Effectiveness of silicon platelet-rich fibrin and autologous bone on bone regeneration in rabbit calvarian defects: a radiological and histological study
Abstract
Repairing bone defects in Oral Surgery often requires the use of bone regeneration techniques. Silicon is an element that has been employed as regeneration material in several studies. In our study, silicon was combined with autologous bone and platelet-rich fibrin (PRF) membranes to analyse the behaviour of this element in bone regeneration. Four circumferential defects were created in the cranial vault of 5 New Zealand rabbits. The following elements were applied to the regeneration of the defects: (P): PRF; (S): silicon and (B): autologous bone, with the following distribution of study groups: Group 1 (PSB); Group 2 (PS); Group 3 (SB) and Group 4 (CONTROL): unregenerate group. The animals were sacrificed after 3 weeks. Computed microtomography studies (μ-CT) were carried out, as well as histomorphometric ones. The ANOVA statistical test was used with a Bonferroni post-hoc test to compare the results (p≤ 0.05). Radiologically, groups PSB and SB were better as far as quantity and percentage of healthy bone observed, but not significantly compared to the control group. The PS group was significantly worse. The histological test revealed that the PSB group was the one to present the largest area, percentage and perimeter of mineralised bone. On evaluating the forming bone (osteoid), no difference was observed across the groups with the exception of the bone perimeter, where the SB group was significantly better. The bone height variable showed no significant differences. In conclusion we can state that the combination of PRF, autologous bone and silicon provides good results at 3 weeks whilst the PS group shows the worst results. This highlights the importance of autologous bone forming part of the graft material in order for the bone to mineralise.
Further details about PRF application in regenerative surgery are accessible for you to learn on our website.
1. Introduction
Bone defects in Oral Surgery are common and sometimes difficult to repair, requiring bone regeneration techniques to ensure the success of restorative treatment. Scientific research continues to make advances in the understanding of the biological and physiological processes involved in bone healing, regeneration and remodelling [1]. In vivo tissue engineering covers regeneration and reconstruction of tissues and organs in the body itself. The basic premise is that controlled manipulation of the extracellular microenvironment may lead to controlling the ability of the cells to organise themselves, grow, differentiate, and form a functioning extracellular matrix and finally new functional tissue [2].
Regenerative techniques have become routine procedures and with proven clinical results, but their predictability and wait time are still some of the drivers for continuing to investigate the development of new and improved materials for bone regeneration. Autologous bone and platelet-rich plasma are two widely used materials in the field of implantology for regenerating soft and hard tissue. However, a material that combines all the advantages of each of them separately has not yet been developed.
The use of autologous bone is widely described in the literature since it is considered the gold standard for bone regeneration. Notwithstanding, despite the fact that it has excellent biological properties of osteoinduction, osteoconduction and osteogenesis, autograft presents considerable morbidity and an elevated and unpredictable resorption rate [3] but has shown that its behaviour improves on combination with other bone substitutes, obtaining a more abundant and consistent volume over time and quicker bone healing [4,5].
Together with autologous bone, platelet-rich blood derivatives are also frequently used in tissue regeneration. Amongst these derivatives is platelet-rich fibrin [PRF]. It is a regenerative biomaterial widely used in various medical applications. Furthermore, it has gained greater popularity compared to platelet-rich plasma [PRP] because it a single-step technique to which no chemical substances need to be added. Another advantage is greater gradual release of growth factors over time [6]. A single fibrin membrane contains a large amount of growth factors and cytokines involved in bone regeneration and soft tissue maturation [7]. It is an easily manipulated biomaterial which in Oral Surgery is applied in periodontal therapy, alveolar preservation, surgical bone augmentation procedures and, in combination with bone grafts, in maxillary sinus elevation surgery [8-11]. Its latest application, still not clinically tested, is as a decontaminant for rough titanium surfaces due to its antimicrobial properties [12,13].
Finally, there are several publications which state that bioinorganic ions such as zinc, manganese, magnesium or silicon are essential in bone metabolism [14,15]. Silicon is the second most abundant element in the Earth’s crust and in the human body it is found mainly in areas of bone mineralisation and growth [16]. It plays a vital role in bone and connective tissue biology, and although its mechanism of action is not understood exactly, thanks to the research by Carlisle and Schwarz it is known that it acts by producing greater mineralisation on bone matrix through the synthesis and stabilisation of the crosslinking of collagen fibres [17-20]. Silicon is an initiating factor in bone mineralisation since it is present in elevated concentrations in the osteoid matrix of immature bone and, as the bone matures, silicon concentration is diminished by the concentration of calcium [17,21]. It has been used over the years as bone regenerative material in the form of dental implant coating as well as being incorporated in ceramic biomaterials [hydroxyapatite, tricalcium phosphate or glass ceramics] providing good results regarding bone regeneration in in vitro and in vivo studies [22-24]. This good behaviour is due to its strong bond with bone because of the formation of a biologically active layer, similar to apatite, on its surface [25-27].
Silicon has been employed together with other biomaterials such as glass ceramics [28] or apatite [29,30] or with tricalcium phosphate [TCP] [28] to enhance the biological properties of the materials with which it bonds. However, as far as we know, no studies have been found on the use of silicon in combination with autologous bone or with platelet-rich fibrin membranes for use in bone regeneration.
In our study, silicon was combined with autologous bone and PRF membranes in order to analyse the role of this element in bone regeneration when combined with other widely used biomaterials. The main aim was to assess the bone regeneration obtained in the different groups analysed (these being (P): PRF; (S): silicon and (B): autologous bone, with a study group distribution as follows: Group 1 (PSB); Group 2 (PS); Group 3 (SB) and Group 4 (CONTROL): unregenerate group) over a period of 3 weeks in an animal testing model.
2. Materials and Methods
2.1. Test animal specimens
The experimental study was carried out on the parietal bone of 5 laboratory New Zealand rabbits aged 6 months and weighing between 3.5-4 kg. The animals were fed daily (ad libitum) with a laboratory animal diet using Harlan-Teklad (2030). The animals were subjected to surgery under general anaesthesia at the Jesús Usón Minimally Invasive Surgical Centre, [Cáceres, Extremadura, Spain]. The experiment was conducted in accordance with the guidelines of the Spanish National Health Institute (NIH) and European Directive 86/609/EEC on the care and use of experimental animals.
The study also complied with European Directive 2010/63/EU on the protection of animals used for experimental purposes and all local laws and regulations. The researchers obtained approval of the Ethical Committee of the Institution (CCMI- Ref 028/16). Identification of the animals comprising the groups to be evaluated was undertaken using a chip. During the experimental period the specimens were kept in individual cages.
2.2. Surgical procedure
Before initiating the surgical procedure, immobilisation of the rabbits was performed, and their vital signs were recorded. The anaesthetics employed were intravenous midazolam (0.25 mg / kg) and propofol (5 mg / kg) and inhaled 2.8% sevoflurane gas. Two analgesics were used: tramadol (3 mg / kg) and ketorolac (1.5 mg / kg). After sedation, a retro-orbital blood sample was drawn from each rabbit using a butterfly needle. Said samples were placed in test tubes without anticoagulant and were centrifuged for 12 minutes at a speed of 2700 rpm at room temperature to obtain PRF membranes. Once the membranes had been obtained, these were cut into small portions of approximately 2mm in diameter to be divided into three parts which were used subsequently for the three experimental groups. 0.01mg per cc silicon versenate was used. (Natural Energy Laboratory of Venezuela, Caracas, Venezuela).
Four non-self-healing bone defects were achieved (diameter: 9 mm; depth: 3 mm approximately, until reaching the dura mater) on the parietal bone, on each side of the median line of the cranium, using a trephine (Helmut-Zepf Medical GmbH, Seitingen, Germany) mounted on a surgical micromotor at 2000 rpm under irrigation with saline solution. Piezoelectric instruments were used to remove the internal table and medullary bone of each defect. Depth was controlled with a periodontal probe. Once the defects had been undertaken, the bone obtained was ground and the material obtained was divided into two equal parts. The configuration of the groups was the following: Group 1 (PSB): mixture of platelet-rich fibrin membrane (P) + silicon (S) + autologous bone (B); Group 2 (PS): platelet-rich fibrin membrane (P) + silicon (S); Group 3 (SB): silicon (S) + autologous bone (B) and lastly Group 4 (CONTROL) in which no regenerative material was placed. The distribution of the groups in parietal bone can be seen in Table 1.
Table 1: Distribution of study groups in parietal bone defects.
After stitching, anti-inflammatory and analgesic agents were administered (carprofen 1 ml / 12.5 kg and buprenorphine 0.05 mg / kg). Finally, the animals were sacrificed using an intravenous overdose of potassium chloride after 3 weeks. The surgical procedure can be seen in Figure 1.
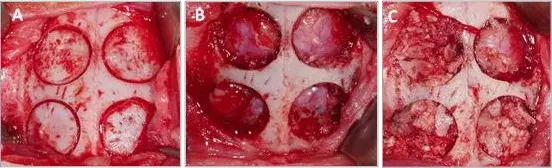 Figure 1: Summary of the surgical procedure followed in the study undertaken.
Figure 1: Summary of the surgical procedure followed in the study undertaken.
The samples obtained of the cranial vault of each specimen were cut on the anatomical sagittal plane, and after separation from the cerebral mass and washed in physiological saline solution, the pieces were cut and marked individually. Each of the samples was immersed in a 10% formalin solution for tomographic and histomorphometric analysis [31].
2.3. Microcomputed tomography (micro-CT)
The samples were analysed using Computed Tomography (CT) with a CT Bruker Albira scanner (Bruker Co., Billerica, MA, USA). Acquisitions were made using the following parameters: 1000 image, in 360° radiographic projection, at 45 kV and 30 minutes acquisition time. Tomographic reconstruction was carried out using Albira Suite software (Bruker Co., Billerica, MA, USA) and standard reconstruction parameters [32] to generate 2D and 3D volumes with 8.3 voxels resolution/mm. The average bone density measured in Hounsfield Units (HU) was evaluated using PMOD software (Bruker Co., Billerica, MA, USA), positioning spherical volumes of interest [VOI] of 2 mm in a rosette formation within each lesion. High resolution reconstructions of a 10 mm3 volume were performed in each lesion using Albira Suite software, giving rise to volumes with a resolution of 20 voxels / mm.
For each of the volumes the following variables were evaluated: (1) The sum total of the Hounsfield values of all the voxels (TOTAL SUM), (2) the average of all the Hounsfield values of the voxels (Averaged) in Hounsfield units and (3) the percentage of healthy bone (%).
These same variables were evaluated for air as a measurement to establish the background noise of the images and for surrounding bone, in which the percentage of expected healthy bone is 100%.
2.4. Histological processing of the sample
Samples were obtained from the cranium of each sample, cutting along the anatomical sagittal plane. The desiccated specimens were immediately submerged in a solution of 4% formaldehyde and 1% calcium embedded in acrylic resin and they were processed for cutting following the Donath and Breuner method for obtaining histological cuts of 5µm in thickness [33]. The samples were stained with Von Kossa (VK) 5% silver nitrate (Sigma-Aldrich Chemical Co., Poole, United Kingdom) to view the mineralised bone after 3 weeks and they were observed using an Olympus BXB61 optical microscope (Olympus, Tokyo, Japan) with 1.5 and 20x lenses. The images were taken using a DSP DS-Fi1 digital signal processor (Nikon, Tokyo, Japan) in conjunction with NIS-Elements 4.0 BR software (Nikon, Tokyo, Japan). An image was taken of each bone defect. One week before sacrificing the specimens (at 2 weeks) a fluorescent marker was administered to the rabbits to observe calcein deposition on the recently deposited bone matrix.
The fluorescent images were taken using a DSP DS-Fi1 camera [Nikon, Tokyo, Japan] in conjunction with NIS-Elements 4.0 BR software [Nikon, Tokyo, Japan].
For both kinds of stainings (VK and immunofluorescence) four variables were analysed: bone height (only on VK) (mm) (BH), bone area (µm2) (BA), percentage of bone area (%) (BA) and bone perimeter (µm) (BP).
2.5. Statistical analysis
For the statistical analysis of the results obtained the ANOVA t test was applied with a subsequent Bonferroni test to compare the results obtained in both study groups, using STATVIEW F-4.5 software. The results were expressed as median ± standard deviation for all the variables analysed. The significance level was set at p ≤ 0.05.
3. Results
3.1. Results for radiological variables
Using Albira Suite software, high definition 2D and 3D reconstructions were obtained (Figure 2) and bone quantification variables were measured, in which after 3 weeks there were no statistically significant differences across the PSB, SB and CONTROL groups in the Total SUM, Averaged and % Healthy Bone variables. The PS group was the one which obtained the least significant values for these three variables (Tables 2 and 3).
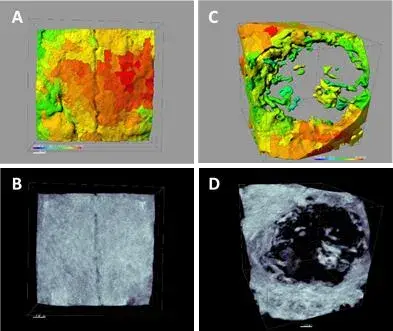 Figure 2: 3D reconstructions of the control (A, B) and SB group after 3 weeks (C, D). The images present a calibration of 1 µm. The colour range oscillates between 250-1,000 HU.
Figure 2: 3D reconstructions of the control (A, B) and SB group after 3 weeks (C, D). The images present a calibration of 1 µm. The colour range oscillates between 250-1,000 HU.
Table 2: Radiological results (Total HU of bone density of the area studied per group, Median of said measurement per group and % of regenerated bone per group) after 3 weeks. The statistically significant differences are shown in the same row by pairs of similar symbols (* *, ; p<0.05).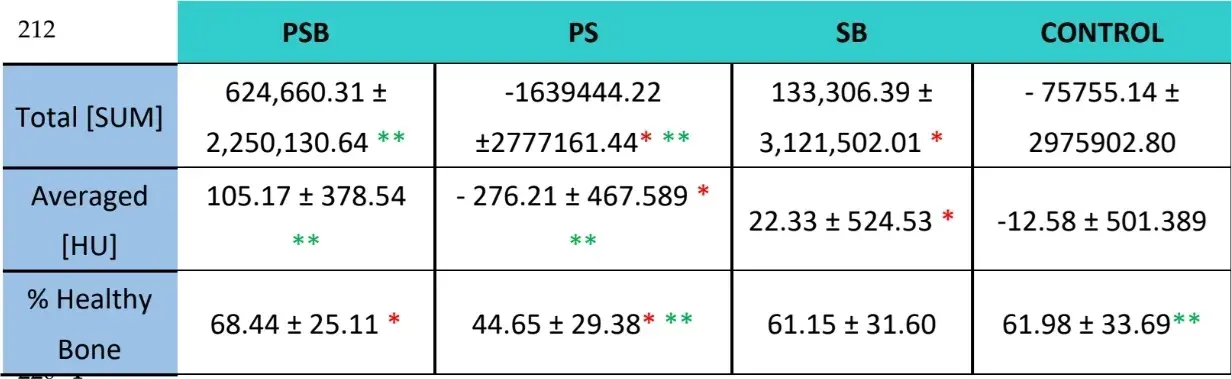
Table 3: Radiological results after 3 weeks of bone and air. Both groups have been analysed for reference use but were not compared with the rest of the study groups.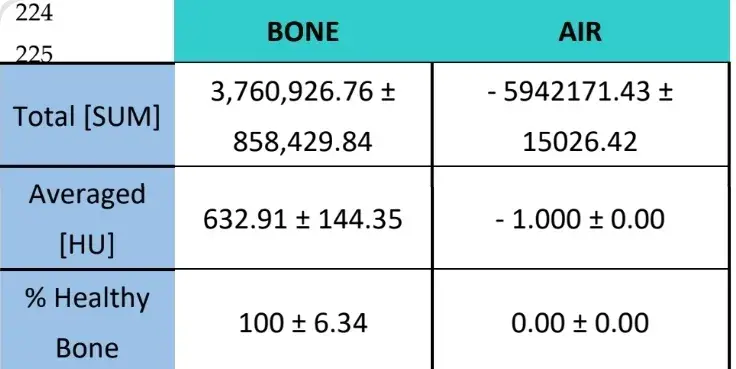
Three weeks later histological evaluations were made of the four groups (Table 4). The bone height variable showed no statistically significant differences across the groups after 3 weeks of healing.
Two histological stainings, Von Kossa and fluorescence, were carried out (Figure 4). The first was used to observe mineralised bone. The bone area [BA], area percentage [%BA] and bone perimeter [BP] were observed. The Von Kossa staining revealed that the PSB group presented statistically higher BA and BP than the other groups. The %BA was higher in the CONTROL group although not significantly compared to all the groups, the sample size being very small.
The fluorescence staining was employed to measure the aforementioned variables in newly deposited bone. No statistically significant differences were observed across the groups for the BA and %BA variables after 3 weeks. In the SB group the bone perimeter was significantly higher, the PSB group being the one which showed a significantly lower value.
Table 4: Histological results after 3 weeks (BH, bone height; BA, Bone area; BP, Bone perimeter. The statistically significant differences are shown in the same row by pairs of similar symbols (* *, ; p<0.05).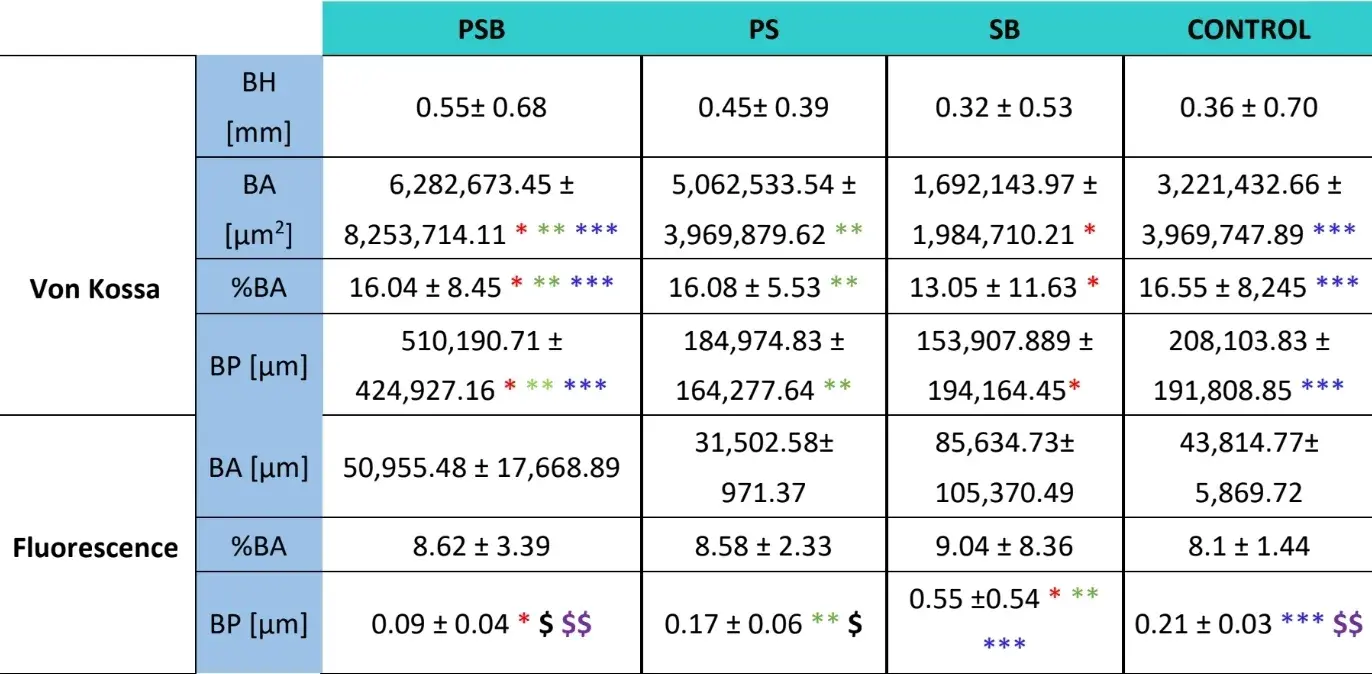
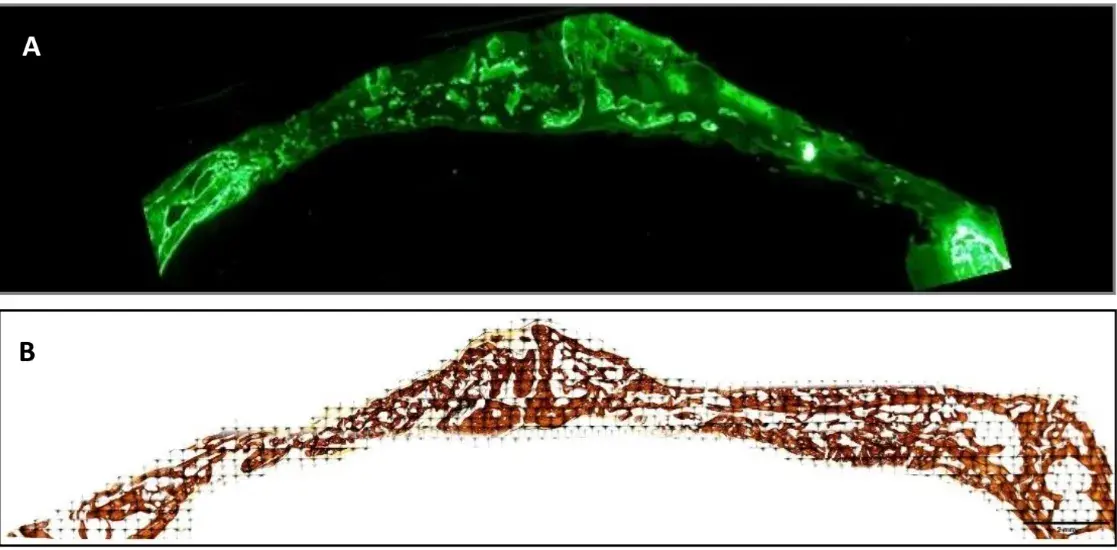 Fig. 3 Images of histological sections made using fluorescence [A] and Von Kossa staining [B] after 3 weeks. Pointers indicate areas of calcein deposition. The images present a calibration of 2 mm.
Fig. 3 Images of histological sections made using fluorescence [A] and Von Kossa staining [B] after 3 weeks. Pointers indicate areas of calcein deposition. The images present a calibration of 2 mm.
4. Discussion
In this radiological and histological study, we observed the bone formed after the placing of different bone regenerative materials to which silicon was added in order to evaluate its role as a stimulator of bone formation.
In the radiological study (Table 2) it was observed that groups PSB and SB showed higher values in terms of the amount and percentage of healthy bone observed. However, these differences were not statistically significant with regard to the CONTROL group. On the other hand, the PS group did present significantly lower values compared to the rest of the groups.
The histological study (Table 4) revealed that the PSB group was the one that presented the greatest area, percentage and perimeter of mineralised bone compared to the other groups. Conversely, on evaluating calcein deposition on the bone matrix, no difference was observed across groups with regard to bone area and area percentage. The SB group presented a significantly larger bone perimeter. The bone height variable showed no differences across the groups.
Silicon is a bioinorganic ion which has been employed as a regenerative material in several studies, usually with other inorganic biomaterials such as calcium phosphate or hydroxyapatite [34]. The advantages they offer are a low cost and a longer useful life [35].
The bioactivity of silicon will depend on the accumulation of silicon ions when it is exposed to body fluids, such as blood as in the case of this study. This phenomenon was to give rise to the formation of a layer similar to biologically active apatite. [36]
As far as we know, there is no evidence in the literature of the use of silicon versenate as a regenerative material, thus the comparison with other studies must be undertaken with caution.
The method usually employed is carried out by substituting hydroxyapatite or calcium phosphate with silicon by precipitation methods, enabling regenerative biomaterials with better properties to be obtained. [37,38] Several in vitro studies have shown that silicon is a biocompatible and bioinert element [36]. Furthermore, it promotes angiogenesis, osteoblastic differentiation and increases bone mineral density, by accelerating its formation [16,17,22,23,27,39,40]. In a recent study defects were regenerated on the cranial vaults of rats and, after 4 weeks, it was observed that significantly larger bone formation occurred when compared with a similar mixture to which silicon had not been added. [30]
Likewise, mixed in vitro and in vivo studies have been undertaken in which it has been possible to compare the behaviour of silicon in both situations. Mao et al carried out a study in which silicon was added to a mixture of calcium phosphate and glass ceramic to regenerate cranial vault defects in rabbits. Cellular viability was observed similar to the one which presented in spongy bovine bone, but with a greater rate of cellular proliferation. All this was attributed to the inclusion of silicon ions, which was to also boost other cellular functions such as cellular adhesion [41]. In the histological study undertaken, increased new bone formation was observed in the experimental group.
In accordance with the reviewed literature, a greater amount of mineralised bone would be expected to be found in the groups containing silicon. In our study, the PS and SB groups showed no difference compared to the control group, the combination of silicon, platelet-rich fibrin and autologous bone [the PSB group] being the one which enabled a significantly higher level of mineralised bone than the rest.
Autologous bone is still deemed the gold standard material for regeneration since it is the only one with osteogenic, osteoinductive and osteoconductive properties and this gives it an advantage in comparison with the rest of available materials. Notwithstanding, its limited availability, morbidity and its higher resorption rate have prompted the search for new biomaterials [35].
In our study, autologous bone combined with silicon [SB] showed no significant difference compared to the PS and control groups with regard to calcified bone. However, it was, after the PSB group, the one which presented a greater amount of bone and the one which presented greater bone perimeter of osteoid bone. There are no publications in which any of the study groups have employed autologous bone with silicon; and our preliminary results suggest that we would need to see how the autologous bone behaves over longer periods.
Fibrin-rich plasma has been used before in bone regeneration as the only filler material [42-44], or in combination with autologous bone [45], xenografts [46] or alloplastic grafts [47] providing better results regarding bone regeneration and faster healing compared to negative control groups. However, there is limited evidence on the combination of silicon and platelet-rich concentrates. Only two studies have been found in the literature which combine both materials. Their results differ and with ours too. In the first study a combination of PRP/silicon was subsequently gelled with calcium chloride. It was applied on a cell culture of osteoblasts, and greater proliferation, greater cell viability and greater cell deposition of calcium was observed in the experimental group than in the group without silicon [48]. Conversely, in the second study a cell-generating factor (CGF) was combined with sodium orthosilicate on human osteoblasts. No statistically significant differences were observed in respect of cellular proliferation or growth compared to the groups in which only orthosilicate or only platelet concentrate was administered. Conversely, a greater production of type I collagen was observed [49]. In both studies it was concluded that the osteoconductive properties of the silicon would be boosted by the growth factors in the CGF. Both results differed in our study, in which the PS group was the one that obtained significantly lower radiological values, although histologically it showed no difference compared to the control group. The comparison with both publications must be made with caution because they were in vitro studies with a different material. Notwithstanding, our results would be in accordance with two trials undertaken on rabbit bone in which it was observed that the PRF did not significantly improve bone regeneration when compared with the negative control group [47,50].
In our study, greater formation of mineralised bone occurred in the PSB group and this could be due to two factors: the first of these is that the silicon and the PRF would boost its osteoconductive capacity on combining with each other and at the same time it would accelerate bone mineralisation. This could explain the better performance of the PSB group in our study. The second factor is the addition of autologous bone to the biomaterial which, as mentioned earlier, is deemed the reference material in bone regeneration.
As limitations of the study, it is worth noting the low number of specimens in the sample, as well as the time period evaluated. The PSB and SB study groups offered good regeneration results, however, radiologically they did not present a significant difference compared to the control group.
A way of improving the study would be to evaluate longer time periods, which would perhaps offer more revealing results on the behaviour of these regenerative materials. Furthermore, the use of a positive control group (filling the defect with autologous bone without silicon or a mixture of just autologous and heterologous bone) could have been contemplated. Another limitation of the study is the difficulty in comparing due to the type of material employed (silicon versenate). Silicon is an element that is employed in very different forms in the literature such as silicon dioxide [51], silicon acetate [52] and sodium silicate [27], and there is still no validated protocol. Granulated silicon was used in our study. Under physiological conditions, bio-available silicon is found in the form of orthosilicate acid, whilst in the studies in which hydroxyapatite substituted with silicon has been employed, the silicon used is in the form of SiO4, although, on the other hand, it has recently been shown that silicon in its physiological format could not be concentrated at the inorganic mineral stage of bone without metabolising it beforehand to orthosilicate [53].
In the radiological study, no statistically significant difference was observed across the study groups in comparison with the control group and this could be due to the lack of membrane use or fixing agents to coat the defect so as to stabilise coagulation and prevent delay in complete ossification of the area, although the period studied (3 weeks) would be insufficient to observe it.
You have the opportunity to gather more in-depth information about bone regeneration in our courses "Bone augmentation: current techniques" and "Bone augmentation 2.0. New techniques and protocols".
5. Conclusions
Silicon is a promising element as a material to be included with other bone regenerative materials due to its low cost and good properties observed in vitro and in vivo, although the definition of its properties and optimal conditions of use are still being investigated [54,55]. The combination of platelet-rich fibrin, autologous bone and silicon offers goods results after 3 weeks. The PSB and SB groups being the ones offering good results, the PSB group presents greater bone mineralisation speed compared to SB and this could be due to the inclusion of platelet-rich plasma and silicon. The results obtained in this study reveal the importance of autologous bone forming part of the graft material in order for the bone to mineralise.
Within the limits of this research, the histological analysis of regenerated tissues could provide useful information on the nature and amount of bone formed with the use of silicon, platelet-rich fibrin and autologous bone. Further studies are needed to gain knowledge of the true regenerative potential of bioinorganic ions such as silicon.
Authors:
Argimiro Hernández-Suarez, María Rizo-Gorrita, Dubraska Suárez-Vega, Gladys Velazco, Ivan Rodriguez Gelfenstein, María-Ángeles Serrera-Figallo6 and Daniel Torres-Lagares
References
Ferres E. Estudio del efecto de la adición de silicio al beta-fosfato tricálcico e hidroxiapatita en la neoformación ósea en defectos críticos en calotas y conejo. Tesis Doctoral. Universidad de Murcia.2017.
Morales D. Ingeniería tisular como puntal de la medicina regenerativa en estomatología. Revista Cubana de Estomatología 2014;51(3):288-304
Oppenheimer AJ, Tong L, Buchman SR. Craniofacial Bone Grafting: Wolff's Law Revisited. Craniomaxillofac Trauma Reconstr. 2008;1(1):49-61.
Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, Dickinson IC, Choong PF, Schuetz MA, Hutmacher DW. Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective. Bone Res. 2013 Sep 25;1(3):216-48.
Mordenfeld A, Johansson CB, Albrektsson T, Hallman M. A randomized and controlled clinical trial of two different compositions of deproteinized bovine bone and autogenous bone used for lateral ridge augmentation. Clin Oral Implants Res. 2014 Mar;25(3):310-320.
Masoudi E, Ribas J, Kaushik G, Leijten J, Khademhosseini A. Platelet-Rich Blood Derivatives for Stem Cell-Based Tissue Engineering and Regeneration. Curr Stem Cell Rep. 2016 Mar;2(1):33-42.
Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006 May;24(5):227-34.
Amaral Valladão CA Jr, Freitas Monteiro M, Joly JC. Guided bone regeneration in staged vertical and horizontal bone augmentation using platelet-rich fibrin associated with bone grafts: a retrospective clinical study. Int J Implant Dent. 2020 Oct 17;6(1):72.
Cho YS, Hwang KG, Jun SH, Tallarico M, Kwon AM, Park CJ. Radiologic comparative analysis between saline and platelet-rich fibrin filling after hydraulic transcrestal sinus lifting without adjunctive bone graft: A randomized controlled trial. Clin Oral Implants Res. 2020 Nov;31(11):1087-1093.
Areewong K, Chantaramungkorn M, Khongkhunthian P. Platelet-rich fibrin to preserve alveolar bone sockets following tooth extraction: A randomized controlled trial. Clin Implant Dent Relat Res. 2019 Dec;21(6):1156-1163.
de Almeida Barros Mourão CF, de Mello-Machado RC, Javid K, Moraschini V. The use of leukocyte- and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial. J Craniomaxillofac Surg. 2020 Apr;48(4):452-457.
Castro AB, Herrero ER, Slomka V, Pinto N, Teughels W, Quirynen M. Antimicrobial capacity of Leucocyte-and Platelet Rich Fibrin against periodontal pathogens. Sci Rep. 2019 Jun 3;9(1):8188.
Schuldt L, Bi J, Owen G, Shen Y, Haapasalo M, Häkkinen L, Larjava H. Decontamination of rough implant surfaces colonized by multispecies oral biofilm by application of leukocyte- and platelet-rich fibrin. J Periodontol. 2020 Aug 27.
Zofková I, Nemcikova P, Matucha P. Trace elements and bone health. Clin Chem Lab Med. 2013 Aug;51(8):1555-61
Zhang D, Wong CS, Wen C, Li Y. Cellular responses of osteoblast-like cells to 17 elemental metals. J Biomed Mater Res A. 2017 Jan;105(1):148-158.
Arora M., Arora E. The Promise of Silicon: bone regeneration and increased bone density. J Arthroscopy and Joint Surgery 2017;4(3):103-105. 430
Carlisle EM. Silicon. A possible factor in bone calcification. Science 1970;167:279–280.
Carlisle EM. Silicon as an essential trace element in animal nutrition. Ciba Found Symp. 1986;121:123-39.
Schwarz K, Milne DB. Growth-promoting effects of silicon in rats. Nature. 1972 Oct 6;239(5371):333-4.
Dong M, Jiao G, Liu H, Wu W, Li S, Wang Q, Xu D, Li X, Liu H, Chen Y. Biological Silicon Stimulates Collagen Type 1 and Osteocalcin Synthesis in Human Osteoblast-Like Cells Through the BMP-2/Smad/RUNX2 Signaling Pathway. Biol Trace Elem Res. 2016 Oct;173(2):306-15.
Price CT, Koval KJ, Langford JR. Silicon: a review of its potential role in the prevention and treatment of postmenopausal osteoporosis. Int J Endocrinol. 2013;2013:316783.
Zou S, Ireland D, Brooks RA, Rushton N, Best S. The effects of silicate ions on human osteoblast adhesion, proliferation, and differentiation. J Biomed Mater Res B Appl Biomater. 2009 Jul;90(1):123-30.
Uribe P, Johansson A, Jugdaohsingh R, Powell JJ, Magnusson C, Davila M, Westerlund A, Ransjö M. Soluble silica stimulates osteogenic differentiation and gap junction communication in human dental follicle cells. Sci Rep. 2020 Jun 18;10(1):9923.
Kim, E. J., Bu, S. Y., Sung, M. K. & Choi, M. K. Effects of silicon on osteoblast activity and bone mineralization of MC3T3-E1 cells. Biol. Trace Elem. Res. 2013; 152(1):105–12
Hench LL, Xynos ID, Polak JM. Bioactive glasses for in situ tissue regeneration. J Biomater Sci Polym Ed. 2004;15(4):543-62.
Chowdhury M. The Silica-based Formulations for Drug Delivery, Bone Treatment, and Bone Regeneration. ChemBioEng Rev 2016; 3(5):229-246.
Byun IS, Sarkar SK, Anirban Jyoti M, Min YK, Seo HS, Lee BT, Song HY. Initial biocompatibility and enhanced osteoblast response of Si doping in a porous BCP bone graft substitute. J Mater Sci Mater Med. 2010 Jun;21(6):1937-47.
Mao Z, Gu Y, ZhangJ, Shu W.W., Cui Y. Superior biological performance and osteoinductive activity of Si-containing bioactive bone regeneration particles for alveolar bone reconstruction. Ceram. Int. 2020. 46(2020):353-364.
Wang X, Ito A, Sogo Y, Li X, Oyane A. Silicate-apatite composite layers on external fixation rods and in vitro evaluation using fibroblast and osteoblast. J Biomed Mater Res A. 2010 Mar 1;92(3):1181-9.
Roh J, Kim JY, Choi YM, Ha SM, Kim KN, Kim KM. Bone Regeneration Using a Mixture of Silicon-Substituted Coral HA and β-TCP in a Rat Calvarial Bone Defect Model. Materials (Basel). 2016 Feb 6;9(2):97.
Toledano M, Toledano-Osorio M, Osorio R, Carrasco-Carmona Á, Gutiérrez-Pérez JL, Gutiérrez-Corrales A, Serrera-Figallo MA, Lynch CD, Torres-Lagares D. Doxycycline and Zinc Loaded Silica-Nanofibrous Polymers as Biomaterials for Bone Regeneration. Polymers (Basel). 2020 May 25;12(5):1201.
Sánchez F, Orero A, Soriano A, Correcher C, Conde P, González A, Hernández L, Moliner L, Rodríguez-Alvarez MJ, Vidal LF, Benlloch JM, Chapman SE, Leevy WM. ALBIRA: a small animal PET∕SPECT∕CT imaging system. Med Phys. 2013 May;40(5):051906.
Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982 Aug;11(4):318-26.
Garbo C, Locs J, D'Este M, Demazeau G, Mocanu A, Roman C, Horovitz O, Tomoaia-Cotisel M. Advanced Mg, Zn, Sr, Si Multi-Substituted Hydroxyapatites for Bone Regeneration. Int J Nanomedicine. 2020 Feb 13;15:1037-1058.
Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017 Jun 7;2(4):224-247.
Götz W, Tobiasch E, Witzleben S, Schulze M. Effects of Silicon Compounds on Biomineralization, Osteogenesis, and Hard Tissue Formation. Pharmaceutics. 2019 Mar 12;11(3):117.
Gibson IR, Best SM, Bonfield W. Effect of silicon substitution on the sintering and microstructure of hydroxyapatite. J Am Ceram Soc. 2002;85(11):2771–2777
Porter AE, Botelho CM, Lopes MA, Santos JD, Best SM, Bonfield W. Ultrastructural comparison of dissolution and apatite precipitation on hydroxyapatite and silicon-substituted hydroxyapatite in vitro and in vivo. J Biomed Mater Res A. 2004 Jun 15;69(4):670-9.
Li H, Chang J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater. 2013 Jun;9(6):6981-91.
Shie MY, Ding SJ, Chang HC. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011 Jun;7(6):2604-14.
Thian E.S., Huang J., Best S.M., et al., The response of osteoblasts to nanocrystalline silicon-substituted hydroxyapatite thin film. Biomaterials 2006; 27(13):2692–2698
Lee, S. H., Kim, S. W., Lee, J. I., Yoon, H. J. The effect of platelet-rich fibrin on bone regeneration and angiogenesis in rabbit cranial defects. Tissue Engineering and Regenerative Medicine 2015. 12(5):362-370. 482
Jeon YR, Kim MJ, Kim YO, Roh TS, Lee WJ, Kang EH, Yun IS. Scaffold Free Bone Regeneration Using Platelet-Rich Fibrin in Calvarial Defect Model. J Craniofac Surg. 2018 Jan;29(1):251-254.
Sindel A, Dereci Ö, Toru HS, Tozoğlu S. Histomorphometric Comparison of Bone Regeneration in Critical-Sized Bone Defects Using Demineralized Bone Matrix, Platelet-Rich Fibrin, and Hyaluronic Acid as Bone Substitutes. J Craniofac Surg. 2017 Oct;28(7):1865-1868.
Kökdere NN, Baykul T, Findik Y. The use of platelet-rich fibrin (PRF) and PRF-mixed particulated autogenous bone graft in the treatment of bone defects: An experimental and histomorphometrical study. Dent Res J (Isfahan). 2015 Sep-Oct;12(5):418-24.
Karayürek F, Kadiroğlu ET, Nergiz Y, Coşkun Akçay N, Tunik S, Ersöz Kanay B, Uysal E. Combining platelet rich fibrin with different bone graft materials: An experimental study on the histopathological and immunohistochemical aspects of bone healing. J Craniomaxillofac Surg. 2019 May;47(5):815-825.
Knapen M, Gheldof D, Drion P, Layrolle P, Rompen E, Lambert F. Effect of leukocyte- and platelet-rich fibrin (L-PRF) on bone regeneration: a study in rabbits. Clin Implant Dent Relat Res. 2015 Jan;17 Suppl 1:e143-52.
Sani F, Mehdipour F, Talaei-Khozani T, Sani M, and RazbanV. Fabrication of platelet-rich plasma/silica scaffolds for bone tissue engineering Bioinspired, Biomimetic and Nanobiomaterials 2018 7:2, 74-81.
Bonazza V, Borsani E, Buffoli B, Parolini S, Inchingolo F, Rezzani R, Rodella LF. In vitro treatment with concentrated growth factors (CGF) and sodium orthosilicate positively affects cell renewal in three different human cell lines. Cell Biol Int. 2018 Mar;42(3):353-364.
Faot F, Deprez S, Vandamme K, Camargos GV, Pinto N, Wouters J, van den Oord J, Quirynen M, Duyck J. The effect of L-PRF membranes on bone healing in rabbit tibiae bone defects: micro-CT and biomarker results. Sci Rep. 2017 Apr 12;7:46452.
Fielding GA, Smoot W, Bose S. Effects of SiO2, SrO, MgO, and ZnO dopants in tricalcium phosphates on osteoblastic Runx2 expression. J Biomed Mater Res A. 2014 Jul;102(7):2417-26.
Kamitakahara M, Tatsukawa E, Shibata Y, Umemoto S, Yokoi T, Ioku K, Ikeda T. Effect of silicate incorporation on in vivo responses of α-tricalcium phosphate ceramics. J Mater Sci Mater Med. 2016 May;27(5):97.
Chappell HF, Jugdaohsingh R, Powell JJ. Physiological silicon incorporation into bone mineral requires orthosilicic acid metabolism to SiO44. J R Soc Interface. 2020 Jun;17(167):20200145.
Gutiérrez-Prieto SJ, Fonseca LF, Sequeda-Castañeda LG, Díaz KJ, Castañeda LY, Leyva-Rojas JA, Salcedo-Reyes JC, Acosta AP. Elaboration and Biocompatibility of an Eggshell-Derived Hydroxyapatite Material Modified with Si/PLGA for Bone Regeneration in Dentistry. Int J Dent. 2019 Dec 5;2019:5949232.
Szurkowska K, Szeleszczuk Ł, Kolmas J. Effects of Synthesis Conditions on the Formation of
Si-Substituted Alpha Tricalcium Phosphates. Int J Mol Sci. 2020 Dec 1;21(23):9164.
