Comparison Between Single Early-Loaded Implants With Sandblasted Acid-Etched (Sa) Surface Versus Sa Surface Modified With Ph Buffering Agent (Soi): Four-Month Data From A Split-Mouth, Multicentre Randomized Controlled Trial
Purpose. To compare implant survival and success rates and implant stability quotient (ISQ) values of early-loaded single implants with sandblasted acid-etched (SA, control group) surface versus implants with SA surface modified with pH buffering agent (SOI, test group).
Materials and methods. This study was designed as multicentre, split-mouth, randomized controlled trial to evaluate implant and prosthesis survival rates, complications, and implant stability quotient (ISQ) in any partially edentulous subject requiring at least two single implant-supported crowns. A one-stage implant placement procedure was used, and implants were randomized after implant site preparation. ISQ values were evaluated for each implant, at baseline and then every week up to 8 weeks after surgery, and finally at definitive crown delivery (12 weeks after implant placement).
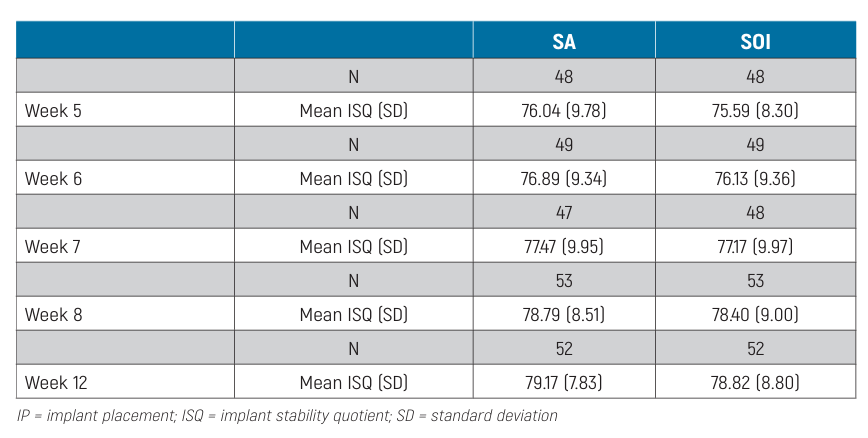
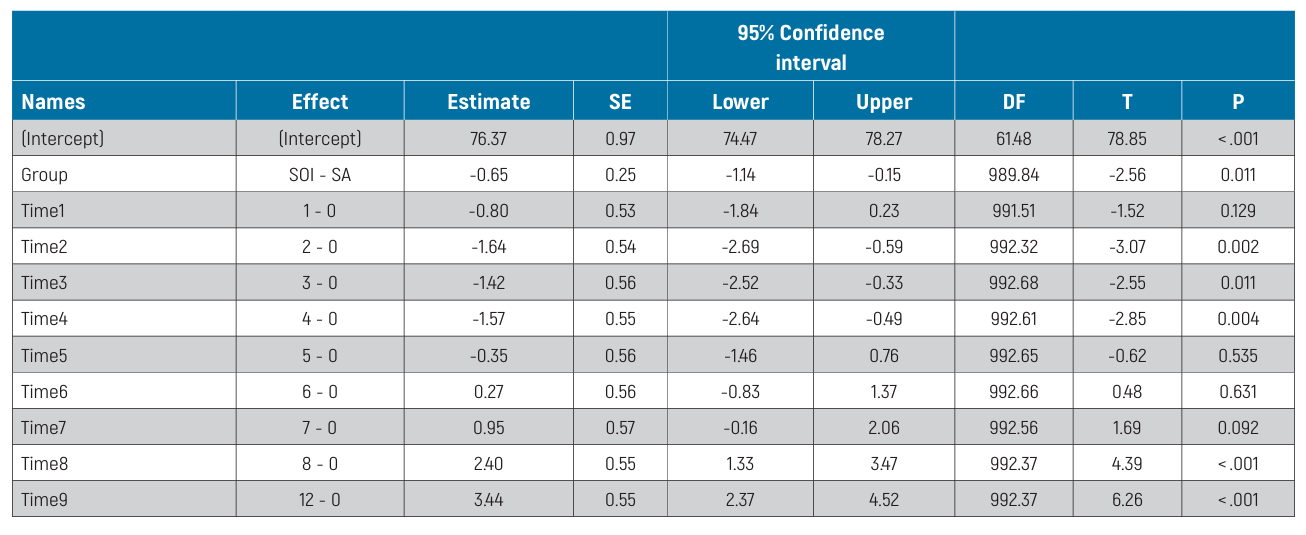
Results. Overall, 62 patients from 9 centres were enrolled in this study. One patient dropped out from the study at 8 weeks. In the first 12 weeks of observation, 2 implants failed, both in the SA group, the difference not being statistically significant (P = 0.5). No prosthesis failure occurred up to 4 months after fitting. Five complications were experienced, 3 in the SA group and 2 in the SOI group. The difference between groups was not statistically significant (OR = 0.66, 95% CI: 0.11 to 4.07; P = 0.650). Of these complications, loss of stability without rotation was observed in 2 implants from the SOI group and 2 implants from the SA group, all in the third and fourth weeks of measurements. All the implants were submerged and successfully osseointegrated at the twelfth week. The last complication was an SA implant screw loosening, which was resolved chair-side. The baseline mean ISQ values were 76.57 ± 7.54 (95% CI 74.69 to 78.44) in the SA group and 75.92 ± 7.69 (95% CI 73.89 to 77.73) in the SOI group. The mean ISQ values at 12 weeks were 79.17 ± 7.83 (95% CI 77.03 to 81.29) and 78.82 ± 8.80 (95% CI 76.42 to 81.21) in the SA and SOI groups, respectively. Mixed-effects modelling revealed a statistically significant difference between groups over time, with slightly lower ISQ values for the SOI group (-0.65; 95% CI -1.14 to -0.15). Statistically significant differences were also estimated among centres (P <0.001).
Conclusions. Within the limitations of the present preliminary report, it is possible to conclude that both implants can be successfully loaded early.
Introduction
Dental implants are considered a reliable tool for oral rehabilitation, and several recent studies have sufficiently proven their longevity and stability. Various techniques have been tried to enhance the appeal and acceptance of implants by patients. Among these, a shortened healing time has become one major focus of implant research. Different surgical approaches have been attempted to provide immediate aesthetic and functional rehabilitation. Although a Cochrane systematic review has failed to find any convincing evidence of a clinically significant difference in implant and prosthesis failures associated with different implant loading times, as compared with the conventional procedure, immediate implant placement into fresh extraction sockets has been associated with a higher incidence of implant failures. Hence, over the years different implant macro and micro designs have been introduced in the attempt to increase the bone-to-implant contact during healing time, making osseointegration faster. Modification techniques have experienced constant development in recent years, the purpose being to alter the roughness of the implant surface in order to create a favourable environment for osseointegration. Surface modification techniques can be divided into three categories, physical and chemical utilized individually, or a combination of both. Chemical surface modification techniques tested to date include micro-rough sandblasting and large-grit acid-etching, coating the titanium surface with a resorbable nano-layer of hydroxyapatite, and coating with a pH buffering agent. A combination of physical and chemical techniques, respectively grit blasting with alumina followed by acid etching, produces one of the best documented implant surfaces in the dental field, with successful long-term follow-ups. However, a Cochrane review failed to show any relevant clinical difference between different implant types.
Nonetheless, Tallarico and co-authors have demonstrated that the physiological implant stability quotient (ISQ) diminishes less during the bone remodelling phase with sandblasted acid-etched implants with a bioabsorbable apatite nanocoating than with solely sandblasted acid-etched implants.
Recently, Osstem (Osstem Implant, Osstem Global, Seoul, South Korea) introduced a new surface, modified with pH buffering agent to improve osseointegration. This has shown promising basic research results, but despite the positive preliminary findings achieved by one independent randomised controlled trial (RCT)17, a systematic review found little difference between sandblasted and acid-etched dental implants and the pH-buffered surface.
In order to provide further useful data, this split-mouth, multicentre randomized controlled trial was designed to compare implant survival, success rates, and ISQ values of early-loaded TSIII (Osstem Implant) implants with sandblasted acid-etched (SA) surface versus an SA surface modified with pH buffering agent (SOI) in the rehabilitation of single implant-supported crowns. The two implants used were identical in terms of shape, dimensions and geometry, the only difference being the surface preparation: the SOI implant surface is hydrophilic, while the SA surface is hydrophobic (Fig. 1). The null hypothesis tested was that there would no difference between groups against the alternative hypothesis of a difference. The manuscript was prepared according to the CONSORT statement guidelines for improving the quality of reports of randomized trials (http://www.consort-statement.org/).

Materials and methods
This study was designed as a spilt-mouth, multicentre, randomized controlled trial with blind outcome assessment, with the exception of complications and failures, which were reported by the treating dentists. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki for Biomedical Research Involving Human Subjects, as amended in 2018, and was registered with clinicaltrial.gov as number NCT04073654. The research protocol received ethical approval from the coordinating centre, located in Albania (protocol number 1/2018). Before starting treatment, all the patients were duly informed about the nature of the study, and signed an informed written consent form for surgical and prosthetic procedures. Patients were to be enrolled and treated in 10 public and private centres in Europe and South Africa between September 2019 and June 2021.
Inclusion/exclusion criteria
Any partially edentulous subject requiring at least two single implant-supported crowns, being at least 18 years old and able to sign informed consent, was screened for eligibility. Broad inclusion criteria were used, including any type of bone, location, smoking habits, etc. A minimal bone volume was required to allow the placement of implants at least 8.5 mm long and 3.5 mm wide, with a minimal insertion torque of 30 Ncm. Post-extraction sockets or augmented bone were allowed if at least four months had passed from the extraction or augmentation procedures. Smokers were categorized as either moderate (up to 10 cigarettes/ day) or heavy smokers (more than 10 cigarettes/day), according to their declaration.
Patients were not admitted to the study if any of the following exclusion criteria were present:
- General contraindications to implant surgery;
- Less than 4 mm of keratinized gingiva at the implant sites;
- Immunosuppression or compromise;
- Irradiation of the head and/or neck in the previous 5 years;
- Uncontrolled diabetes;
- Pregnancy or lactation;
- Untreated periodontal disease;
- Poor oral hygiene and motivation (full mouth bleeding and full mouth plaque index higher than 25%);
- Addiction to alcohol or drugs;
- Psychiatric problems and/or unrealistic expectations;
- Acute infection or suppuration at the site intended for implant placement;
- Any form of tissue augmentation required at implant placement;
- Immediate post-extraction (implants could be placed after a 4-month healing period);
- Previous or ongoing treatment with intravenous aminobisphosphonates;
- Referral for implant placement alone (no follow-up possible at the treatment centre);
- Participation in other studies, if the present protocol could not be fully adhered to.
Preoperative radiographs (periapical radiography and/or cone-beam computed tomography) were obtained for every potentially eligible patient to quantify bone volumes at the planned implant sites. Patients having sufficient bone volumes to receive two single implants were invited to join the trial and were informed of its nature. Only after they fully understood the nature of the trial (including procedures, follow-up evaluations, and any potential risks involved) were they asked to join and signed informed written consent. For patients with more than two suitable implant sites, operators were free to choose those sites with the most similar characteristics at the screening visit, preferably non-adjacent. The selected implant sites were then coded as number 1 (the lowest according to the FDI World Dental Federation notation) and number 2 (the highest).
Clinical procedures
About 10 days prior to implant placement, all patients underwent a professional oral hygiene session. All patients received prophylactic antibiotic therapy: 2 g of amoxicillin 1 hour prior to the intervention, or clindamycin 600 mg 1 hour before implant placement if allergic to penicil- lin. All patients rinsed with chlorhexidine mouthwash 0.2% for 1 minute prior to any surgical procedure, and were treated under local anaesthesia using articaine with epinephrine 1:100,000. Depending on the anatomy of the site and the clinician’s preference, flapless or miniflap (crestal flap without vertical incisions) access was obtained (the same technique in each patient). Implant sites were prepared simultaneously using taper drills (800–1200 RPM) with copious saline irrigation, according to the drilling protocol recommended by the manufacturer (122 Taper kit, Osstem Implant) and bone density. This was assessed during the drilling phase, and classed, based on the clinician’s experience as: “hard”, “normal” or “soft”. Operators were free to choose implant lengths according to the clinical indications and their preferences. If possible, two implants of the same length and diameter were to be chosen for each patient. Tapered TSIII implants with sandblasted acid-etched (SA) surface (SA group) or SA surface modified with pH buffering agent (SOI group) were placed via a one-stage protocol at bone level or slightly subcrestally, with a minimum insertion torque of 30 Ncm. Implants inserted with lower torque were to be excluded from further ISQ measurements and left to heal undisturbed for 4 months before crown fitting. The treatment sequence is reported in Figs. 2A-H.
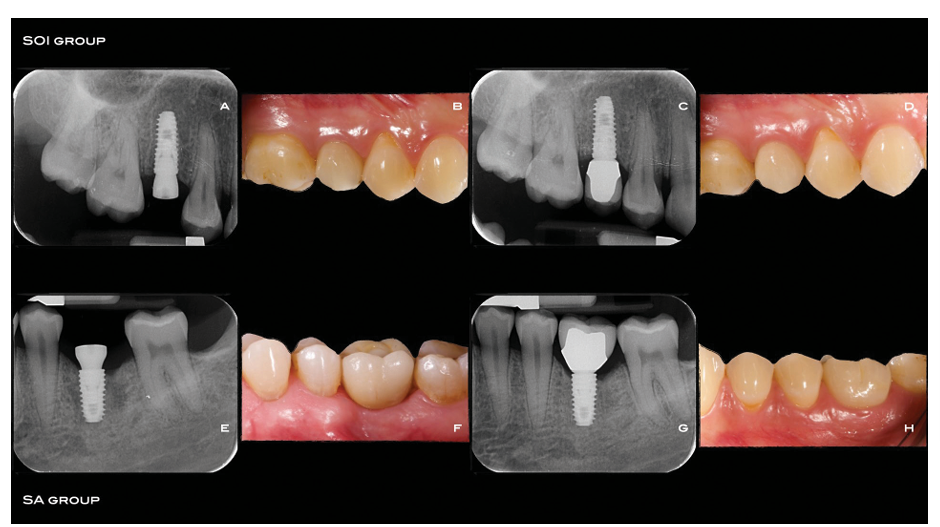
After implant placement, appropriate multipegs (Hiossen, Englewood Cliffs, NJ, USA) were connected to the implants one at time, and the implant stability quotient (ISQ) was measured using resonance frequency analysis by means of IS3 Monitor device (Hiossen). Healing abutments were connected to the implants using a finger driver, and any flaps were closed with sutures. Post-surgical analgesic treatment with ibuprofen 600 mg as needed was advised. Antibiotics (1 g of amoxicillin, or clindamycin 600 mg if patients were allergic to penicillin) were administered twice a day for 5 days. The ISQ measurements were continued weekly for 8 weeks after implant placement, and then at the twelfth week, as per a previously published report. At each time-point, the healing abutments were unscrewed, appropriate multipegs were attached to the implants, one at time, and two measurements were made: buccopalatal and mesiodistal. Finally, the healing abutments were screwed back in place using a finger driver, after cleaning and disinfection with chlorhexidine 0.2% and an ultrasonic cleaner. Definitive impressions were taken eight weeks after implant placement, and definitive crowns were delivered twelve weeks after implant placement. Implants were to be restored as single units. Investigators were free to fit definitive metal-ceramic or full ceramic crowns, which could be either cemented or screw-retained. Nevertheless, identical procedures and materials were to be used for implants from both groups in the same patient. Definitive crowns/ abutments were screwed twice at 20 or 30 Ncm (according to the manufacturer’s instructions and considering the implant platform), with an interval of 10 minutes. Occlusion was checked, and oral hygiene instruction reinforced if necessary. Periapical radiographs and intraoral pictures were taken at implant placement and definitive crown fitting.
Primary outcome measures were implant and prosthesis failures, and any complications were recorded.
- Implant failure was defined as an implant rotating during abutment screw tightening/ loosening, fracture, and/or any infection dictating implant removal or other mechanical complication rendering the implant unusable.
- Crown replacement for any reason was considered a prosthesis failure.
- Any biological (pain, swelling, suppuration, etc.) and/or mechanical (screw loosening, chipping of the ceramic materials, etc.) complication was recorded.
The secondary outcome measure was the implant stability quotient (ISQ), as recorded by blind outcome assessors using resonance frequency analysis. Buccopalatal and mesiodistal measurements were taken and averaged, with the result being displayed by the device in ISQ units ranging from 1 to 100. The ISQ values were recorded at the time of implant placement (baseline) and then weekly up to the eighth week, and finally at the twelfth week after implant placement during definitive crown fitting.
The following secondary outcome measures will be assessed at one year of follow up: marginal bone levels (MBL); probing pocket depth (PPD), bleeding on probing (BOP), plaque index (PI); and pink aesthetic score (PES). A blind outcome assessor from each centre will collect the data, and a single blind assessor will evaluate MBL and PES.
Data analysis
The appropriate sample size was estimated as 65 implants in each group, to be enrolled across 10 different centres, given an effect size d = 0.6383489, ß err prob 0.05, and power (1-ß err prob = 0.95). Effect size was determined based on a previous similar study reporting ISQ values of 71.2± 4.07 for conventional surface implants and 74± 4.68 for those with the modified surface17. Due to the split-mouth design of the trial, each patient provided both test (SOI) and control (SA) implants. In order to avoid underpowered results (<95%) due to possible drop-outs, 35 patients were added, making a total planned sample size of 100 patients (200 implants). To this end, each centre was to place 10 test implants (SOI) and 10 control implants (SA) in 10 enrolled patients.
Accordingly, ten computer-generated restricted randomization lists were created. Only one person, not involved in the research, was aware of the randomization sequence and had access to the randomization lists, which were stored on a password-protected laptop. The randomization codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes, which were opened sequentially after both implant sites had been prepared.
All data analysis was carried out according to a pre-established analysis plan by two of the investigators (MC and JB) without knowing group allocation. Patient data was recorded on an Excel spreadsheet (Microsoft Corporation, Redmond, Washington, USA). Implant and prosthesis failures, as well as complications were noted (dichotomous outcomes). The ISQ values (continuous outcome) were analysed at all time-points (0, 1, 2, 3, 4, 5, 6, 7, 8 and 12 weeks). The McNemar test was conducted on implant failures. Mixed-effects models were created for the continuous dependent variable (ISQ), and generalized mixed models for dichotomous dependent variables (complications), considering patients as random effects and time and group as fixed effects. A further mixed-effects model was created to estimate differences among centres, considering patients as random effects and time and centre as fixed effects. Post-hoc analyses and effects plots were produced. Jamovi Version 1.8.0.0 (Jamovi Project, Sydney, Australia) statistics software was used for all the analyses. The data are presented as mean ± standard deviation with 95% confidence interval (CI) and frequency and percentage for dichotomous variables. P-values of <0.05 were considered statistically significant.
Results
Patients were to be recruited and treated using similar procedures in 10 different centres, and each centre was supposed to recruit and treat 10 patients (20 implants). However, one centre failed to recruit any patient. The 9 remaining centres were located as follows: three in Italy (MT, FG, LM), and one each in Albania (EX), Bulgaria (DE), Romania (MG), Switzerland (NW), South Africa (AdW) and Poland (LZ). Patients were assessed to establish their eligibility for the trial, and only 2 centres (MT and EX) recruited 10 patients, while 1 centre (MG) recruited 9 patients, 2 centres (NW and AdW) 8 patients, 2 centres (LM and LZ) 6 patients, and the remaining 2 centres recruited 3 (DE) and 2 (FG) patients, respectively. Ninety-one patients were originally screened for eligibility, but only 62 participants (23 men and 39 women) were consecutively enrolled in the trial by the nine participating centres. Reasons for not including 29 patients were: frequent check-up visits (18 patients), need for guided bone regeneration (11 patients). One patient dropped out of the trial at the eighth week having lost one implant, although this was replaced, the patient preferred to withdraw from the trial. Twenty-two deviations from the original protocol occurred in 6 centres, as reported in Table 1.
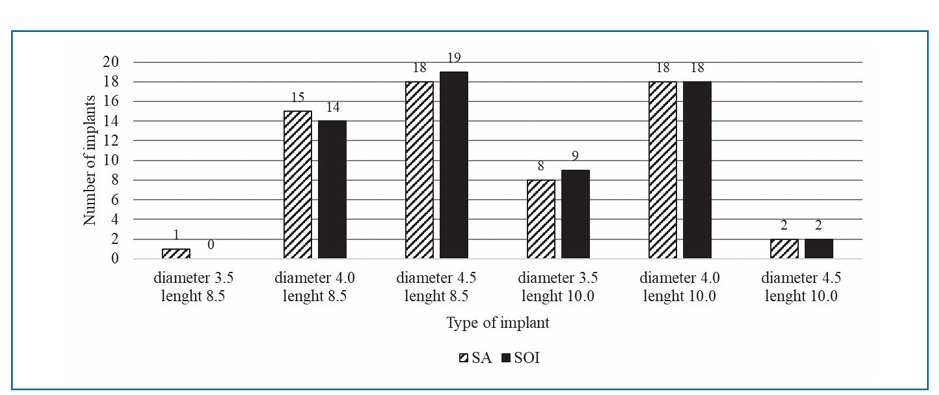
The mean age of the patients was 52.1±14.3 years, 66.1% of whom were non-smokers, while 27.4% smoked up to 10 cigarettes per day and 6.5% smoked more than 10 cigarettes per day. A total of 124 implants were placed, distributed equally between SA and SOI groups. The number of different implant sizes used are reported in Fig. 3. All centres fitted same type of prosthesis at both sites in all patients, with one exception (Patient 2 at Centre 3 received one screw- and one cement-retained crown). Materials were similar in both groups. In particular, screwed-retained protheses were in the great majority in both SA and SOI groups (69.4% and 68.1%, respectively) and 56.5% of crowns were metal-free in both groups.
Two implants from the SA group were lost, while no implant failed in the SOI group. Both of the former were lost (mobility without pain) between the 3rd and 4th week, and immediately replaced; the remaining SOI implant in the patient from Centre 7 was continuously followed up, while the patient from Centre 10, withdrew from the study at the 8th week. The difference in implant failures between groups was not statistically significant (P = 0.5). No prosthesis failure occurred in either group in the 12-week study period.
Overall, five complications were experienced, three in the SA group and two in the SOI group. At Centres 1, 7 and 8, 4 implants (2 from each group) manually displayed slight horizontal mobility but no implant rotation. Implants were submerged and successfully osseointegrated, and then loaded at 12 weeks in line with the trial protocol. A later complication occurred 8 weeks after crown fitting, specifically a screw loosened at one implant-supported crown in the SA group (Centre 3). As estimated by a mixed-effects model, the difference in complications between groups was not statistically significant, being OR = 0.66, 95% CI 0.11 to 4.07; P=0.650.
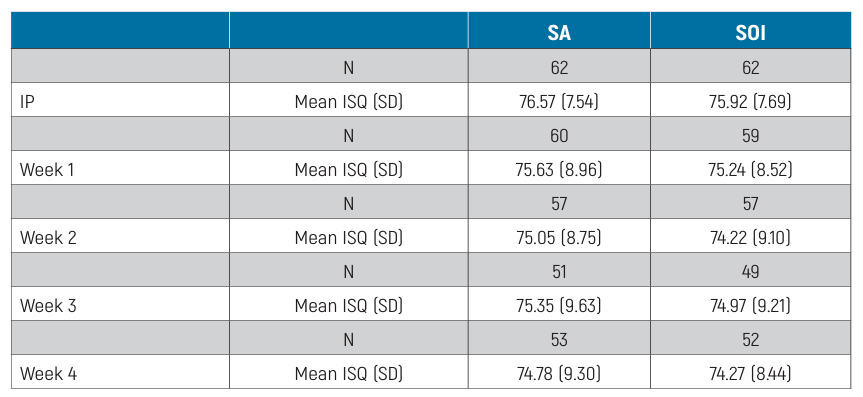
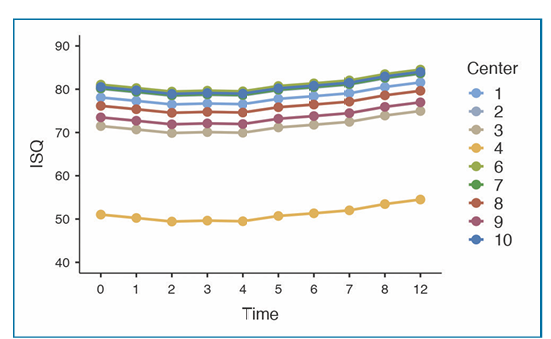
The distribution of ISQ measurements is reported in Table 2. The baseline mean ISQ values were 76.57 ±7.54 (95% CI 74.69 to 78.44) at SA implants and 75.92 ±7.69 (95% CI 73.89 to 77.73) at SOI implants. The mean ISQ values at 12 weeks were 79.17 ±7.83 (95% CI 77.03 to 81.29) and 78.82 ± 8.80 (95% CI 76.42 to 81.21) in the SA and SOI groups, respectively. The mixed-effects model revealed a statistically significant difference between groups over time, with lower ISQ values in the SOI group (-0.65; 95% CI -1.14 to -0.15; Table 3). The effects plot over time is shown in Fig. 4.
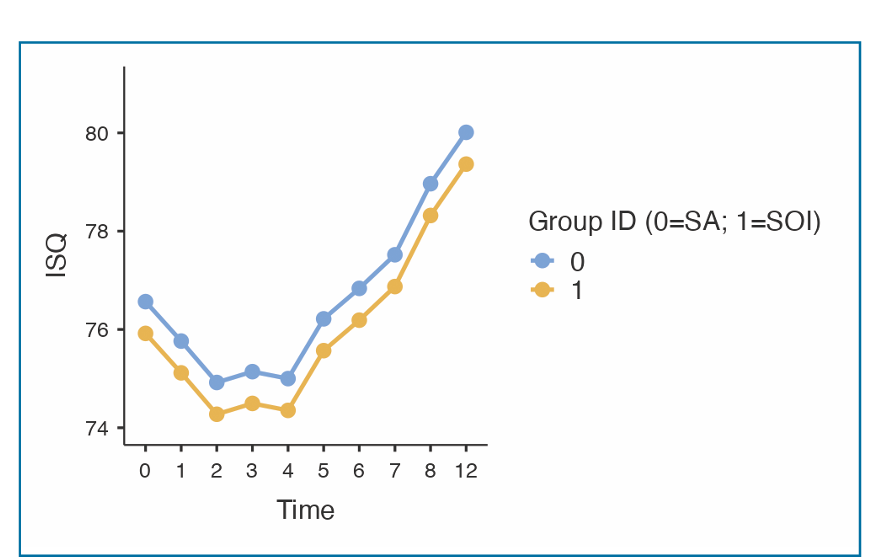
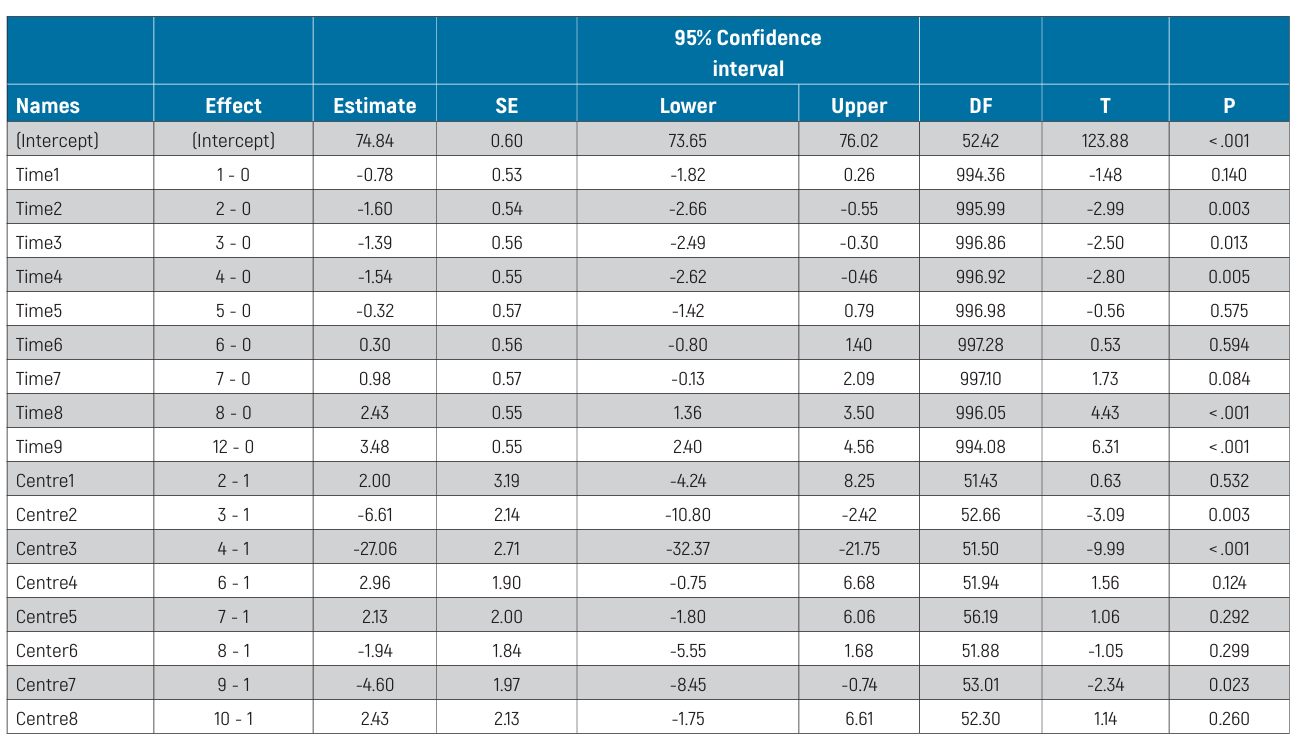
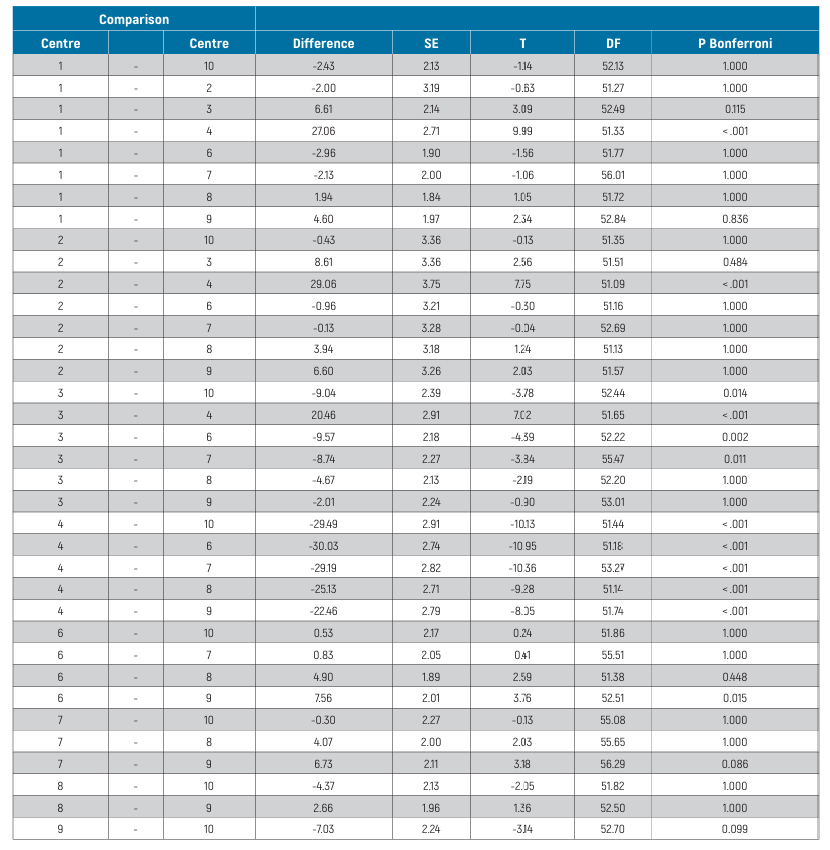
The mixed-effects model for between-centre differences is reported in Table 4. Statistically significant differences were estimated among centres (P <0.001). In particular, Centre 4 displayed significantly lower ISQ values (P <0.001) than all other centres. All pair-wise differences from post-hoc analysis are reported in Table 5. The effects plot over time is shown in Fig. 5.
Discussion
This randomized controlled trial was designed to provide preliminary data on the clinical performance and implant stability quotient of Osstem TSIII implants with SA surface modified with a pH buffering agent (SOI) used for the rehabilitation of single implant-supported crowns, as compared to the conventional SA surface. This split-mouth trial revealed a statistically significant difference in favour of the SA group, allowing the null hypothesis of no difference in ISQ between groups to be rejected. However, it should be noted that the difference was only 0.65, which is not clinically significant.
Furthermore, when comparing ISQ values among centres, there were also some statistically significant differences in some pairwise comparisons. One possible explanation for these differences could be the different numbers of patients treated at each centre. Alternatively, the discrepancies noted could also reflect differences in primary implant stability and procedures used by the dentists at the different centres. In this regard, even though the same implant designs and drilling protocols were applied, there are several other factors that may affect ISQ measurement data, including but not limited to bone quality and quantity. This raises some doubts as to the clinical relevance of ISQ values for the purposes of rapid implant stability assessment.
Nonetheless, only two out of the entire sample of implants failed, both in the SA group between the third and fourth weeks, while all the implants in the SOI group integrated successfully; this difference was not statistically significant, and indicates that both types of implant tested can be loaded early. That being said, it should be noted that four other implants, 2 per group, presented clinically detectable mobility during the third and fourth weeks, and were therefore submerged, eventually osseointegrating without failing. Even though the submerged technique is not a prerequisite for osseointegration, it is the authors’ opinion that repeated unscrewing of the healing abutment should be avoided during the early weeks of this process. This procedure was included in the trial protocol for research purposes only.
One of the main limitations of this study is the small sample size; the planned sample size was not achieved so the study may be underpowered for the purposes of testing the hypothesis.
Furthermore, several measurements were discontinued due to the COVID-19 quarantine. However, it is worth noting that the measurements were discontinued in both groups due to the split-month design of the study. Another limitation of the present research is the short follow-up. However, the main aim of this paper is to report the trend in stability of both types of implant during the osseointegration period.
Indeed, although a large part of research in implant dentistry is currently focused on the study of bioactive surfaces, only a few reports of human trials are available, with most of the literature being based on animal research. Ethical restrictions do not allow for histological analysis in humans, but there is a general opinion generated from animal and in-vitro studies that implant surface modification may improve early osseointegration. Nonetheless, the results of this preliminary study are in agreement with the conclusions drawn from a previous systematic review and randomized controlled trial in humans, which both failed to find any statistically significant difference between conventional and modified implant surfaces. The purpose of modified surfaces is to enhance contact osteogenesis around dental implants, accelerating osseointegration in the early healing phase. In the present study, implants were placed in healed bone, with a direct bone-to-implant contact, with high primary implant stability. Even though histological analysis around implants placed in humans are not feasible for ethical reasons, further randomized controlled trials are needed to evaluate the performance of implants with modified surface in cases with a gap between implants and bone or poor bone quality.
Conclusions
Despite the limitations of the present trial, it appears that implants with a surface modified with pH buffering agent (SOI) can be safely used for the early rehabilitation of single implant-supported crowns, but that these offer no advantages over implants with a conventional sandblasted acid-etched (SA) surface. Further studies are needed to confirm or refute these preliminary results.
Łukasz Zadrożny, Erta Xhanari, Mircea Gheorghita, Andre De Waal, Nicolas Widmer, Leonardo Muzzi, Elitsa Deliverska, Fulvio Gatti, Marta Czajkowska
References
- Howe M-S, Keys W, Richards D. Long-term (10- year) dental implant survival: A systematic review and sensitivity meta-analysis. J Dent 2019;84:9-21.
- French D, Ofec R, Levin L. Long term clinical performance of 10 871 dental implants with up to 22 years of follow-up: A cohort study in 4247 patients. Clin Implant Dent Relat Res 2021;23:289-97.
- Testori T, Galli F, Capelli M, Zuffetti F, Buti G, Esposito M. Immediate nonocclusal versus early loading of dental implants in partially edentulous patients -15-year follow-up of a multicentre randomised controlled trial. Clinical Trials in Dentistry 2021;03:5-20.
- Testori T, Gaul F, Capelli M, Zuffetti F, Esposito M. Immediate nonocclusal versus early loading of dental implants in partially edentulous patients: 1-year results from a multicenter, randomized controlled clinical trial. Int J Oral Maxillofac Impl 2007;22:815-22.
- Fernández R, Peñarrocha-Oltra D, Peñarrocha-Diago M, Buti J, Xhanari E, Esposito M. Natural or palatal positioning of immediate post-extraction implants in the aesthetic zone? Five-year outcomes of a multicentre randomised controlled trial. Clinical Trials in Dentistry 2020;02:59-71.
- Esposito M, Grusovin MG, Maghaireh H, Worthington HV. Interventions for replacing missing teeth: different times for loading dental implants. Cochrane Database Syst Rev 2013:CD003878.
- Felice P, Barausse C, Buti J, Gessaroli M, Esposito M. Immediate, early (6 weeks) and delayed (4 months) single post-extractive implants: 3-year post-loading data from a randomised controlled trial. Clinical Trials in Dentistry 2019;01:5-23.
- Atieh MA, Payne AG, Duncan WJ, Cullinan MP. Immediate restoration/loading of immediately placed single implants: is it an effective bimodal approach? Clin Oral Implants Res 2009;20:645- 59.
- Chen J, Cai M, Yang J, Aldhohrah T, Wang Y. Immediate versus early or conventional loading dental implants with fixed prostheses: A systematic review and meta-analysis of randomized controlled clinical trials. J Prosthet Dent 2019;122:516-36.
- Mello C, Lemos C, Verri F, Dos Santos D, Goiato M, Pellizzer E. Immediate implant placement into fresh extraction sockets versus delayed implants into healed sockets: A systematic review and meta-analysis. Int J Oral Maxillofac Surg 2017;46:1162-77.
- Morton D, Bornstein MM, Wittneben JG, Martin WC, Ruskin JD, Hart CN, Buser D. Early loading after 21 days of healing of nonsubmerged titanium implants with a chemically modified sandblasted and acid-etched surface: Two-year results of a prospective two-center study. Clinical Implant Dent Relat Res 2010;12:9-17.
- Tallarico M, Baldini N, Gatti F, Martinolli M, Xhanari E, Meloni SM, Cervino G, Lumbau Aurea I. Role of new hydrophilic surfaces on early success rate and implant stability: 1-year post-loading results of a multicenter, split-mouth, randomized con- trolled trial. Eur J Dent 2021;15:1-7.
- Park C-J, Lim JH, Tallarico M, Hwang K-G, Choi H, Cho G-J, Kim C, Jang S, Song J-D, Kwon AM, Jeon SH, Park H-K. Coating of a sand-blasted and acid-etched implant surface with a pH-buffe- ring agent after vacuum-UV photofunctionalization. Coatings 2020;10:1040.
- Kang JH, Kim S-K, Pae HC, Park JY, Cha J-K, Choi S-H. Influence of implant surface coated with pH buffering agent on early osseointegration. J Korean Dental Sci 2018;11:5-13.
- Buser D, Janner SF, Wittneben JG, Brägger U, Ramseier CA, Salvi GE. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clinical Implant Dent Relat Res 2012;14:839-51.
- Esposito M, Ardebili Y, Worthington HV. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database Syst Rev 2014:CD003815.
- Schätzle M, Männchen R, Balbach U, Hämmerle CH, Toutenburg H, Jung RE. Stability change of chemically modified sandblasted/acid-etched titanium palatal implants. A randomized-controlled clinical trial. Clin Oral Implants Res 2009;20:489-95.
- Chambrone L, Shibli JA, Mercurio CE, Cardoso B, Preshaw PM. Efficacy of standard (SLA) and modified sandblasted and acid-etched (SLActive) dental implants in promoting immediate and/or early occlusal loading protocols: a systematic review of prospective studies. Clin Oral Implants Res 2015;26:359-70.
- Tallarico M, Vaccarella A, Marzi GC, Alviani A, Campana V. A prospective case-control clinical trial comparing 1-and 2-stage Nobel Biocare TiUnite implants: resonance frequency analysis assessed by Osstell Mentor during integration. Quintessence Int 2011;42:635-44.
- Huang H, Wu G, Hunziker E. The clinical significance of implant stability quotient (ISQ) measurements: A literature review. J Oral Biol Craniofac Res 2020;10:629-638.
- Tallarico M, Vaccarella A, Marzi GC. Clinical and radiological outcomes of 1-versus 2-stage im- plant placement: 1-year results of a randomised clinical trial. Eur J Oral Implantol 2011;4:13-20.
- Pae H-C, Kim S-K, Park J-Y, Song YW, Cha J-K, Paik J-W, Choi S-H. Bioactive characteristics of an implant surface coated with a pH buffering agent: an in vitro study. J Periodont Impl Sci 2019;49:366- 81.
- Nicolau P, Guerra F, Reis R, Krafft T, Benz K, Jackowski J. 10-year outcomes with immediate and early loaded implants with a chemically modified SLA surface. Quintessence Int 2018;50:2-12.
