound healing. During this phase, platelets interact with the fibrin matrix to form a hemostatic plug and induce a slow release of cytokines to stimulate cell migration and proliferation. This process is able to subsequently improve wound healing. PRF contains platelets and leukocytes (including monocytes, lymphocytes, and granulocytes). It is reported that interaction between these cells and the fibrin matrix stimulates a slow release of growth factors that may result in a better wound healing in the early phase of this process.
The first delivered procedure for the PRF preparation that includes a centrifugation step at 708 g of relative centrifugation force-max × 12 min, is called L-PRF. This protocol was developed to activate the ex vivo coagulation process. The generated fibrin matrix had solid consistence and a dense structure with minimal space between fibrin fibers. Several inflammatory cells are present within the matrix, but histologically they are located to the distal part of the clot. Recently, the protocols for the preparation of platelet fibrin concentrates have been modified, following the “Low-speed centrifugation concept” theory. The most recent of these protocols is the A-PRF + method (RCF-max: 208 g × 8 min). Studies showed that the A-PRF + preparation resulted in a higher number of platelets and leukocytes in the fibrin mesh, more widespread in the material. The generated A-PRF + fibrin matrix showed a more porous structure, allowing more space for trapped platelets and immune cells and therefore a higher and more sustained release of growth factors, compared with L-PRF. In contrast, other recent investigations showed different results, reporting better histological and mechanical characteristics in favor of L-PRF. It is still unclear whether the morphological differences that may affect the difference in growth factor release from generated PRF membranes will have a real impact on the fibroblast cells involved in the periodontal reparative and regenerative processes during wound healing. Therefore, the aim of the present study was to compare the effects of released factors by A-PRF + and L-PRF membranes on in vitro proliferation and migration of gingival and periodontal ligament fibroblast cells.
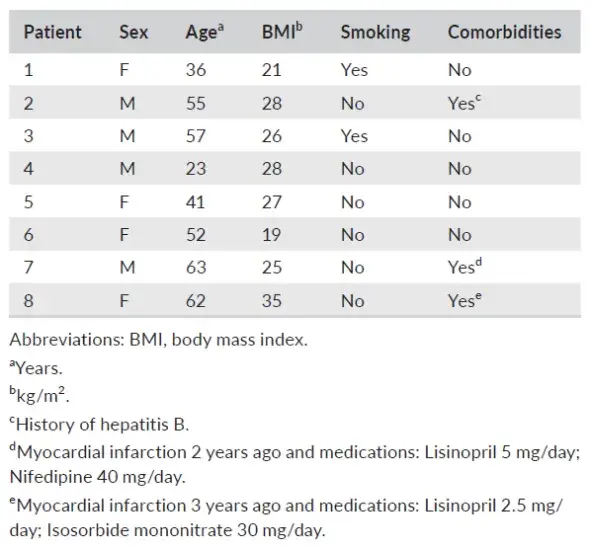
TABLE 1. Periodontitis patient background characteristics
 2 MATERIAL AND METHODS
2 MATERIAL AND METHODS
2.1 PRF preparation
The study was performed in accordance with the Medical Ethical Committee of VU Medical Center, Vrije Universiteit Amsterdam (study protocol reference 2016.530). Blood samples were collected from the antecubital fossa of 8 periodontitis patients attending the Department of Periodontology at the Academic Center for Dentistry Amsterdam (Table 1). They were selected for periodontal surgery using PRF materials, aged 25 to 65 years, and signed an informed consent (inclusion criteria: eligible for periodontal surgery, aged 18-80; exclusion criteria: pregnancy or lactation, uncontrolled diabetes, HIV positive, leukopenia, diseases related to reduced wound healing, use of anticoagulants, corticosteroids, and immunosuppressants). From each patient, seven tubes of blood were sampled; the first one was discarded, and the other tubes were taken within 60 s. Two Tubes were addressed for surgical use and, therefore, excluded from the study. The following four sampled tubes were two A-PRF + tubes (10 mL tube, glass, PRF Processe); two L-PRF tubes (9 mL tube, glass coated plastic tubes, Intra-Lock). Subsequently, the two 10 mL tubes were centrifuged at 208 g × 8 min for the A-PRF+; while the two 9 mL tubes were centrifuged at 708 g × 12 min for L-PRF p reparation. Original brand centrifuges were used for the preparation of the materials (A-PRF duo Centrifuge, PRF Process or L-PRF Intra-spin, Intra-Lock). After the designated centrifugation steps, the tubes were kept vertical for 5 min.
The fibrin clot was located in the middle part of the tube, between the red corpuscular phase at the bottom of the tube and the acellular plasma at the top. The fibrin clots from the A-PRF + and L-PRF tubes were extracted with sterile tweezers and placed in a dedicated sterile metal box device (Xpression Fabrication Kit, Intra-Lock), engineered to optimize the consistency and the thickness of the matrix via weighted homogenous pressure, for 5 min. When fibrin clots were obtained, they were transported to the laboratory and further processed under sterile conditions.
The PRF clots were removed from the compressive metal box in a sterile environment (airflow cabinet) and placed into a six-well dish with 3 mL of Dulbecco' Modified Eagle Medium (DMEM, Invitrogen Life Sciences), enriched with 10% FCI (Fetal clone I, HyClone Laboratories) and 1% PSF (Antibiotics Penicillin/Streptomycin/Amphotericin B, Sigma-Aldrich). The clots were incubated for 48 h in a 37°C incubator with 5% CO2. Subsequently, the L-PRF and A-PRF + conditioned medium was removed and immediately added to the fibroblasts cells cultures for the following 24 h (see paragraph 2.3, Wound Healing experiment).
2.2 | Fibroblasts and periodontal ligament cells isolation
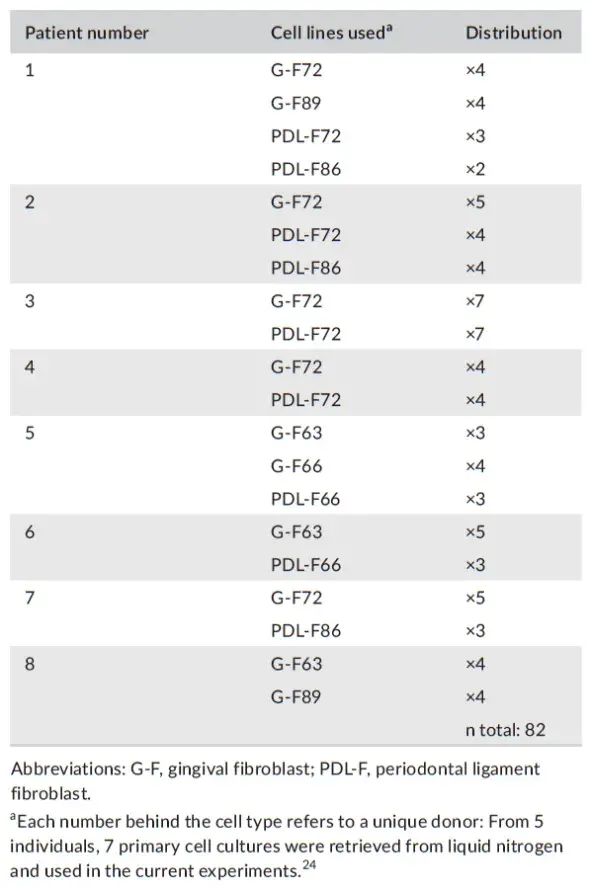
Primary G-F and PDL-F cells were obtained from extracted wisdom teeth, also used in a previous study (from five donors) which were stored in stocks in liquid nitrogen at passage 3. Signed informed consent to use these cells obtained from this operational waste material was obtained. All cell cultures were immediately after the extraction anonymized for the researcher using cell cultures, as required by Dutch law. The week before every experiment, cells were retrieved and seeded in 75 cm2 culture flasks. The experiments were performed with cells from passages 5-7. Different primary cell cultures were used per experiment in the study (Distribution of cells and patients per experiment, Table 2). The day before the experiment, the periodontal fibroblasts (G-F or PDL-F) were seeded in special cell migration culture plates having a silicon insert that separates two compartments (Culture-insert 2 Well 24, ibiTreat, Ibidi, Martinsried, Germany), in a concentration of 3.3 × 105 cells/mL. 70 μL of the cell suspension was placed in each half of the well delimited by the silicon insert, according to the manufacturer instructions, and left incubating for 1 day at 37°C incubator with 5% CO2.
TABLE 2. Patients and cell lines used
 2.3 Wound healing experiments
2.3 Wound healing experiments
To start the actual experimental conditions, the silicon inserts from the special migration plates (Culture-insert 2 Well 24, ibiTreat) were removed, leaving a gap of 500 ± 50 μm (according to manufacturer) between the fibroblasts attached to the plastic bottom that were until this moment, separated by the silicon insert. Now, these fibroblasts were incubated with the 2-day conditioned medium from either the A-PRF + or L-PRF clots or the controls. As control conditions, either 1 mL of medium (DMEM + 10% FCI + 1% PSF) or 25 ng/mL of human FGF II in 1 mL DMEM + 10% FCI + 1% PSF was used. From this moment, cell migration was monitored with a time frame microscope (Carl Zeiss Observer Z1, Carl Zeiss) at 10 × magnification, under stable pressure of 5% CO2 in air at 37°C (Tokia Hit Incubation system, Tokai Hit Co.) (See experimental flowchart, Figure 1). The cell migration was recorded at two random fields per well at 20 min time intervals for 24 h (Figure 2).
After 24h, the fibroblasts were trypsinized, and the number of cells and relative viability was determined using the protocol of Kahn et al25 that allows discrimination between life and dead cells. Cells were incubated with mix-and-read Muse Count & Viability reagent according to manufacturer (Muse Cell analyzer, Merck Millipore, Darmstadt, Germany).
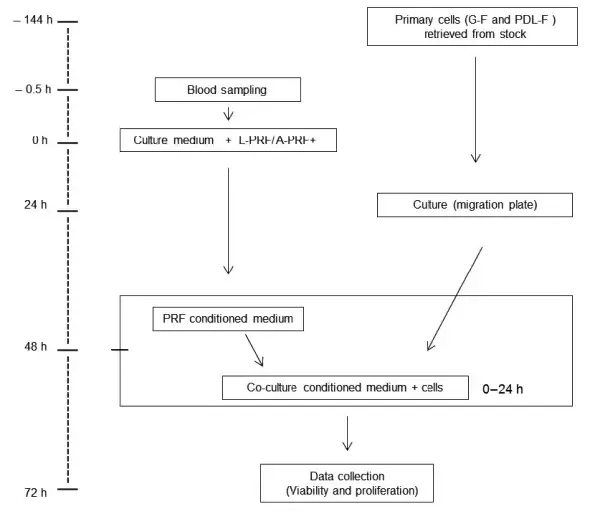 FIGURE 1. Flowchart and time line (in hours) of the experimental procedures. Abbreviations: h, hours; G-F, gingival fibroblasts; PDL-F, periodontal ligament fibroblasts; PRF, platelet-rich fibrin
FIGURE 1. Flowchart and time line (in hours) of the experimental procedures. Abbreviations: h, hours; G-F, gingival fibroblasts; PDL-F, periodontal ligament fibroblasts; PRF, platelet-rich fibrin
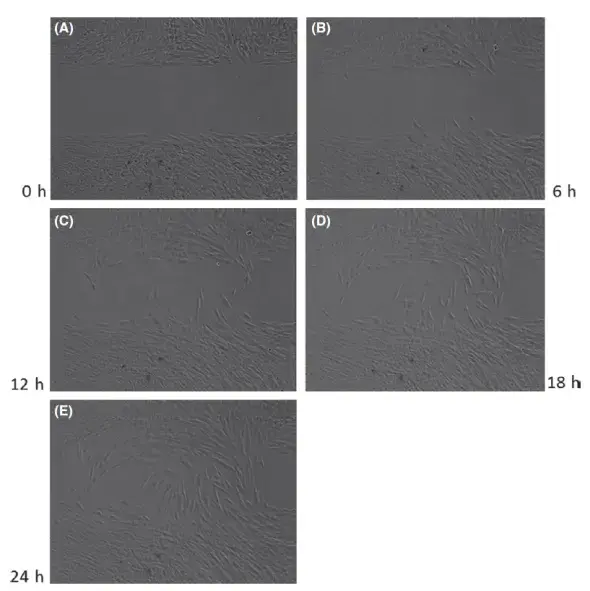 FIGURE 2. Examples of events during an experiment (Experiment 1, PDL-F72 cells incubated with condition medium from L-PRF from patient 1). Images of the artificial wound (after insert removal) were obtained every 20 mins, from 0 h to 24 h. Wound Closure was observed via time frame microscope (Carl Zeiss Observer Z1, Carl Zeiss, Goettingen, Germany) at 10 × magnification, under stable pressure of 5% CO2 in air at 37°C. (A) time = 0 h; (B) time = 6 h; (C) time = 12 h; (D) time = 18 h; (E) time = 24 h
FIGURE 2. Examples of events during an experiment (Experiment 1, PDL-F72 cells incubated with condition medium from L-PRF from patient 1). Images of the artificial wound (after insert removal) were obtained every 20 mins, from 0 h to 24 h. Wound Closure was observed via time frame microscope (Carl Zeiss Observer Z1, Carl Zeiss, Goettingen, Germany) at 10 × magnification, under stable pressure of 5% CO2 in air at 37°C. (A) time = 0 h; (B) time = 6 h; (C) time = 12 h; (D) time = 18 h; (E) time = 24 h
2.4 Image analysis
Cell migration images were analyzed with an image software (Fiji, V1.49, open source). Images were normalized in terms of white balance and transformed in Binomial (White and Black), by which the white area represented the fibroblasts and the black area the artificial wound (gap). Image closure was calculated on the raw light intensity per image. Raw light intensity was different for every image stack. To normalize the values between images stacks, the light intensity was calculated in percentage from 0% to maximum 100%. The reduction of the gap due to the migrating cells was scored in every image stack and recorded with a specific software tool (Time Series Analyzer, V3.0). Speed of closure was expressed in μm2/min, measured on the basis of the percentage of the cell closure during time, considering that the initial measured area of the gap was of 1 mm2.
On the basis of the artificial closure graph (Figure 5), three different patterns of migration were identified (Phase I: maximal migration. Phase II: linear migration. Phase III: stoppage). At the end of the experiments (total of eight experiments, one plate with 24 migration wells per experiment), 82 series of images from the behavior of seven different fibroblast cultures were available (4 G-F and 3 PDL-F).
2.5 Statistical analysis
Means and standard deviations were calculated, and data for different conditions were compared. To test normal distribution of data, Kolmogorov-Smirnov tests were used for all the data sets. The Kruskal-Wallis test was applied to test proliferation and viability differences between the conditions. To compare differences between G-F and PDL-F primary cell cultures, Wilcoxon tests were performed. In terms of migration analysis, the Friedman test was performed, corrected with Dunn's test for multiple comparisons. The speed of cell migration was tested by 1-way ANOVA corrected with the Bonferroni test for multiple comparison. Statistical analyses and graphical representation (means and standard error of the mean) were made with SPSS (IBM Corporation), GraphPad (GraphPad Software), and Excel (Microsoft Corporation).
3 RESULTS
3.1 Number of samples
PRF membranes were obtained from 8 periodontitis patients who were undergoing periodontal surgery. Patient's background characteristics are listed in Table 1. Per patient, 2 A-PRF + and 2 L-PRF membranes were obtained. Each PRF membrane was cultured for 48 h, and the conditioned medium from these cultures was added to the fibroblast cells cultures (4 G-F primary cell, 3 PDL-F cell) (see distribution in Table 2). This amounted to n = 23 experiment per A-PRF + medium, n = 20 per L-PRF, n = 21 for FGF, and n = 18 for control medium.
3.2 Effect of A-PRF+, L-PRF, FGF, and control medium on cell proliferation
Twenty-four hours before the addition of the fibrin membrane conditioned medium, the fibroblasts were seeded in the migration plates (density of 3.3 × 105 cells/mL a nd 0 .46 × 105 cells/well). The number of cells was counted again at the end of the 24 h experiment. Compared with control medium (NEG) (set at 100%, SD ± 27.7%), the proliferation of the PDL and GF combined increased by 69.2 ± 31.3% for the A-PRF+ (P = .0078), 49.6 ± 24.9% for the L-PRF (P = .0367), and 26.7 ± 22.8% for the FGF (the latter was a non-significant increase) (data presented in Figure 3). Compared with each other, although not significant, A-PRF + showed a trend for higher proliferation than L-PRF and FGF (increased proliferation 19.6% and 42.5%, respectively). Concerning L-PRF, it showed 22.9% more proliferation than FGF. However, the differences between the two types of PRF conditions and FGF failed to were not statistically significance (P > .05) (Figure 3A). These results were obtained for G-F and PDL-F combined. The results with the two fibroblasts were analyzed together, since no differences in the responses between them were observed (Figure 3B).
3.3 | No effect of A-PRF+, L-PRF, FGF, and control medium on cell viability
Fibroblast viability was tested, and no differences were observed for the periodontal fibroblasts after the 24 h incubation with A-PRF+, L-PRF, FGF, or control medium (Figure 4). And, cell viability was similar for both G-F and PDL-F separately (Data not shown).
3.4 | Both A-PRF + and L-PRF showed higher cell migration than FGF and control
The speed of closing the artificial wound of 500 ± 50 μm was recorded, and images were made every 20 minutes and analyzed by an image software program. The data are presented as percentages wound closure (Figure 5). The statistical analysis showed that A-PRF + and L-PRF induced faster migration of fibroblast cells (P < .01), compared with FGF and control (NEG) (Figure 5).
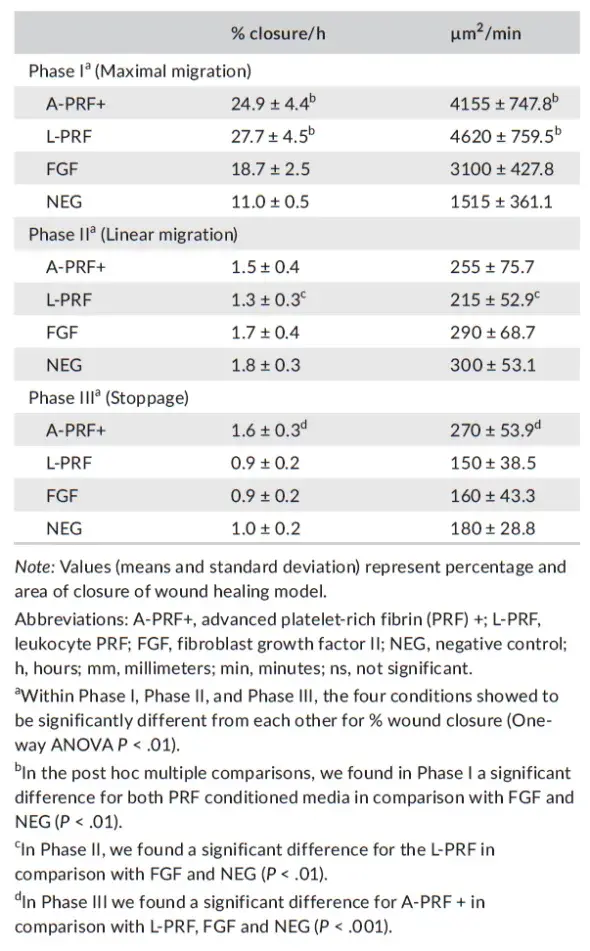
The induced cell speed of the different conditioned media showed that in phase I, the two PRF media were inducing faster cell migration compared with the negative and positive controls (P < .01), but were not different from each other. In phase II, a significantly slower speed was registered for L-PRF, compared with the FGF and control (NEG) (P < .01). In the stoppage phase (Phase III), a higher speed (ie, fibroblast cell migration) was registered for the A-PRF+, compared with all the other culture conditions (P < .01) (Table 3).
4 DISCUSSION
The aim of our study was to investigate the effects of two different PRF preparations on the migration and proliferation of G-F and PDLF. For this purpose, we designed an artificial wound healing experiment where a 48 h conditioned medium obtained from 2 different preparation techniques of PRF membranes was added to fibroblasts. Our results showed that both PRF preparations were able to promote artificial wound closure compared with controls (medium alone or human FGF-II). Furthermore, the effect of A-PRF + conditioned medium also accelerated the gap closure for a longer period of time (up to 24 h) when compared with the L-PRF conditioned medium (Phase III of the artificial wound closure).
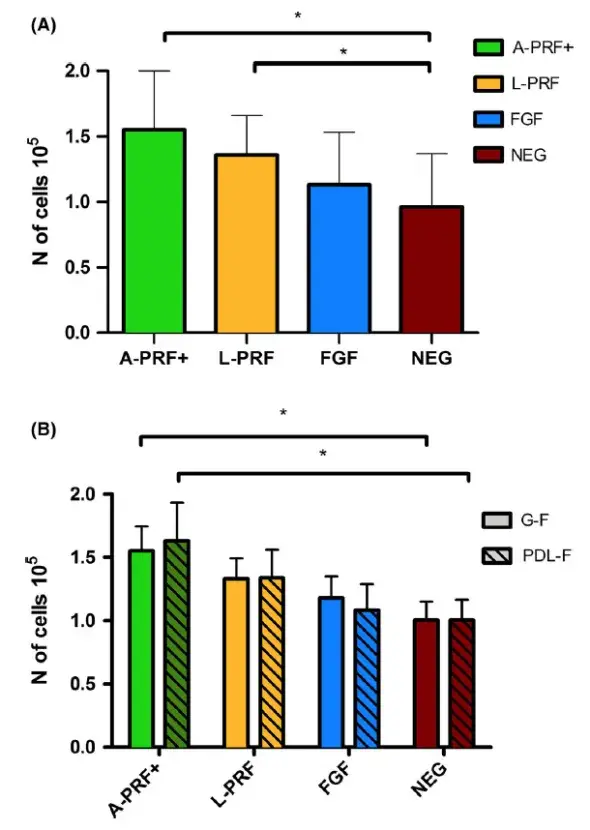
FIGURE 3. Fibroblast proliferation (results for G-F and PDL-F combined) (Panel A) and proliferation per cell type (Panel B) for different culture conditions. Absolute cell concentration after 24h (cell concentration at t0 was 3.3 × 105 cells/mL). PRF medium shows higher proliferation compared with NEG. Means ± SEM. * = P < .05
These results suggest that A-PRF + conditioned medium contains a different and/or more enriched mix of growth factors and chemokines that promote migration, and/or that the amount of inhibitory components present is less. The reason for this result may be due to the structure of the PRF prepared according to the different techniques. L-PRF is obtained with the use of centrifugation with a relatively high g-force, that produces a thick three-dimensional fibrin network, with some leukocytes trapped in it, mostly on the apical side of the matrix. A more recently adapted protocol (A-PRF+) makes use of a centrifugation step using a lower g-force (and 33% reduced time which is also of influence). The latter protocol has been demonstrated to give a higher and more evenly distributed number of platelets throughout the fibrin matrix, but a lower number of leukocytes, although more evenly dispersed in the matrix.
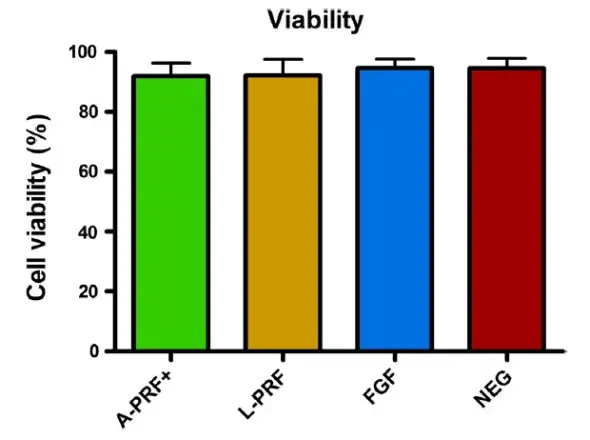 FIGURE 4. Fibroblast viability in the different culture conditions (results for G-F and PDL-F combined) after 24h. Cells viability is comparable in every culture condition. Means ± SEM
FIGURE 4. Fibroblast viability in the different culture conditions (results for G-F and PDL-F combined) after 24h. Cells viability is comparable in every culture condition. Means ± SEM
Comparing A-PRF + and L-PRF, some recent studies reported a higher growth factor release for A-PRF+. Kobayashi and colleagues m easured different growth factor release from L-PRF, PRP, and A-PRF + up to 10 days, showing higher release for A-PRF+. The same group found also increased fibroblasts migration and proliferation in cultures with A-PRF + conditioned medium. Cabaro et al reported that L-PRF and A-PRF + contained similar numbers of viable leukocytes and released a comparable quantity of inflammatory cytokines, but A-PRF + stimulated cultures secreted 3-, 1.6-, 3-, and 1.2- fold higher levels of eotaxins, CCL5, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), respectively. Eotaxins and CCL5 are pro-inflammatory chemokines chemotactic for T cells and leukocytes, and they are overexpressed during the early phase of tissue injury. PDGF and VEGF promote the chemotaxis of macrophages and can indeed favor the migration of fibroblasts. Similar results are reported by El Bagdadi et al and Masuki et al, who observed also an enhanced proliferation of human periosteal cells for the A-PRF + stimulated cultures. Our results are in agreement with the aforementioned research groups. The only paper reporting better performance for L-PRF is from the group of Dohan and colleagues; in that study, authors showed bigger clot size, better mechanical characteristics, and higher growth factors expression for L-PRF, in comparison to A-PRF.
Based on this literature, the use of A-PRF could be advisable in terms of soft tissue healing. The enhanced effect on fibroblasts reported in the current study demonstrates that independently from the amount of growth factors released, a possible higher wound closure can be achieved with this technique. Nevertheless, there are no clinical studies that support the use of one or the other technique for PRF preparation. Therefore, time is still needed to reach the level of sufficient knowledge for the clinical relevance of optimal PRF preparation protocol. The only clinical study comparing the two techniques is a recent publication from Caymaz et al, reporting that the use of A-PRF after mandibular third molar extraction significantly reduces postoperative pain, compared with L-PRF.
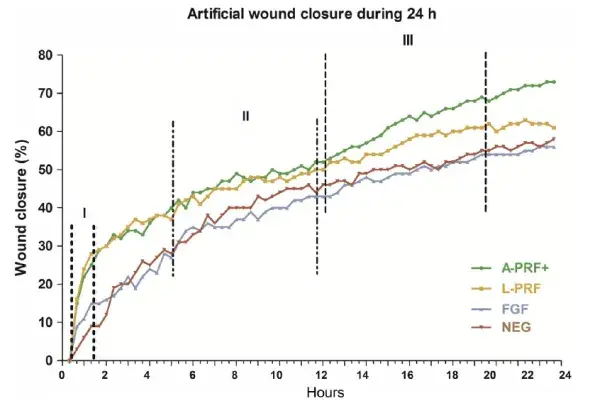 FIGURE 5. Cell migration of G-F and PDL-F over a period of 24 h. n experiments = 82. PRF medium shows higher migration compared with controls. Migration phases were defined in the figure to compare cell speed in Table 3. I: Phase 1, maximal migration phase. II: Phase 2, linear migration phase. III: Phase 3, stoppage phase. A-PRF + and L-PRF shows more migration compared with FGF and NEG (P = .01)
FIGURE 5. Cell migration of G-F and PDL-F over a period of 24 h. n experiments = 82. PRF medium shows higher migration compared with controls. Migration phases were defined in the figure to compare cell speed in Table 3. I: Phase 1, maximal migration phase. II: Phase 2, linear migration phase. III: Phase 3, stoppage phase. A-PRF + and L-PRF shows more migration compared with FGF and NEG (P = .01)
The strength of our study is the relative high number of PRF donors and primary cell cultures. We limited donor-related PRF differences, obtaining both L-PRF and A-PRF + from the blood of the same donor at the same time. The analysis of cellular migration and artificial wound closure was as well standardized. The use of specific culture plates offered a standard gap for each experiment, and the use of the live cell monitoring, never used in PRF research before, gave us the possibility of following the behavior of cells over time, such as the relative migration in a given time frame. Based on this, we could spot a different cell behavior for the A-PRF + induced migration, supported by a software program image analysis.
TABLE 3. Presentation of the observed speeds of artificial wound closure in percentage and μm2/min
 A shortcoming of our study is that we did not use the real fibrin matrix to induce migration, but only conditioned medium containing a mixture of growth factors, chemokines, and cytokines that were released from the clot. We used this design because some cross reaction between leukocytes from patients and cells from stocks from different donors were possible, which could influence the results. The fibrin matrix itself is also an important feature of PRF materials, and it could orchestrate growth factors release from the living cells present inside. We used a 24-h protocol, but we realize that a longer incubation time could have been necessary in order to show complete closure of the artificial wound in all the cell cultures. However, growth factors and cytokines present in the conditioned medium can be depleted without replacement during experiments beyond 24 h, thus suppressing further PDL-F and G-F activities. A third critical point is the absence of a mechanistic investigation in our model for the cell behavior. In this regard, our research group is involved in a follow-up study aimed to elucidate the secretome of these two PRF materials.
A shortcoming of our study is that we did not use the real fibrin matrix to induce migration, but only conditioned medium containing a mixture of growth factors, chemokines, and cytokines that were released from the clot. We used this design because some cross reaction between leukocytes from patients and cells from stocks from different donors were possible, which could influence the results. The fibrin matrix itself is also an important feature of PRF materials, and it could orchestrate growth factors release from the living cells present inside. We used a 24-h protocol, but we realize that a longer incubation time could have been necessary in order to show complete closure of the artificial wound in all the cell cultures. However, growth factors and cytokines present in the conditioned medium can be depleted without replacement during experiments beyond 24 h, thus suppressing further PDL-F and G-F activities. A third critical point is the absence of a mechanistic investigation in our model for the cell behavior. In this regard, our research group is involved in a follow-up study aimed to elucidate the secretome of these two PRF materials.
To conclude, both A-PRF + and L-PRF materials induce positive proliferation and migration responses in vitro by fibroblasts. Interestingly, both G-F and PDL-F responded similarly. In the initial phase, the artificial wound closure speed was similar for A-PRF + and L-PRF, but in the stoppage phase, A-PRF + induced faster cell migration than L-PRF. This work may be useful for choosing the best PRF preparation procedure for periodontal wound healing and periodontal regeneration procedures. The results from the current in vitro study support the clinical application of L-PRF and A-PRF+, with A-PRF + perhaps having an advantage; however, clinical efficacy and clinical significance is still lacking.
Further details about periodontal diseases treatment are accessible for you on our website.
List of authors:
Luciano Pitzurra
Ineke D. C. Jansen
Teun J. de Vries
Michel A. Hoogenkamp
Bruno G. Loos
References
1. Tonetti MS, Claffey N. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol. 2005;32(Suppl 6):210-213.
2. Amaliya A, Laine ML, Delanghe JR, Loos BG, Van Wijk AJ, Van der Velden U. Java project on periodontal diseases: periodontal bone loss in relation to environmental and systemic conditions. J Clin Periodontol. 2015;42(4):325-332.
3. Heitz-Mayfield LJ, Lang NP. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol 2000. 2013;62(1):218–231.
4. Avila-Ortiz G, De Buitrago JG, Reddy MS. Periodontal regeneration - furcation defects: a systematic review from the AAP Regeneration Workshop. J Periodontol. 2015;86(2 Suppl):S108-130.
5. Cortellini P. Reconstructive periodontal surgery: a challenge for modern periodontology. Int Dent J. 2006;56(4 Suppl 1):250-255.
6. Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human infrabony defects. II. Re-entry procedures and bone measures. J Periodontol. 1993;64(4):261-268.
7. Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human infrabony defects. I. Clinical measures. J Periodontol. 1993;64(4):254-260.
8. Tonetti MS, Pini-Prato G, Cortellini P. Periodontal regeneration of human intrabony defects. IV. Determinants of healing response. J Periodontol. 1993;64(10):934-940.
9. Wood W, Eming SA. Repair or regenerate–how can we tip the balance? EMBO Rep. 2012;13(12):1040-1042.
10. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37-44.
11. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45-50.
12. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e51-55.
13. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-646.
14. Schar MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015;473(5):1635-1643.
15. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e56-60.
16. Davis VL, Abukabda AB, Radio NM, et al. Platelet-rich preparations to improve healing. Part II: platelet activation and enrichment, leukocyte inclusion, and other selection criteria. J Oral Implantol. 2014;40(4):511-521.
17. Davis VL, Abukabda AB, Radio NM, et al. Platelet-rich preparations to improve healing. Part I: workable options for every size practice. J Oral Implantol. 2014;40(4):500-510.
18. Miron R, Choukroun J, Ghanaati S. Controversies related to scientific report describing g-forces from studies on platelet-rich fibrin: Necessity for standardization of relative centrifugal force values. Int J Growth Factor Stem Cell Dentist. 2018;1(3):80-89.
19. El Bagdadi K, Kubesch A, Yu X, et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept). Eur J Trauma Emerg Surg. 2017.
20. Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88(1):112-121.
21. Ghanaati S, Booms P, Orlowska A, et al. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40(6):679-689.
22. Masuki H, Okudera T, Watanebe T, et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (APRF), and concentrated growth factors (CGF). Int J Implant Dent. 2016;2(1):19.
23. Cabaro S, D’Esposito V, Gasparro R, et al. White cell and platelet content affects the release of bioactive factors in different blood-derived scaffolds. Platelets. 2018;29(5):1-5.
24. de Vries TJ, Schoenmaker T, Micha D, et al. Periodontal ligament fibroblasts as a cell model to study osteogenesis and osteoclastogenesis in fibrodysplasia ossificans progressiva. Bone. 2018;109:168-177.
25. Khan A, Gillis K, Clor J, Tyagarajan K. Simplified evaluation of apoptosis using the Muse cell analyzer. Postepy Biochem. 2012;58(4):492-496.
26. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an opensource platform for biological-image analysis. Nat Methods. 2012;9(7):676-682.
27. Miron RJ, Chai J, Zheng S, Feng M, Sculean A, Zhang Y. A novelmethod for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A. 2019;107(10):2257-2271.
28. Kobayashi E, Flückiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20(9):2353-2360.
29. Dohan Ehrenfest DM, Pinto NR, Pereda A, et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets. 2018;29(2):1-14.
30. Caymaz M, Uyanik L. Comparison of the effect of advanced platelet-rich fibrin and leukocyte- and platelet-rich fibrin on outcomes after removal of impacted mandibular third molar: A randomized split-mouth study. Nigerian J Clin Pract. 2019;22(4):546-552.

