Comparison of the effects of Platelet Rich Fibrin (A-PRF+) and Enamel Matrix Derivative (EMD) on PROs (patient-reported outcomes) in regenerative periodontal micro-surgery: A randomized controlled trial – Pilot study
Abstract
Aim: To investigate self-reported short-term morbidity after the application of advanced platelet rich fibrin (A-PRF+) in combination with periodontal regenerative microsurgery in molar furcations and compare this the use of enamel matrix derivative application (EMD) or open flap debridement (OFD, control).
Materials and Methods: A prospective randomized controlled trial was designed to study effectsof A-PRF+, EMD or control for pocket closure of molar furcations grade II. Patients were randomly allocated in one of three treatment groups: OFD with application of A-PRF+, OFD with application of EMD and OFD alone. In all patients, venous blood was drawn from the antecubital fossa. A minimally invasive microsurgical approach was performed in all groups. Morbidity and patient-reported outcomes (PROs) were assessed by questionnaires up to 6 weeks postoperatively.
Results: 15 patients (n=5 per treatment allocation) completed the six-week follow-up. No differences in pain medications and PROs were observed between the groups, except that patients in EMD group scored statistically significant higher for postoperative morbidity compared to OFD group during the first week. All the patients except one reported to undergo the treatment again.
Conclusion: The use of A-PRF+ was not associated with a different post-operative pain and discomfort compared to EMD or OFD.
Further information about PRF application in dentistry are accessible for you to learn on our website.
Introduction
Periodontitis is a common inflammatory disease that, if left untreated, leads to progressive breakdown of the supporting tissues of the teeth, namely connective tissue attachment and alveolar bone (Pihlstrom et al., 2005). The goal of the periodontal treatment is to arrest the progression of attachment and bone loss and, if possible, regain the lost tissues. This could be achieved by means of periodontal regenerative surgical procedures, and many different materials have been proposed to facilitate this (Cortellini et al., 2015). The materials studied fall into two categories, namely autologous materials (obtained from the patient self ) and heterologous materials (from animals, other humans or of synthetic origin).
Currently, platelet rich fibrin (PRF) is considered to be a promising autologous material in terms of clinical outcomes in periodontal surgery, and as such PRF, Leukocyte rich-PRF (L-PRF) and Advanced-PRF+ (A-PRF+) can be proposed (Agrawal, 2017). The most recently introduced protocols have the objective of incrementing the number of platelets and leukocytes in the fibrin clot. This can be achieved by reducing the Relative Centrifugation Force (RCF), following the “Low-speed centrifugation concept” theory. As result, the generated A-PRF + fibrin matrix shows a more porous structure, in which platelets and leucocytes remain trapped during the centrifugation phase. Due to the higher number of trapped cells, a higher and more sustained release of growth factors has been reported, compared with other PRF centrifugation protocols (El Bagdadi et al., 2017; Masuki et al., 2016; Fujioka-Kobayashi et al., 2017). Therefore, among PRF materials, it is verisimilar that A-PRF+ has the highest potential in promoting wound healing (Sousa et al., 2020; Fujioka-Kobayashi et al., 2017; Miron et al., 2020). These сharacteristics seem to be favourable especially in the early phase of the wound healing process. Numerous clinical trials showing promising results are available about the use of different types of PRF in regenerative periodontal surgery (Castro et al., 2017). For example, compared to open flap debridement (OFD), Leucocyte-PRF (L-PRF), can significantly improve both vertical and horizontal clinical attachment levels (CAL) (Kanoriya et al., 2017; Bajaj et al., 2013; Sharma et al., 2011; Castro et al., 2017). The application of L-PRF in some oral surgical procedures such as third molar extractions and mucogingival surgery is reported to be associated with better patient-reported outcomes (PROs) than controls (Femminella et al., 2016; He et al., 2017). For the various PRF preparations, the clinician needs to draw venous blood (approximately 4 to 6 tubes of 10 ml) immediately before the periodontal regenerative procedure. To date, patient’s levels of acceptance of the procedure in regenerative periodontal surgery have not investigated.
From the family of heterologous materials, enamel matrix derivative (EMD) has been proposed as one of the gold standards as regenerative material (Miron et al., 2016). Due to its ready to use packaging, EMD does not require a blood drawing procedure. The principal component of EMD is amelogenin of porcine origin. In humans, amelogenins have been shown to be expressed in teeth during root development and serves as precursor to acellular cementum deposition (Hammarstrom, 1997). There is evidence that EMD can be beneficial in wound healing favouring soft tissue regeneration and angiogenic activity. Additionally, it is shown that EMD has also an effect on a plethora of cell types that are involved in (i) hard tissue healing, (ii) alter the expression of pro-inflammatory markers and (iii) antibacterial action (Miron et al., 2015). Many randomized clinical trials also showed a superiority of the clinical use of EMD compared to other regenerative techniques as guided tissue regeneration (GTR) and OFD with primary closure or coronally advanced flap, in periodontal regenerative surgery of furcation defects ( Jepsen et al., 2004; Peres et al., 2013). Moreover, EMD is shown to have a positive effect in terms of reducing post-operative pain in some clinical trials (Zucchelli et al., 2002; Wennstrom et al., 2002). However, in terms of patient acceptance, there is still limited evidence about the superiority of EMD over any other material. Furthermore, not all patients consent to receive heterologous material derived in particular from pig, and in current times some patients are concerned about animal welfare.
Few clinical studies related to periodontal regeneration procedures investigated PROs and self-perception. Askar (2019) reports that there is a low percentage of patients that after periodontal surgical procedures such as GTR, OFD and osseous surgery experience post-operative discomfort that can impair patients’ daily routine (Askar et al., 2019). Factors such as smoking and diabetes mellitus may be related to an increased rate of post-operative complications of healing and discomfort (Larrazabal et al., 2010). The methodological addition of assessing PROs to solely clinical outcomes, is expected to provide critical information to better understand the acceptance of periodontal surgical therapies (Avila-Ortiz et al., 2015). In general, data related to morbidity of the microsurgical approach for regenerative procedures are scarce. To date, no study has compared directly PROs after either PRF, EMD and OFD.
The current pilot study aimed to investigate shortterm morbidity after the application of A-PRF+ in combination with periodontal regenerative microsurgery in molar furcations in comparison to EMD application or OFD.
Materials and methods
Trial design
The present manuscript has been written according to the CONSORT statement for improving the quality of reports of parallel-group randomized trials (http://consort-statement.org/). This pilot study is part of a randomized controlled trial (RCT) (Platelet rich fibrin in periodontal surgery: a double blind, randomized controlled trial) conducted at Academic Centre for Dentistry Amsterdam (ACTA), Department of Periodontology. The study was approved by the medical ethical committee of the VU Medical Center, Vrije Universiteit Amsterdam (study protocol number: 6265602917). This RCT has been terminated prematurely (March 2020) due to the corona crisis; the initial lockdown and the subsequent partial capacity of the clinic limited patients recruitment, surgeries and follow-ups.
Participants and inclusion and exclusion criteria
Screening for eligibility was carried out on patients who completed active non-surgical periodontal therapy. The stage of periodontitis was defined according to the classification of 2017 (Papapanou et al., 2018). Patients who met the inclusion criteria were asked for participation in this research. Patients received an information sheet with the content and the aim of the research. A 2-week time period was given to contemplate about participation in the study. After agreement, patients were asked to sign an informed consent.
To be included, the patients should be aged between 18 and 80 years old, have at least one molar with furcation involvement grade II (Hamp et al., 1975) with horizontal CAL >3 mm and a residual pocket depth after non-surgical therapy ≥5 mm, full mouth plaque score (FMPS) <20% and full mouth bleeding score (FMBS) <30%. During recruitment the following exclusion criteria were applied: uncontrolled diabetes, HIV (human immunodeficiency virus) positive patients, leukopenia, or any systemic diseases related to reduced wound healing potential, allergy to any medication or material related with the study protocol, pregnancy or lactation, daily use of any medication suppressive for the immune system like corticosteroid or other immunosuppressant and antibiotic use at least 3 months before the study enrollment. Exclusion criteria on a site level were the following: third molars, lingual furcation sites of mandibular molars, bone loss extending up to the apex, endodontically and non-endodontically treated teeth with periapical radiolucency, teeth with vertical fractures or cracks, mobility more than 1 and furcation involvement grade I or III (Hamp et al., 1975).
In order to score morbidity and acceptance of the therapy, the questionnaires were given to the patient before the surgery. The patient was informed by an oral explanation about the content of the questionnaires and he/she was advised to complete each of them between 20:00 and 21:00 h.
Blood collection before PRF preparation
In order to maintain patient blindness, blood was collected from all the participants by a periodontist. The blood drawing procedure was performed by venipuncture of the antecubital fossa immediately before the surgery in order to prepare the A-PRF+. In total 4 tubes of blood (40 ml) were taken from each patient. Two of them were used for the preparation of the A-PRF+ (sterile plain glass-based vacuum tubes). Two other tubes (with EDTA), not to be used for the preparation of the A-PRF+, were used for the analysis of peripheral blood markers (results not shown in this paper). The A-PRF+ was prepared according to the spinning protocol, requiring 208 g of relative centrifugation force (RCF max) for 8 minutes (Miron et al., 2020; Fujioka-Kobayashi et al., 2017). After centrifugation, the fibrin clot (A-PRF+) was gently taken from the tube and separated from the red element phase at the base of the tube with the use of pliers. The A-PRF+ clot was then adapted in the proper PRF box (APRF, Nice, France), that provided a constant compression due to the lid (cover of the PRF box) on top of the clot. The compression lasted 5 minutes, after which it was possible to retrieve A-PRF+ membranes, equal in size and thickness. The whole procedure was performed in the surgical room, with sterile instruments in a sterile setting (Figure 1), and in presence of the patient sitting on the dental chair.
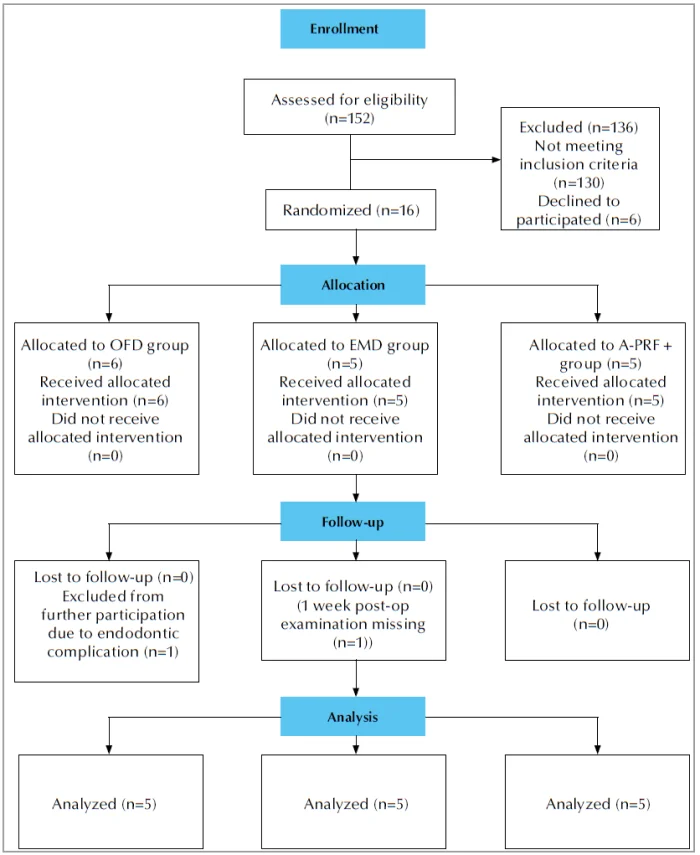 Figure 1. CONSORT flow chart of the study.
Figure 1. CONSORT flow chart of the study.
Surgical procedure and postoperative instructions and protocol
The surgical procedure was performed by an experienced periodontist (SB). All surgeries were performed with a microsurgical approach with a modified or simplified papilla preservation technique depending on the width of the inter-dental space (Cortellini et al., 1999; Cortellini et al., 1996). If deep pockets were present at the distal site (retromolar area) the access was performed with a trap technique, leaving as much tissue as possible in the middle of the ridge. This allowed a better coverage and stabilization of the flap. The flap was elevated in the most atraumatic way possible, leaving interdental tissues intact. In addition, the flaps were extended apically to the defects and furcation entrances. If necessary, releasing incisions were performed on the mid- buccal or mid-lingual side of the most mesial tooth involved in the flap. Degranulation was thoroughly performed with curettes, including removal of granulation tissue from the furcation involved areas. After having access to the bone anatomy, ultrasonic debridement of roots was performed with saline cooling, together with hand instruments. At this point, the person in charge for the randomization had to open an envelope containing the treatment allocation and the surgeon was informed about which of the three approaches was to be used (A-PRF+ or EMD or OFD). If the patient was allocated to OFD group, the area was rinsed with saline (NaCl 0.9 %), followed by a pause of 1 minute, after which the rinsing was repeated with the same solution. The latter was performed in order to resemble the application of regenerative materials. In case of A-PRF+ allocation, sterile prepared A-PRF+ membranes were minced by sterile scissors and compressed, as much as possible, into the furcation II defect of the molars involved in the surgery. In order to guarantee clot stabilization, an additional A-PRF+ membrane was applied covering the graft material and protecting the furcation entrance around the tooth profile. A-PRF+ was intended to be applied in less than 1 hour from the beginning of the surgery. In case of EMD, roots were dried and EMD (Emdogain®, Institut Straumann AG, Basel, Switzerland) was applied for 2 minutes. Subsequently, the flap was sutured. The papilla was repositioned in the original buccal position. Propylene sutures 5-0 were used with the intention to achieve a primary, tension free closure. The suture technique was horizontal mattress with a Laurel loop. Additional single sutures were applied if necessary.
After the surgery, every patient was asked to avoid any form of brushing in the operated area and to rinse firstly with hydrogen peroxide for 2 mins and later with chlorhexidine 0.12 % (PerioAid 0.12%, Dentaid, Barcelona, Spain) for 1 min twice per day. These instructions were continued for 4 weeks. Analgesics use (Paracetamol, also known as acetaminophen, 500 mg, maximum intake of 6 grams per day) was advised but not prescribed. The patient was requested to report the consumption of the medication in the provided questionnaires. Sutures were removed after 14 days. A prophylaxis protocol via gentle polishing with rubber cups and brushes was performed on day 7, week 2 and week 6 after surgery. Each patient was instructed to perform oral hygiene with interdental brushes and an electric toothbrush from 4 weeks post-operatively onward, at which point the chemical plaque control was ceased.
Assessment of post-operative morbidity
Post-operative morbidity was assessed with the completion of a validated questionnaire for post-operative discomfort (Gobbato et al., 2016). The patients were asked to complete the questionnaires on a standardized time (between 20:00 and 21:00 h) on the day of the surgery, every day during the first week, at 2 week and at week 6 post-operatively. The questionnaires were designed to evaluate pain experience allowing the patient to rate the pain experience from ‘no pain’ to ‘worst possible pain’ using a visual analogue scale (VAS) of 10 cm. The patient was asked to draw a vertical line with a pen on the VAS under each question. The distance between the left edge of the VAS and the vertical line that was drawn by the patient was measured with a ruler by the examiner. This value (mm) ranged from 0 to 100. All the questionnaires that were referring to the post-operative period were given to the patients the day of the surgery. The patient was asked to bring the questionnaires at the follow-up appointments. Each questionnaire included ten questions (Appendix 1). Two questions were not included in the questionnaire of the day of the surgery (questions 4 and 6) since these questions aimed at the pain in the morning and throughout the day, therefore not applicable at the same day of the intervention. Besides pain in response to various stimuli, other parameters were investigated in a dichotomous fashion (yes or no): these parameters were experienced post-operative bleeding, consumption of analgesic medication and whether the patient would undergo a similar procedure in the future if recommended by the dentist. The patient was also asked to indicate the type of analgesic medication that he used and the quantity.
Randomization, blinding and treatment allocation
A randomization scheme was made on the basis of the number of patients needed for the main study (Platelet rich fibrin in periodontal surgery: a double blind, randomized controlled trial), and was calculated by a sample size calculation. The current study is the first analysis of the parent study focusing on the first weeks and it is considered as pilot because no specific sample size was calculated for the PROs. Smokers were also included in the study cohort and their distribution was stratified. Closed numbered envelopes were prepared with the description of the material that was going to be used (A-PRF+, EMD, OFD) and stored in a locked drawer. Care was taken not to reveal to the patient the content of the envelope. The surgeon was blinded for the technique until the intra-surgical moment of material application. The examiner of this study that evaluated the short-term PROs was blinded about treatment allocation throughout the study.
Data analysis
The Statistical Package for the Social Sciences (IBM SPSS Statistics Data Editor, Chicago, Illinois, US) and GraphPad software (GraphPad Prism Version 8.4.1, San Diego, California, US) were used for data analyses. For age, which was the only normally distributed (Shapiro-Wilk test), differences between groups were analyzed by One-Way Analysis of Variance (ANOVA) was used. For all other variables, differences between groups were explored by non-parametric tests (Kruskal-Wallis test). Differences in frequencies of smoking and sex per group were tested with Chi squared test. For all other variables, the Kruskal Wallis test was used to compare differences across the three groups. Bonferroni correction was done at the pairwise sub-analysis. The level of statistical significance was set at p<0.05.
Results
Participants and baseline data
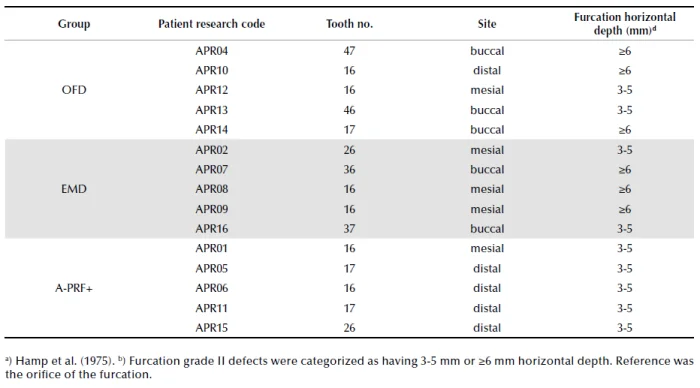
Recruitment of patients started in June 2018. From October 2018 to March 2020, 16 patients participated in the study and received surgery, and were seen for follow-up for minimally 6 weeks. One patient, at 5 weeks post-operatively, was excluded from further participation in the study due to endodontic complications at the tooth that was investigated. Thus, 15 patients were analysed for this pilot study (see CONSORT flow-chart, Figure 2). Table 1 shows the patient and periodontal characteristics at baseline. The mean age was 64.4 (SD 6.0), 56.0 (SD 15.0) and 50 (SD 12.3) for the A-PRF+, EMD and OFD groups respectively (not statistically significant different across the groups). Fourteen patients were classified as stage III periodontitis, one as stage IV. Table 2 presents the tooth characteristics as recorded on the day of the surgery. Based on randomization, in total 11 upper (5 in A-PRF+, 3 in EMD and 3 in OFD group) and 4 lower molars (2 in EMD and 2 in OFD group) were included in our analysis. Five patients had buccal (3 allocated in EMD and 2 in OFD group) and 10 interproximal furcations (5 in A-PRF+ group, 3 in EMD and 2 in OFD) included. Regarding the furcation depth at baseline, none of the patients in A-PRF+, 3 patients in EMD and 3 patients in OFD group had furcations with a horizontal attachment loss ≥6 mm (Table 2).
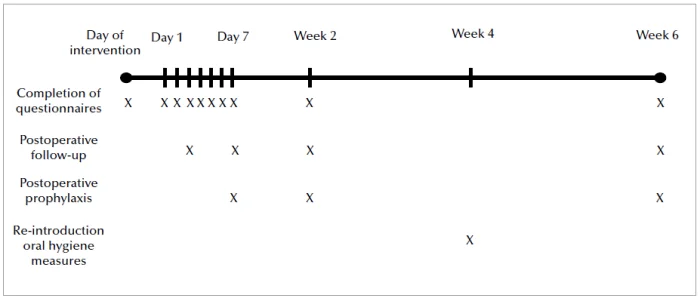 Figure 2. Flowchart and timeline of the study.
Figure 2. Flowchart and timeline of the study.
Table 1. Patient and periodontal characteristics.
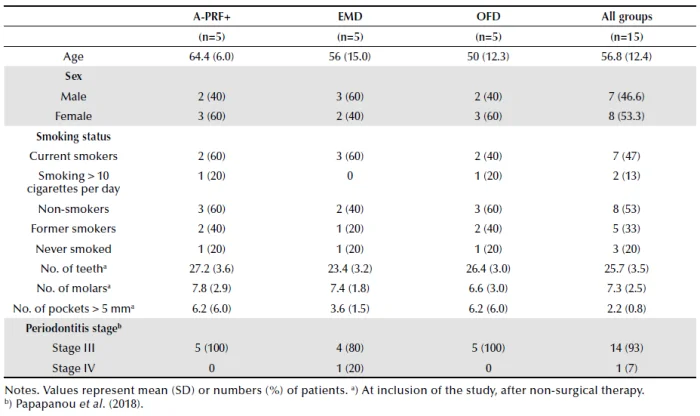 Table 2. Tooth characteristics at baseline (day of the surgery). All teeth had furcation grade IIa.
Table 2. Tooth characteristics at baseline (day of the surgery). All teeth had furcation grade IIa.
 Surgical procedures
Surgical procedures
No adverse events during the surgeries were reported by the surgeon. In all patients, primary intention healing was achieved. In one patient (APR02) a releasing incision at the palatum was needed. The handling of the PRF membranes and EMD was uneventful. Time of the surgery was recorded for 12 out of 15 subjects and it ranged from 23 to 72 minutes. Healing was uneventful for all participants of the study. One patient (APR07, EMD group) did not attend the 1-week follow-up examination because of reported illness, not related to post-operative complications.
Morbidity
Figure 3 shows the mean values of each group per question based on the VAS scale during the post-operative follow-up period. In the EMD group, patients scored significantly higher on the question “Pain experienced in the mouth as a whole” on days 5 and 6 compared to OFD group. In the EMD group, patients scored significantly higher for the question “Pain experienced throughout the day” on days 3, 4, 5 and 6 compared to OFD. The EMD group scored higher on days 3, 4 and 6 for the question “Pain experienced in the morning”, a significant difference was again only between OFD and EMD. In the EMD group, the VAS score was higher on day 6 for the question “Pain experienced at night” compared to OFD group and patients from EMD group experienced more swelling on day 4 compared to OFD group. No statistically significant differences in the morbidity questions were observed between EMD and A-PRF+ groups or OFD and A-PRF+ groups for any of the follow-up time points. Tables S1, S2, S3, S4, S5, S6 and S7 for each question are provided as supplementary material.
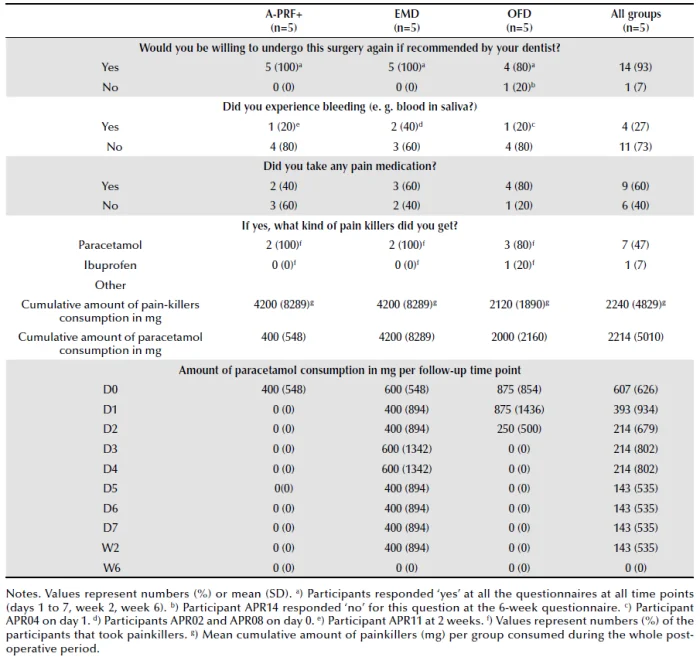
Table 3 shows the number and proportion of patients who were willing to undergo the surgery again if recommended by their dentist, who experienced bleeding during the post-operative follow-up period and who used painkillers as well as the type and the mean consumption per group. All patients were willing to undergo the surgery again if recommended by their dentist. Only one patient (APR14) from OFD group, at the 6-week follow-up, responded negatively for this question. One patient (APR04) from the OFD group on day 1, 2 patients from EMD group (APR02, APR08) on day 0 and one patient (APR11) from A-PRF+ group at 2 weeks reported bleeding. The mean amount (in mg) of consumption of painkillers during the post-operative period was 2120 mg (SD 1890) in OFD group, 4200 mg (SD 8289) in EMD group and 400 mg (SD 548) in A-PRF+ group. No statistical significant differences were found between groups per post-operative day. Only one patient (APR13), used ibuprofen instead of paracetamol as analgesic of choice. We compared also the daily consumption of painkillers per follow-up day across groups and no statistical significant differences found (Table 3).
Table 3. Dichotomous questions of the questionnaire and pain-killer consumption during the follow-up period.
 Compliance
Compliance
Two patients (APR13 and APR16) sent a photograph of the questionnaires electronically to the examiner, as the scheduled appointments were cancelled due to restrictions of the COVID-19 pandemic. Therefore, the analogue of the VAS score of the photographs was taken. The compliance of the patients regarding the time of filling in the questions was checked during the clinical appointments. One patient (APR15) filled the 6-week questionnaire one week earlier than the scheduled date, indicating 0 VAS score to all of the questions and no use of medications.
Discussion
Within the limitation of the low numbers of participants (5 per group) in this study, we observed that A-PRF+ use was related to low levels of pain or discomfort and a high level of patient acceptance. Although this study has to be considered a pilot, to the best of our knowledge this is the first study that investigates patient acceptance and post-operative morbidity after the use of A-PRF+ in periodontal micro-surgery in furcation involved teeth, and also compares the results with a widely used material in periodontal regeneration (EMD).
Patients that received A-PRF+ experienced mild post-operative pain and discomfort, scoring low numbers at all the VAS questions. Post-operative bleeding was a rare event and the painkiller consumption among all subjects was limited to acetaminophen. The overall mean consumption of painkillers was less (as a trend) in A-PRF+ group compared to the other groups and the paracetamol consumption was limited to 1 gram only at the day of the surgery. In general, the additional procedure of drawing blood for PRF preparation, did not affect the overall perception of the procedure of the patient. All the patients of the study were willing to undergo the same procedure again if necessary. This report suggests that the pre-surgical blood sampling for the preparation of any blood-derived material is a procedure accepted by patients and does not represent a limit in terms of invasiveness or discomfort. In accordance with our findings, there are few studies that suggest that PRF (L-PRF) in oral surgery might be related to less post-operative pain (He et al., 2017; Femminella et al., 2016). According to Femminella (2016) PRF significantly reduced the patient discomfort after its use in the healing of palatal wounds compared to controls that received a gelatine sponge. Moreover, it seems that PRF is beneficial during the early wound healing after lower third molar extractions in terms of patients’ pain relief (He et al., 2017).
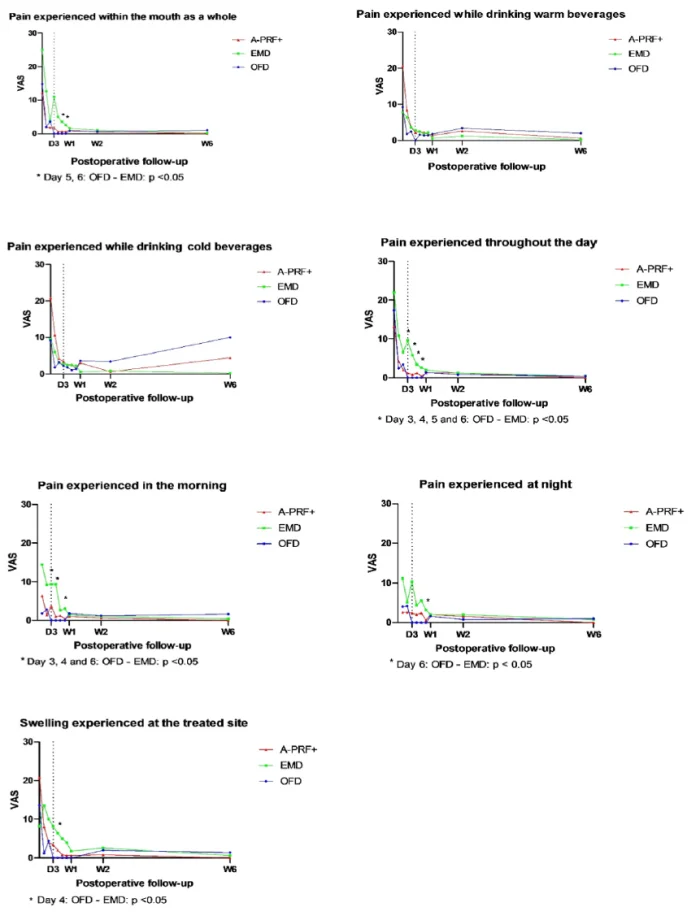 Figure 3. Mean scores of visual analogue scale (VAS) of each group per question during the post-operative follow-up. Patient APR01, after the first week, was excluded from the analysis of the first VAS question “Pain experienced within the mouth as a whole” because another periodontal surgery was performed at day 7. Statistical significant differences across groups are depicted with an asterisk (*). VAS is 0-100. Statistical significance level is set at p <0.05.
Figure 3. Mean scores of visual analogue scale (VAS) of each group per question during the post-operative follow-up. Patient APR01, after the first week, was excluded from the analysis of the first VAS question “Pain experienced within the mouth as a whole” because another periodontal surgery was performed at day 7. Statistical significant differences across groups are depicted with an asterisk (*). VAS is 0-100. Statistical significance level is set at p <0.05.
These clinical results are related to the biological properties of PRF, where the interaction between the fibrin network and the cytokines and chemokines released by leukocytes and platelets are promoting essential early tissue healing events (Miron et al., 2017). These events would happen anyhow during the healing process, but research shows a faster progression in tissues treated with PRF derivatives. Angiogenic properties have been reported, explained by the slow and simultaneous release of PDGF, TGF-β1, IGF, and VEGF (Schar et al., 2015; Kobayashi et al., 2016). The same mediators are able to recruit pericytes and stem cells, promoting the tissue repair. The fibrin matrix is furthermore able to express integrins of various nature, promoting cells adhesion via fibrin, fibronectin and vitronectin (Masuki et al., 2016; Cabaro et al., 2017). Moreover, fibrin degradation products are able to promote migration of neutrophils in the wound and facilitate their transmigration into the vascular endothelium (Strauss et al., 2020). The proteases release facilitates the permeability of extracellular matrix for the formation of newly formed blood vessels. Furthermore, the degradation of the fibrin clot releases neutrophils and macrophages trapped in the fibrin itself, contributing to the production of oxygen radicals and enzymes able to fight bacterial contamination of the wound (Nasirzade et al., 2020). All these events may contribute to a lower pain perception from patients.
Patients in EMD group scored significantly higher compared to OFD group at the questions that referred to the pain during the first week. This was somewhat to our surprise, as EMD has been reported as a patient friendly material that may show an advantage in terms of patient acceptance compared to more complicated techniques such as GTR ( Jepsen et al., 2004). In that respect, our findings are more similar to the findings of the study of Tonetti et al. (2004), where EMD was used for the treatment of infrabony defects compared to OFD: patients in EMD group experienced higher levels of sensitivity with a peak at 3 weeks (Tonetti et al., 2004). However, both studies did not use questionnaires to specify the type of stimulus that elicited the sensitivity. Although in our study we did not perform root preconditioning with EDTA, post-operative sensitivity remains a problem in this patient group. Another interesting finding is that when EMD was used under flaps in periodontal surgery, patients experienced higher levels of swelling compared to controls (Hagenaars et al., 2004). Similarly, the present EMD group scored significantly higher for the question referring to perceived swelling on day 4 post-operatively.
Despite some high scores for the EMD group, we found that all three procedures were related to low levels of post-operative pain. The events of post-operative bleeding were rare and 14 out of 15 patients were willing to undergo the procedure again if recommended by the dentist. Again, we like to stress that apparently blood drawing was not an objection. Therefore, our study indicates that periodontal regenerative surgery appears to be a well-tolerated procedure. This is in accordance with previous studies that investigated the morbidity after periodontal surgery (Mei et al., 2016; Askar et al., 2019; Curtis et al., 1985).
Another point to be considered is the impact of smoking during the healing phase. In smokers the healing can be less favourable, and this may result in higher post-operative pain perception (Guo et al., 2010). In our cohort, almost half of the patients were smokers, very few (3 in total, 1 per group, never smoked). This may have made the results in our cohort comparable between patients. Furthermore, we didn’t find differences in pain response in the subgroup of patients that were smoking.
The strengths of this study include the setting, the design and the intense follow-up. Double blinding was achieved throughout the study (for both patients and examiner) and the strict recall program allowed us to ensure the compliance of the patients with the protocol. The daily monitoring of patient-centred data with the questionnaires helped us to detect differences at the first post-operative days that could have been missed in case that the questionnaires were given only on a weekly basis. There are also limitations to the present study. A sample size calculation was not possible because of the lack of the studies that investigated similar outcomes in the literature. The number of patients that are included in our study is low and therefore the interpretation of our findings should be done with caution. Maxillary and mandibular sites may as well have a different post-operative perception, and due to the low number, we had to group the operated sites. While we used a post-operative questionnaire already used in a publication regarding post-operative discomfort after regenerative periodontal surgery (Gobbato et al., 2016), it does not clarify whether the quality of life of the patient was affected. For this aim, another validated questionnaire like the OHIP-14 may have been indicated. Nevertheless, due to the broader range of applications of the OHIP-14, it seems less precise to define some of the parameters we aimed to investigate in our study. Another limitation may be the compliance of the patients in answering the questions at the suggested time and day. It was not possible to be precisely assessed and we were dependent on patient’s cooperation and honesty. As such, digital surveys would give better insights in compliance.
Patient related factors, such as age and sex, are stated in the literature as factors that could affect the perception of pain after surgery (Tighe et al., 2015). Namely, female sex and young age scored higher pain in postoperative values in the aforementioned study. In our study the PRF group had the highest mean age, although not significantly different from the other two groups.
There are additional details about periodontal surgery that you can find on our website.
Conclusions
In conclusion, we observed that the application of a PRF preparation (A-PRF+) was related to low levels of pain and discomfort and a high level of patient acceptance during and after the surgical regenerative treatment of molars furcations. The use of A-PRF+ was not related to increased morbidity compared to the two other treatment modalities. In fact, EMD was related to increased levels of pain and swelling during the first week compared to the other treatments, but the pain levels could be considered mild as they were scored at the lower part of the VAS. Because of the pilot nature of the study, the results cannot be generalized and should be considered as observations. Further RCTs are needed to draw a definitive conclusion regarding the effects of A-PRF+ on PROs when applied in periodontal regeneration procedures.
List of authors:
Dimitros Vasdravellis
Luciano Pitzurra
Sergio Bizzarro
Bruno G. Loos
References
Agrawal AA. Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World journal of clinical cases 2017; 5:159-171.
Askar H, Di Gianfilippo R, Ravida A, Tattan M, Majzoub J and Wang HL. Incidence and severity of postoperative complications following oral, periodontal, and implant surgeries: A retrospective study. J Periodontol 2019; 90:1270-1278.
Avila-Ortiz G, De Buitrago JG and Reddy MS. Periodontal regeneration - furcation defects: a systematic review from the AAP Regeneration Workshop. J Periodontol 2015; 86:S108-130.
Bajaj P, Pradeep AR, Agarwal E, et al. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: a randomized controlled clinical trial. J Periodontal Res 2013; 48:573-581.
Cabaro S, D’Esposito V, Gasparro R, et al. White cell and platelet content affects the release of bioactive factors in different blood-derived scaffolds. Platelets 2017; 1-5.
Castro AB, Meschi N, Temmerman A, et al. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol 2017; 44:67-82.
Cortellini P, Pini Prato G and Tonetti MS. The modified papilla preservation technique with bioresorbable barrier membranes in the treatment of intrabony defects. Case reports. Int J Periodontics Restorative Dent 1996; 16:546-559.
Cortellini P, Prato GP and Tonetti MS. The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int J Periodontics Restorative Dent 1999; 19:589-599.
Cortellini P and Tonetti MS. Clinical concepts for regenerative therapy in intrabony defects. Periodontology 2000 2015; 68:282-307.
Curtis JW, Jr., McLain JB and Hutchinson RA. The incidence and severity of complications and pain following periodontal surgery. J Periodontol 1985; 56:597-601.
El Bagdadi K, Kubesch A, Yu X, et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept). European Journal of Trauma and Emergency Surgery 2019; 45: 467-479.
Femminella B, Iaconi MC, Di Tullio M, et al. Clinical comparison of platelet-rich fibrin and a gelatin sponge in the management of palatal wounds after epithelialized free gingival graft harvest: a randomized clinical trial. J Periodontol 2016; 87:103-113.
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y and Choukroun J. Optimized platelet-rich fibrin with the lowspeed concept: growth factor release, biocompatibility, and cellular response. J Periodontol 2017; 88:112-121.
Gobbato L, Nart J, Bressan E, Mazzocco F, Paniz G and Lops D. Patient morbidity and root coverage outcomes after the application of a subepithelial connective tissue graft in combination with a coronally advanced flap or via a tunneling technique: a randomized controlled clinical trial. Clinical Oral Investigations 2016; 20:2191-2202.
Guo S and Dipietro LA. Factors affecting wound healing. J Dent Res 2010; 89:219-229.
Hagenaars S, Louwerse PH, Timmerman MF, Van der Velden U and Van der Weijden GA. Soft-tissue wound healing following periodontal surgery and Emdogain application. J Clin Periodontol 2004; 31:850-856.
Hammarstrom L. Enamel matrix, cementum development and regeneration. J Clin Periodontol 1997; 24:658-668. Hamp S, Nyman S and Lindhe J. Periodontal treatment of multi-rooted teeth. Results after 5 years. Journal of Clinical Periodontology 1975; 2:126-135.
He Y, Chen J, Huang Y, Pan Q and Nie M. Local application of platelet-rich fibrin during lower third molar extraction improves treatment outcomes. Journal of Oral and Maxillofacial Surgery 2017; 75:2497-2506.
Jepsen S, Heinz B, Jepsen K, et al. A randomized clinical trial comparing enamel matrix derivative and membrane treatment of buccal Class II furcation involvement in mandibular molars. Part I: Study design and results for primary outcomes. Journal of Periodontology 2004; 75:1150-1160.
Kanoriya D, Pradeep AR, Garg V and Singhal S. Mandibular degree II furcation defects treatment with platelet-rich fibrin and 1% alendronate gel combination: a randomized controlled clinical Trial. J Periodontol 2017; 88:250-258.
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 2016; Larrazabal C, Garcia B, Penarrocha M and Penarrocha M. Influence of oral hygiene and smoking on pain and swelling after surgical extraction of impacted mandibular third molars. J Oral Maxillofac Surg 2010; 68:43-46.
Masuki H, Okudera T, Watanebe T, et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). International journal of implant dentistry 2016; 2:19.
Mei CC, Lee FY and Yeh HC. Assessment of pain perception following periodontal and implant surgeries. J Clin Periodontol 2016; 43:1151-1159.
Miron RJ, Dard M and Weinreb M. Enamel matrix derivative, inflammation and soft tissue wound healing. J Periodontal Res 2015; 50:555-569.
Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M and Choukroun J. Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue engineering. Part B, Reviews 2017; 23:83-99.
Miron RJ, Sculean A, Cochran DL, et al. Twenty years of enamel matrix derivative: the past, the present and the future. J Clin Periodontol 2016; 43:668-683.
Miron RJ, Xu H, Chai J, et al. Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~ 700 g) and low (~ 200 g) relative centrifugation forces. Clin Oral Investig 2020; 24:1171-1182.
Nasirzade J, Kargarpour Z, Hasannia S, Strauss FJ and Gruber R. Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro. J Periodontol 2020; 91:244-252.
Peres MF, Ribeiro ED, Casarin RC, et al. Hydroxyapatite/beta-tricalcium phosphate and enamel matrix derivative for treatment of proximal class II furcation defects: a randomized clinical trial. J Clin Periodontol 2013; 40:252-259.
Pihlstrom BL, Michalowicz BS and Johnson NW. Periodontal diseases. Lancet (London, England) 2005; 366:1809-1820.
Schar MO, Diaz-Romero J, Kohl S, Zumstein MA and Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res 2015; 473:1635-1643.
Sharma A and Pradeep AR. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: a randomized clinical trial. J Periodontol 2011; 82:1396-1403.
Sousa F, Machado V, Botelho J, Proença L, Mendes JJ and Alves R. Effect of A-PRF Application on palatal wound healing after free gingival graft harvesting: a prospective randomized study. European Journal of Dentistry 2020; 14:63-69.
Strauss FJ, Nasirzade J, Kargarpoor Z, Stähli A and Gruber R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: a systematic review of in vitro studies. Clin Oral Investig 2020; 24:569-584.
Tighe PJ, Le-Wendling LT, Patel A, Zou B and Fillingim RB. Clinically derived early postoperative pain trajectories differ by age, sex, and type of surgery. Pain 2015; 156:609-617.
Tonetti MS, Fourmousis I, Suvan J, Cortellini P, Bragger U and Lang NP. Healing, post-operative morbidity and patient perception of outcomes following regenerative therapy of deep intrabony defects. J Clin Periodontol 2004; 31:1092-1098.
Wennstrom JL and Lindhe J. Some effects of enamel matrix proteins on wound healing in the dento-gingival region. J Clin Periodontol 2002; 29:9-14.
Zucchelli G, Bernardi F, Montebugnoli L and De Sanctis M. Enamel matrix proteins and guided tissue regeneration with titanium-reinforced expanded polytetrafluoroethylene membranes in the treatment of infrabony defects: a comparative controlled clinical trial. J Periodontol 2002; 73:3-12.

