A randomized controlled evaluation on different implant surfaces
A multicenter, randomized controlled split-mouth trial was aimed to compare SA surface implants (SA group) and implants with a newly developed bioabsorbable apatite nanocoating surface (NH group). Outcomes were the two-years implant and prosthetic survival rates, any biological or mechanical complications, insertion torque at implant placement, and the implant stability quotient (ISQ).
Introduction
Implant stability is one of the most important factors that may affect osseo-integration during the healing period. After that, marginal bone loss has been used for several years to measure the implant success. Implant failure can be classified in early or late, depending on its time of occurrence. Early failure occurs prior the definitive prosthesis delivery, as consequence of a lack of integration with the bone, while, late failures occurs after definitive prosthetic loading.
In the last years, dental industry introduced in the market implants with modified surfaces with the aim to enhance the osseointegration, reducing the risks of implant failure and complications during the osseointegration period (early implant failure). According to the scientific literature, the risk of implant failure is improved in immuno-compromised patients, immediate loading, immediate implants, and posterior maxilla. In these clinical scenario, hydrophilic surface could provide faster and stronger osseointegration, allowing to reduce the overall implant failure. However, a recent systematic review reported no statistically significant differences between conventional and novel hydrophilic surfaces. A possible explanation could be the insufficient data regarding the novel implant surfaces. Hence, the aim of this split-mouth randomized controlled trial was to compare early implant failure and implant stability of one-stage Hiossen ET III implants (Deutsche Osstem GmbH, Germany) with its new hydrophilic (NH) surface, compared with Hiossen ET III implants (Deutsche Osstem GmbH, Germany) with the well-known SA surface at the two years follow-up. The null hypothesis was that there is no difference between groups. The following trial was reported according to the STROBE statement.
Materials and Methods
This study was designed as a multicenter, split-mouth, randomized controlled trial of parallel groups with two arms and independent outcome assessment, conducted between November 2017 and May 2018. The study was registered in the clinicaltrial.gov (NCT03649100) after approval from the Institutional Review Board of the Aldent University, Tirana, Albania (3/2018). The 2013 Helsinki declaration was adhered too.
The inclusion and exclusion criteria were in Table 1 on page 50. Patients were clearly informed about the clinical procedures, the materials to be used, the benefits, potential risks and potential complications, and they gave an informed consent before including in the study. Surgical protocol was reported in a preliminary report of the same study. In brief, a single dose of antibiotic was administered prophylactically one hour before surgery. Hiossen ET III implants were placed under local anesthetic in the planned anatomic sites, according to the drilling protocol recommended by the manufacturer (Deutsche Osstem GmbH, Germany). The SA (SA group) or NH (NH group) were randomized after implant site preparation, immediately before implant placement (one-stage protocol). Two to three months after implants placement patients receive single screw-retained restorations. Periapical radiographs were taken at the definitive prosthesis delivery (Figs. 1 to 6).
The outcome measures were implant and prosthetic survival rates, any biological or mechanical complications that occurred during the entire observation period, the insertion torque at implant placement, and the implant stability quotient during osseointegration.
Success rates of the implants and prostheses were evaluated by an independent assessor according to established criteria. The implant stability quotient (ISQ) was measured and recorded using a smart peg (Type 47 cod. 100478, Osstell, Sweden) connected to the implants, and the Osstell Mentor device (Osstell). Measurements were taken at implant placement, and every week up to 8 weeks after implant placement. A blind outcome assessor collected the data (EX), according to a previously published study.
Complications and failures were compared using the Fisher’s exact test. Comparisons between groups (SA versus NH), and between jaws (maxilla versus mandible) were made by unpaired t-test, while the comparison between baseline (T0) and the last follow-up (T8) was made by paired t-tests to detect any change during the follow-up. Pearson’s correlation coefficient was used to evaluate the correlation between insertion torque at implant placement and ISQ value 8 weeks after implant placement. All statistical comparisons were two-tailed and conducted at the 0.05 level of significance. The patient was used as the statistical unit of analysis.
Results
A total of 29 patients (22 females and seven males, with a mean age at implant insertion of 59.9 ± 11.3 years) were treated according to the allocated interventions, and followed up to two years after loading. No patient dropped out. A total of 58 implants (29 with SA surface and 29 with SA surface with the newly developed bioabsorbable apatite nano-coating) were placed. Eighteen patients were rehabilitated in the maxilla and 11 in the mandible. Two years after loading, no implant and no prosthesis failed. Two weeks after implant placement, two Hiossen ET III SA implants showed a small mobility with an ISQ values lower than 55 (49 and 51, respectively). The healing abutments were replaced with cover screws and the implants were left to heal undisturbed up to eight weeks after their placement. Nevertheless, no statistically significant difference was reached (p = 0.491). In both the implants, the healing abutments were replaced with a cover screw and the implants were left to heal submerged for six weeks (up to eight weeks after implant placement).
The mean insertion torque ranged between 35.0 and 45.0 Ncm (mean of 40.5 ± 3.23 (38.17 – 41.83) Ncm in the SA group and 40.48 ± 3.49 (38.02 – 41.98) Ncm in the NH group). The difference between groups was not statistically significant (p = 0.981).
The comparison between ISQ values were reported n Figure 7.
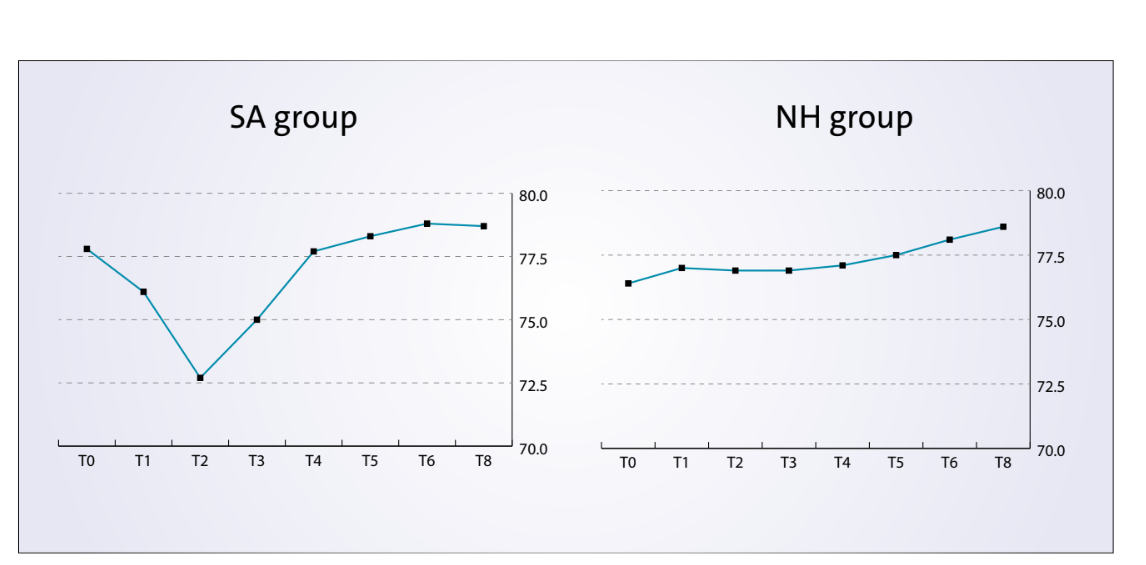
There was a statistically significant difference between groups at the second week after implant placement (T2) with higher values in the NH group (P = 0.041). Similar results were found in the maxilla (P = 0.045), but not in the mandible (P = 0.362). Overall, the ISQ values improved in both groups during the entire follow-up (8 weeks), with statistically significant difference in the NH group (P = 0.019), but not in the SA group (P = 0.266). A positive correlation was found between initial insertion torque and ISQ with higher value in the NH group (0.73 versus 0.66). Correlation was stronger in the mandible (SA = 0.71; NH = 0.86) compared with the maxilla (SA = 0.52; NH = 0.55).
Discussion
The purpose of the present research is to update a previous research providing the two years follow-up data. In this study, the mean ISQ experienced during the osseointegration period improved in both groups, with statistically significant difference only in the NH group. Therefore, it can be assumed that implants with the hydrophilic surface (NH) could reduce possible complications that may occur during the first week of healing, by avoiding the ISQ drop back. This phenomenon could be exploited to improve implant survival and success rates in case of immediate loading, poor bone quality, and immunocompromised patients.
In the literature, there are several researches, including systematic reviews that reported conflicting results. So it is not easy to understand the potentiality of newly developed surfaces. A possible explanation for these results could be different surfaces treatments provided by different implant companies. Moreover, most of these study were performed in animal, or in ideal clinical conditions. New implants surfaces have been introduced in the present days for the purpose of improving the bone to implant interface as well as improving bone integration and reducing the timing of this process. Nevertheless, it would be desirable that researchers will provide well conducted studies even in problematic conditions, were newly developed surfaces could make the differences. These are not only immediate loading, poor bone quality (i.e. posterior maxilla), and immunocompromised patients (i.e. type II diabetic patients), but also in case of indirect bone healing, such as guided bone regeneration and post-extractive implants (20). In these clinical scenarios, the implant surface is not in direct contact with the bone. Therefore, active implant surfaces able to promote healing process could improve the overall osseointegration process, reducing the healing period and, as a consequence, reducing possible biological complications.
Conclusion
The NH implants showed encouraging results in term of implant stability, success and survival rates and are a viable alternative to SA surface, as they seem to avoid the ISQ drop during the remodeling phase.
Implants with NH surface could be finally suggested as gold standard in problematic clinical scenario, such us immediate loading, immediate implants, immunocompromised patients, and one-stage guided bone regenerations. Results remain stable up to two years after loading. Further researches are needed to evaluate the potential benefit of NH surface implants in problematic clinical situations.
Marco Tallarico, Nicola Baldini, Fulvio Gatti, Irene Ieria
