Scanning Electron Microscopy Analyses of Dental Implant Abutments Debonded from Monolithic Zirconia Restorations Using Heat Treatment: An In Vitro Study
Abstract
Aim: The aim of this in vitro study is to present a debonding protocol developed to remove a screw-retained, monolithic, zirconia restoration from its titanium-base abutment, and to microscopically evaluate the abutment integrity at both the prosthetic and connection levels.
Materials and Methods: A total of 30 samples were tested. Each sample consisted of a monolithic zirconia restoration bonded on a titanium link abutment. Five different shapes were designed and fabricated. Randomly, one-third of the Ti-link abutments were subjected to an anodizing process. Then, all the zirconia samples were bonded to the Ti-link abutments according to a pre-established protocol. Forty-eight hours later, the samples were debonded according to the experimental protocol. The outcomes were evaluated by a visual inspection with an optical microscope, scanning electron microscopy (SEM), and chemical composition analysis.
Results: Thirty samples were collected and visually analyzed. Seven samples were randomly evaluated via scanning electron microscopy. In all the examinations, no relevant changes were reported. Chemical composition analysis also relieved no changes in the chemical structure of the titanium.
Conclusions: The titanium-base abutments do not alter the structure and properties of the material, not creating phase changes or the birth of oxides such as to induce fragility. Further clinical studies with longer follow-up periods are needed to confirm these preliminary results.
Introduction
Precision at the implant–abutment interface is one of the most important aspects influencing marginal bone remodeling and the risk of peri-implantitis. Microgaps and bacterial leakage play an important role in peri-implant inflammatory reactions and the subsequent loss of supporting bone to restore the physiological biologic width. Definitive abutment placed at implant insertion and not being removed seems to be an effective prosthetic approach to reduce the physiological marginal bone remodeling. However, in recent decades, screw-retained implant restorations have increased in popularity due to their predictable retention, retrievability, and lack of potentially retained sub-gingival cement. The last point has become very important due to the trend to place implants subcrestally.
Prosthetically driven implant placement is crucial for the long-term success of treatment, allowing the implants to be installed in the most accurate mesio-distal and bucco-lingual position and depth. Some studies show no clinical differences when placing implants 0.5 mm or 1.5 mm subcrestally; therefore, clinicians can choose as they prefer. However, the depth of implant placement should be carefully planned to consider available bone and soft tissue thickness, the type of the implant, and the type and shape of further prosthetic reconstruction. Placing an implant in a subcrestal position may have a positive impact, especially in the aesthetic area, where obtaining a harmonious emergence profile is mandatory. However, the vertical position mostly depends on the type of connection. Implants with internal conical connection and platform switching at the implant–abutment interface have been shown to maintain stable bone levels over a mean follow-up period of two years when placed subcrestally.
Due to their aesthetics, high mechanical properties, and biocompatibility, Yttria stabilized tetragonal zirconia ceramics have gained popularity as the preferred restorative material for implant-supported single crowns in the aesthetic area, with survival rates ranging between 90% and 96% after observation periods of 5 and 10 years, respectively. For these reasons and more, implant companies market several prosthetic options to deliver screw-retained implant-supported restorations. Within these, titanium-base abutments (TBAs) or titanium-link abutments can be considered feasible treatment options for restoring dental implants. The final restoration is a hybrid cemented-screwed, aesthetic solution composed of a metal-free restoration that is bonded outside of the patient’s mouth to an original TBA. The main benefits of this approach include its retrievability, highly precise implant–abutment fit (guaranteed by the manufacturer), and the customization of the emergence profile. Moreover, working on a fully or semi-digital workflow, hybrid prosthetic solutions also potential reductions in production costs compared to the classical workflow.
To create hybrid prosthetic solutions, monolithic zirconia or porcelain fused to zirconia (PFZ) restorations are computer-aided designed (CAD) and computer-aided manufactured (CAM) a with a semi-digital or fully digital approach. Finally, the zirconia restorations are bonded chairside on TBAs, resulting in the hybrid cemented/screw-retained restorations. This approach reduces any inflammatory process due to cement remnants in the peri-implant tissue, maintaining its retrievability. Bonding can also be performed in a dental laboratory under controlled conditions; nevertheless, in case major ceramic corrections are needed (color, contact points, occlusion), the TBA must be debonded from the ceramic restoration before it is placed in the dental ceramic oven at 370°C for five minutes. Moreover, when a zirconia restoration is debonded, the resin cement remains adhered to it, and this must be removed before the restoration can be re-cemented. In the literature, there are several papers concerning bonding protocols and retentive force. However, at the time of writing this manuscript, and to the authors’ knowledge, there are no manuscripts reporting debonding procedures and their impact on the surface of titanium abutments.
The aim of this in vitro study is to present a debonding protocol developed to remove a screw-retained, monolithic zirconia restoration from its TBA and to microscopically evaluate the abutment integrity at both the prosthetic and implant–abutment connection level.
Materials and Methods
A total of 30 samples were considered for this in vitro research. No similar study was found in the literature. For this reason, a priori sample size analysis was not performed. Each sample consisted of a monolithic zirconia restoration (MZR) bonded on a TBA (Ti-link Abutment, Osstem Implant, Seoul, South Korea). All the MZRs were designed (computer-aided design, CAD) and manufactured (computer-aided manufacturing, CAM) in one dental laboratory in Italy using a standardized protocol as recommended by the manufacturer (ST ML, UpCera Shenzhen Dental Technology Co. LTD., Nobil-Metal, Asti, Italy). The percent composition of the used zirconia was ZrO2 + HfO2 + Y2O3 > 98%; Er2O3 < 1.0%; Fe2O3 < 0.3%; Pr2O3 < 0.2%; other oxides < 0.5%. Five different shapes were designed and fabricated, representing the possible extreme clinical variables (Table 1 and Figure 1). The main physical data are reported in Table 2.

Randomly, one-third (10 ut of 30) of the TBAs were subjected to an anodizing process in an anodizing bath heated at 20°C with a solution of 10 g of trisodium phosphate (TPS) in 500 milliliters (mL) of distilled water under a current density of 5 mA × cm−2 due to a stabilized anodizing potential of 65 V (titanium anodizer, Artiglio S.n.c., Parma, Italy). The anodizing process resulted in the formation of a gold-colored oxide layer with a thickness of about 120 µm in 30 s. Subsequently, all the zirconia samples were bonded to the TBSs according to a well-known protocol (Table 3), as follows.

Teflon tape was used to seal the screw hole. Then, the MZRs were bonded to the TBA using PANAVIA SA resin cement (SA Cement Universal, Kuraray Noritake). An oxygen-inhibiting gel (Oxyguard II gel, Kuraray Noritake, Milan, Italy) was used to enable complete curing. Initially, a quick cure of 5 s was performed (Valo, Ultradent, Salt Lake City, UT, USA). After excess cement removal, the samples were put in a dental laboratory curing light and polymerized for 5 min. Finally, the samples were cleaned and polished (Figure 2).

Forty-eight hours later, the samples were debonded according to the experimental protocol reported in Table 4 and as previously published.
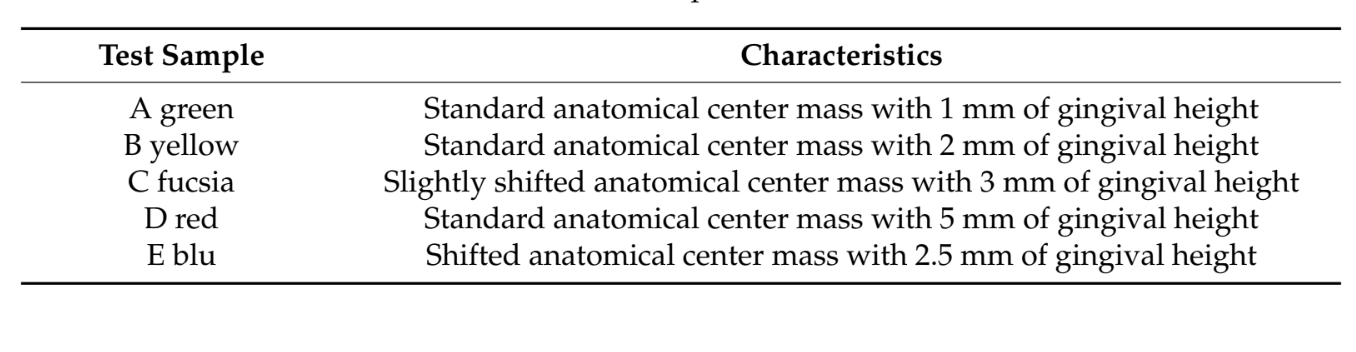
The TBAs were removed from the zirconia restorations using a customized tool inserted inside the abutment screw access hole (Figures 3 and 4).
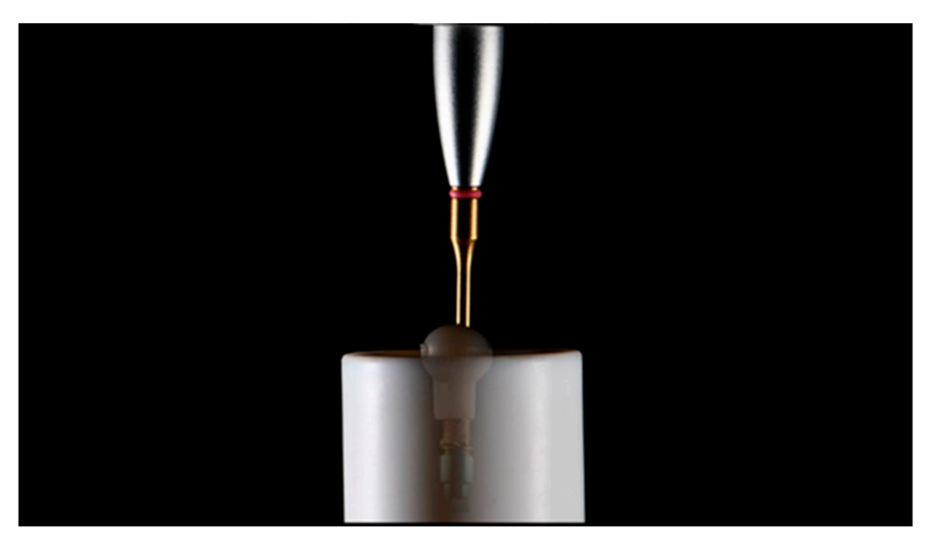
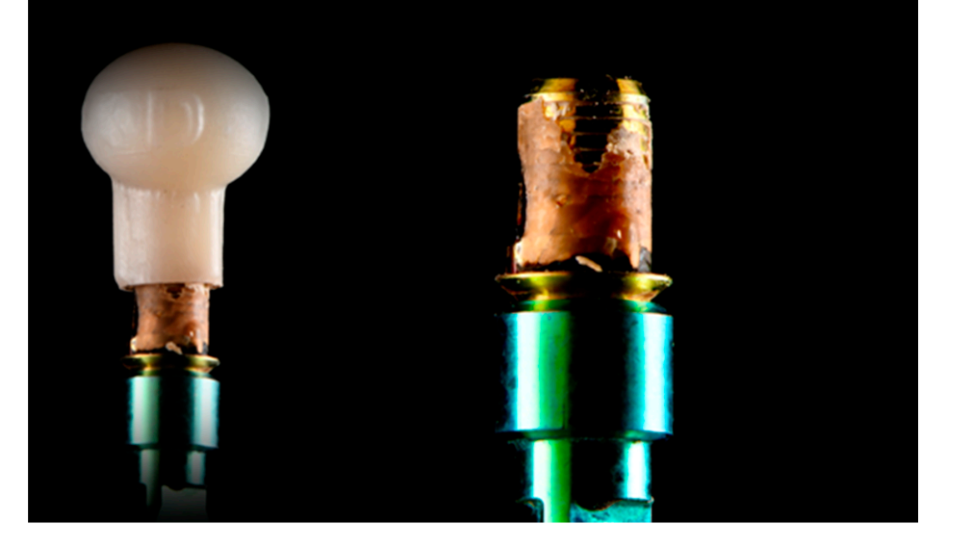
Finally, all the TBAs were cleaned according to an established protocol (Table 5), inspected with the optical microscope, and analyzed via SEM.
The SEM analyses were performed in two centers, one public centre in Warsaw (Warsaw University of Technology, Warsaw, Mazovia, Poland) and another private centre in Villafranca d’Asti, Italy (R&D Nobil Metal SpA). All the collected data were analyzed at the Department of Medicine, Surgery, and Pharmacy, University of Sassari, Italy.
Outcomes
All the MZRs and TBAs were subject to a visual inspection with an optical microscope with different magnifications (up to 40× magnification value, Leica MS5 stereomicroscope, Leica, Milan, Italy) to evaluate the response of the zirconia to the applied debonding protocol, such as fracture and/or microscopic crack.
Randomly, 2 out of 10 anodized TBAs and one new titanium TBA (used as control) were examined via scanning electron microscopy (SEM) using a Zeiss EVO 10 SEM (R&D Nobil Metal SpA, Italy) operated at 20 kV to evaluate any kind of microscopic difference from the test sample.
Randomly, 5 out of 20 non-anodized TBAs (test) and one new TBA (used as control) were examined via scanning electron microscopy (SEM) using a Hitachi SU70 SEM operated at 30 kV to evaluate any kind of microscopic difference from the test sample.
Chemical composition analysis of all the analyzed samples (test and control TBAs) was performed by an EDS probe (Bruker—XFlash Detector, R&D Nobil Metal SpA, Italy) integrated into the Zeiss EVO 10 SEM (R&D Nobil Metal SpA, Italy).
Results
After the debonding procedure, all the MZRs and TBAs were visually inspected with a stereomicroscope. All the samples were debonded according to the aforementioned protocol. Then, all the MZRs were found to be free of complications, such as fractures or crack lines, independently of the shapes. Figure 5 shows a general view of the two TBAs—on the left side, the part is in an initial condition, and the right side shows the TBAs after the described procedures. The color difference between these two can be easily spotted. The initial condition maintains the typical outlook of the titanium, while the second became yellow. This is an expected outcome, as, during the heat exposure at 370°C, an oxide layer is created on the titanium surface.
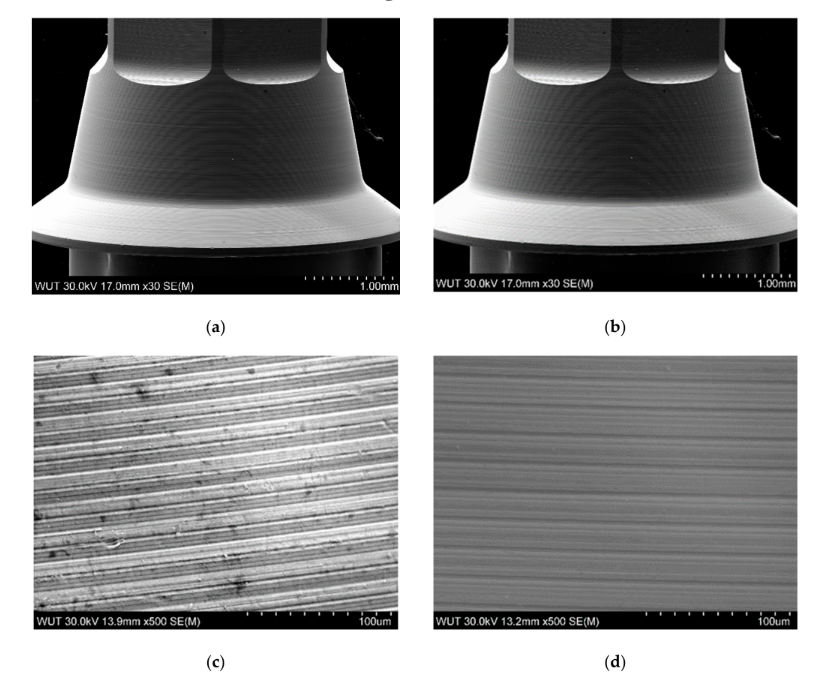
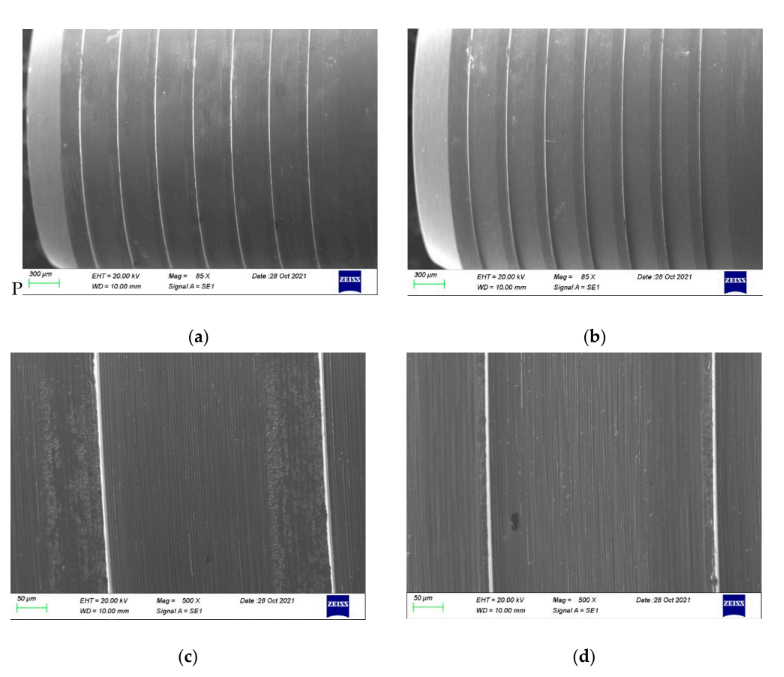
As no damages were found under stereomicroscopic analysis, the samples proceeded directly to the SEM inspection. More details can be found in the SEM images presented in Figure 6a–d. A conical part of the TBAs is compared in Figure 6a,b. As can be noticed, the contrast on the part after the thermal exposure is visibly less prominent, which may be related to a lower conductivity of the specimen or the presence of a very thin layer, which can hinder the escape of secondary electrons during observations. Both features can be linked to the presence of an oxide layer established during the thermal exposure of TBA. Higher magnification of the TBA in its initial condition reveals the patterns from the manufacturing process—the machining. The cementing and removal of the zirconia did not change those patterns, as seen in Figure 6d, but some slight changes on the surface can be noticed. Similar results were found for the anodized TBAs compared with the control and the new titanium-base abutment (Figure 7).
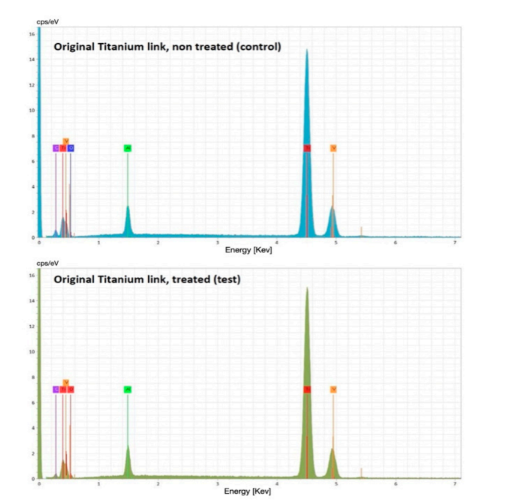
The chemical composition analysis (Energy-Dispersive X-ray Spectroscopy [EDS] graph) showed no differences between the test and control groups (Figure 8).
Discussion
This in vitro study was developed to microscopically evaluate the effect of a debonding protocol applied to remove a screw-retained, monolithic zirconia restoration from its TBA. To the best of the authors’ knowledge, at the time of writing this paper, there are no comparable studies. Therefore, it is impossible to compare the present research results with other studies.
One of the most important features of an implant-supported restoration is its retrievability, which could be necessary for implant complications. The monolithic ceramic restorations fulfill the need for suitable aesthetic reconstructions and reduce the risk of porcelain chipping. However, some complications, such as screw loosening, may still be observed. In addition, interproximal contact could be lost at the implant sites, increasing the risk for periodontal disease. The TBAs were introduced to overcome the risk of abutment fracture of one-piece zirconia restorations, allowing for a hybrid (cemented- and screw-retained), a strong link between the implants and the monolithic zirconia restorations, and finally providing a favorable, long-term, aesthetic outcome and patient satisfaction. Monolithic zirconia restorations bonded on TBAs can be easily retrieved by the patient’s mouth; nevertheless, a strict protocol, such as the one presented in this research, must be applied.
In the present study, the analyzed samples were brought to a maximum temperature of 37°C following standardized parameters avoiding the so-called “stress relief phase”. This prevents structural changes usually obtained by titanium with a higher temperature. In the present study, stereographic microscope and SEM observations show the formation of oxides due to surface color changes to yellow (see Figure 6). From a material point of view, the process of debonding did not change the properties as well as the dimensions of the TBAs. The implant connection is free of any changes. Furthermore, the part where the crown was cemented had some minor but irrelevant changes.
Titanium oxide formation at high temperatures is a well-known fact, and it has been found to be influenced by the annealing temperature. Oxidation occurs due to the high reactivity of titanium with oxygen in the air, even at room temperature. The surface morphology and structural and electrical properties of TiO2 films are influenced by the annealing temperature. It was observed that when the annealing temperature increases up to 900°C, the TiO2 crystallite size is increased. Nevertheless, at about 300° C, TiO2 films crystallize in the anatase phase with poor crystallinity. In the same study, the calculated values of the crystallite size were less than 30 nm. This means the connection part should be up to 30 nanometers bigger than the control TBA. Nevertheless, this is a transformative process that should not influence the overall dimension of the TBAs. Moreover, according to the literature, this might be clinically irrelevant. It has been demonstrated that discrepancies greater than 10 µm result in microleakage and micromovements that allow for bacterial infiltration and mechanical outcomes, such as screw-loosening. A sign of oxidation is discoloration due to a fragile layer enriched with oxygen near the surface (Alpha Case), which could be detrimental to the mechanical properties of the samples.
The main limitation of the present research is the in vitro nature of the study. Moreover, although 30 samples were fabricated and visually analyzed, only 7 out of 30 samples were randomly evaluated with SEM. However, all five randomly chosen samples showed the same results, thereby not justifying additional tests. In addition, the failure modes have not been registered, and no algorithm evaluation was conducted for statistical analysis. For these reasons, the data must be interpreted with caution. Nevertheless, the benefits of this research may be applied in several fields of implant dentistry, including prosthetic rehabilitation on implants.
Although no mechanical or clinical tests were performed, the main clinical considerations are that the TBAs could be reused in the same patient after debonding in those cases where the published protocol was applied. Nevertheless, the authors believe that the TBAs should be bonded chairside after clinical try-on, to avoid the need for debonding.
Conclusions
In light of what was observed from the SEM analysis, the treatment carried out on titanium-base abutments seemed not to alter the structure and properties of the material nor create phase changes or the birth of oxides to induce fragility. According to these results, titanium-base abutments may be reused after debonding. Further clinical studies with longer follow-up times are needed to confirm these preliminary results.
Marco Tallarico, Łukasz Zadrożny, Nino Squadrito, Leonardo Colella, Maurizio Gualandri, Daniele Montanari, Gianantonio Zibetti, Simone Santini, Witold Chrominski, Edoardo Baldoni, Silvio Mario Meloni, Aurea Immacolata Lumbau and Milena Pisano
References
- Tallarico, M.; Canullo, L.; Caneva, M.; Ozcan, M. Microbial colonization at the implant-abutment interface and its possible influence on periimplantitis: A systematic review and meta-analysis. J. Prosthodont. Res. 2017, 61, 233–241. [CrossRef] [PubMed]
- Hermann, J.S.; Buser, D.; Schenk, R.K.; Schoolfield, J.D.; Cochran, D.L. Biologic Width around one- and two-piece titanium implants. Clin. Oral Implants Res. 2001, 12, 559–571. [CrossRef] [PubMed]
- Tallarico, M.; Caneva, M.; Meloni, S.M.; Xhanari, E.; Covani, U.; Canullo, L. Definitive Abutments Placed at Implant Insertion and Never Removed: Is It an Effective Approach? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Oral Maxillofac. Surg. 2018, 76, 316–324. [CrossRef] [PubMed]
- Tallarico, M.; Caneva, M.; Baldini, N.; Gatti, F.; Duvina, M.; Billi, M.; Iannello, G.; Piacentini, G.; Meloni, S.M.; Cicciù, M. Patient-centered rehabilitation of single, partial, and complete edentulism with cemented- or screw-retained fixed dental prosthesis: The First Osstem Advanced Dental Implant Research and Education Center Consensus Conference 2017. Eur. J. Dent. 2018, 12, 617–626. [CrossRef]
- Tallarico, M.; Czajkowska, M.; Cicciù, M.; Giardina, F.; Minciarelli, A.; Zadroz˙ny, Ł.; Park, C.J.; Meloni, S.M. Accuracy of surgical templates with and without metallic sleeves in case of partial arch restorations: A systematic review. J. Dent. 2021, 115, 103852. [CrossRef]
- Zadrożny, Ł.; Czajkowska, M.; Mijiritsky, E.; Wagner, L. Repeatability of Freehand Implantations Supported with Universal Plastic Sleeves—In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 4453. [CrossRef]
- Judgar, R.; Giro, G.; Zenobio, E.; Coelho, P.G.; Feres, M.; Rodrigues, J.A.; Mangano, C.; Iezzi, G.; Piattelli, A.; Shibli, J.A. Biological width around one- and two-piece implants retrieved from human jaws. BioMed Res. Int. 2014, 2014, 850120. [CrossRef]
- Spinato, S.; Galindo-Moreno, P.; Bernardello, F.; Zaffe, D. Minimum Abutment Height to Eliminate Bone Loss: Influence of Implant Neck Design and Platform Switching. Int. J. Oral Maxillofac. Implants 2018, 33, 405–411. [CrossRef]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Race to invade: Understanding soft tissue integration at the transmucosal region of titanium dental implants. Dent. Mater. 2021, 37, 816–831. [CrossRef]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontol. 20002018, 76, 116–130. [CrossRef]
- Esposito, M.; Salina, S.; Rigotti, F.; Mazzarini, C.; Longhin, D.; Grigoletto, M.; Buti, J.; Sbricoli, L.; Gualini, F. Multicentre withinperson randomised controlled trial of 0.5 mm versus 1.5 subcrestal placement of dental implants with internal conical connection: Five-year post-loading results. Clin. Trials Dent. 2020, 2, 77–89. [CrossRef]
- Novaes, A.B., Jr.; Barros, R.R.; Muglia, V.A.; Borges, G.J. Influence of interimplant distances and placement depth on papilla formation and crestal resorption: A clinical and radiographic study in dogs. J. Oral Implantol. 2009, 35, 18–27. [CrossRef] [PubMed]
- Lops, D.; Stocchero, M.; Motta Jones, J.; Freni, A.; Palazzolo, A.; Romeo, E. Five Degree Internal Conical Connection and Marginal Bone Stability around Subcrestal Implants: A Retrospective Analysis. Materials 2020, 13, 3123. [CrossRef] [PubMed]
- Rabel, K.; Spies, B.C.; Pieralli, S.; Vach, K.; Kohal, R.J. The clinical performance of all-ceramic implant-supported single crowns: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, s196–s223. [CrossRef] [PubMed]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implants Res. 2018, 29, s199–s214. [CrossRef]
- Larsson, C.; Wennerberg, A. The clinical success of zirconia-based crowns: A systematic review. Int. J. Prosthodont. 2014, 27, 33–43. [CrossRef]
- Al-Thobity, A.M. Titanium Base Abutments in Implant Prosthodontics: A Literature Review. Eur. J. Dent. 2022, 16, 49–55. [CrossRef]
- Tallarico, M.; Fiorellini, J.; Nakajima, Y.; Omori, Y.; Takahisa, I.; Canullo, L. Mechanical Outcomes, Microleakage, and Marginal Accuracy at the Implant-Abutment Interface of Original versus Nonoriginal Implant Abutments: A Systematic Review of In Vitro Studies. BioMed Res. Int. 2018, 2018, 2958982. [CrossRef]
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral Health 2017, 17, 124. [CrossRef]
- Mühlemann, S.; Kraus, R.D.; Hämmerle, C.H.F.; Thoma, D.S. Is the use of digital technologies for the fabrication of implant-supported reconstructions more efficient and/or more effective than conventional techniques: A systematic review. Clin. Oral Implants Res. 2018, 29, s184–s195. [CrossRef]
- Czajkowska, M.; Walejewska, E.; Zadroz˙ny, Ł.; Wieczorek, M.; S´wie˛szkowski, W.; Wagner, L.; Mijiritsky, E.; Markowski, J.
- Comparison of Dental Stone Models and Their 3D Printed Acrylic Replicas for the Accuracy and Mechanical Properties. Materials 2020, 13, 4066. [CrossRef] [PubMed]
- Zadrożny, Ł.; Rogus´, P.; Pyzlak, M.; Tallarico, M. Full versus semi-digital workflow in case of surgical management of mandibular cyst. J. Dent. 2022, 121, 104013. [CrossRef]
- Zahoui, A.; Bergamo, E.T.; Marun, M.M.; Silva, K.P.; Coelho, P.G.; Bonfante, E.A. Cementation Protocol for Bonding Zirconia Crowns to Titanium Base CAD/CAM Abutments. Int. J. Prosthodont. 2020, 33, 527–535. [CrossRef] [PubMed]
- Burkhardt, F.; Pitta, J.; Fehmer, V.; Mojon, P.; Sailer, I. Retention Forces of Monolithic CAD/CAM Crowns Adhesively Cemented to Titanium Base Abutments-Effect of Saliva Contamination Followed by Cleaning of the Titanium Bond Surface. Materials 2021, 14, 3375. [CrossRef] [PubMed]
- Pitta, J.; Burkhardt, F.; Mekki, M.; Fehmer, V.; Mojon, P.; Sailer, I. Effect of airborne-particle abrasion of a titanium base abutment on the stability of the bonded interface and retention forces of crowns after artificial aging. J. Prosthet. Dent. 2021, 126, 214–221. [CrossRef]
- Pozzi, A.; Tallarico, M.; Barlattani, A. Monolithic lithium disilicate full-contour crowns bonded on CAD/CAM zirconia complete-arch implant bridges with 3 to 5 years of follow-up. J. Oral Implantol. 2013, 41, 450–458. [CrossRef]
- Pozzi, A.; Holst, S.; Fabbri, G.; Tallarico, M. Clinical Reliability of CAD/CAM Cross-Arch Zirconia Bridges on Immediately Loaded Implants Placed with Computer-Assisted/Template-Guided Surgery: A Retrospective Study with a Follow-up between 3 and 5 Years. Clin. Implant Dent. Relat. Res. 2015, 17, e86–e96. [CrossRef]
- Amorfini, L.; Storelli, S.; Mosca, D.; Scanferla, M.; Romeo, E. Comparison of cemented vs screw-retained, customized computer-aided design/computer-assisted manufacture zirconia abutments for esthetically located single-tooth implants: A 10-year randomized prospective study. Int. J. Prosthodont. 2018, 31, 359–366. [CrossRef]
- Gasser, T.J.W.; Papageorgiou, S.N.; Eliades, T.; Hämmerle, C.H.F.; Thoma, D.S. Interproximal contact loss at implant sites: A retrospective clinical study with a 10-year follow-up. Clin. Oral Implants Res. 2022, 33, 482–491. [CrossRef]
- Stimmelmayr, M.; Edelhoff, D.; Güth, J.F.; Erdelt, K.; Happe, A.; Beuer, F. Wear at the titanium-titanium and the titanium-zirconia implant-abutment interface: A comparative in vitro study. Dent. Mater. 2012, 28, 1215–1220. [CrossRef]
- Solá-Ruíz, M.F.; Selva-Otaolaurruchi, E.; Senent-Vicente, G.; González-de-Cossio, I.; Amigó-Borrás, V. Accuracy combining different brands of implants and abutments. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e332–e336. [CrossRef] [PubMed]
- Bakri, A.S.; Sahdan, M.Z.; Adriyanto, F.; Raship, N.A.; Said, N.D.M.; Abdullah, A.A.; Rahim, M.S. Effect of annealing temperature of titanium dioxide thin films on structural and electrical properties. AIP Conf. Proc. 2017, 1788, 030030. [CrossRef]
- Peng, W.; Zeng, W.; Zhang, Y.; Shi, C.; Quan, B.; WU, J. The Effect of Colored Titanium Oxides on the Color Change on the Surface of Ti-5Al-5Mo-5V-1Cr-1Fe Alloy. J. Mater. Eng. Perform. 2013, 22, 2588–2593. [CrossRef]
- Alcisto, J.; Enriquez, A.; Garcia, H.; Hinkson, S.; Hahn, M.; Foyos, J.; Ogren, J.; Lee, E.W.; Es-Said, O.S. The effect of thermal history on the color of oxide layers in titanium 6242 alloy. Eng. Fail. Anal. 2004, 6, 811–816. [CrossRef]
- Gaddam, R.; Sefer, B.; Pederson, R.; Antti, M.-L. Oxidation and alpha-case formation in Ti–6Al–2Sn–4Zr–2Mo alloy. Mater. Charact. 2015, 99, 166–174. [CrossRef]
- Minervini, G.; Fiorillo, L.; Russo, D.; Lanza, A.; D’Amico, C.; Cervino, G.; Meto, A.; Di Francesco, F. Prosthodontic Treatment in Patients with Temporomandibular Disorders and Orofacial Pain and/or Bruxism: A Review of the Literature. Prosthesis 2022, 4, 253–262. [CrossRef]
- Minervini, G.; Romano, A.; Petruzzi, M.; Maio, C.; Serpico, R.; Lucchese, A.; Candotto, V.; Di Stasio, D. Telescopic overdenture on natural teeth: Prosthetic rehabilitation on (OFD) syndromic patient and a review on available literature. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. 1), 131–134.
- Antonelli, A.; Bennardo, F.; Brancaccio, Y.; Barone, S.; Femiano, F.; Nucci, L.; Minervini, G.; Fortunato, L.; Attanasio, F.; Giudice, A. Can Bone Compaction Improve Primary Implant Stability? An In Vitro Comparative Study with Osseodensification Technique. Appl. Sci. 2020, 10, 8623. [CrossRef]
