Classification Systems for Peri-implantitis: A Narrative Review with a Proposal of a New Evidence-Based Etiology Codification
Purpose: To present the different definitions of peri-implantitis proposed in the literature and to propose a new evidence-based etiology-driven classification of peri-implantitis to accurately and fully describe the etiology of peri-implantitis.
Materials and Methods: Full-text papers on the selected topic were obtained for all abstracts and titles that appeared to meet the inclusion criteria. Additional papers were included from the reference lists of the selected studies. No methodologic and reporting quality of the included papers was applied in order to collect the greatest number of articles.
Results: One hundred twenty-two studies were found according to the search criteria. After filter activation, abstract evaluation, and duplicate removal, 16 articles were deemed useful for the aim of the present narrative review. A manual search using personal contact and references of published works and contributions by the authors included another 16 articles, resulting in a total of 32 articles. After full-text article selection and reading, 15 articles were finally included.
Conclusion: There is not a generally accepted classification system of the various degrees of peri-implantitis. An etiology-driven classification was proposed as a tool to assist the clinician in properly detecting and classifying etiology-based peri-implantitis. This classification may also support the assignment of prognosis, and if needed, therapy to arrest/prevent peri-implantitis.
Peri-implant diseases classically present in two forms: peri-implant mucositis and peri-implantitis. The 6th European Workshop on Periodontology (EWOP) (2008) confirmed that peri-implant diseases are infectious in nature, and defined them as “changes in the level of crestal bone, presence of bleeding on probing and/or suppuration; with or without concomitant deepening of peri-implant pockets”. Similarly, the American Academy of Periodontology (AAP) in 2013 defined peri-implantitis as an inflammatory reaction associated with the loss of supporting bone beyond initial biologic bone remodeling around an implant in function.
Some studies suggested that mucositis and peri-implantitis are equivalent to periodontitis, as both represent an imbalance between the host response and the bacterial load.Hence, peri-implant mucositis is the precursor to peri-implantitis, as is gingivitis for periodontitis, and a continuum exists from healthy peri-implant mucosa to peri-implant mucositis and to peri-implantitis. Other authors have refused the hypothesis that peri-implantitis is a disease comparable to periodontitis, due to anatomical differences between the periodontium and the bone-to-implant contact described as osseointegration. As anticipated by the Consensus of the 7th European Workshop on Periodontology, this definition considers that the infection itself is always caused by plaque and its byproducts; however, several etiologic factors are known to be specifically associated with peri-implantitis, such as surgical- and prosthetic-related factors, implant characteristics, smoking, and host response. Recently, Zarb and Koka proposed the term osseo-insufficiency to describe the difference between peri-implantitis and periodontitis-induced bone loss. Then, Albrektsson et al described the peri-implant bone loss as an unbalanced foreign-body reaction, specifically stating that osseointegration is a process whereby bone reacts to the dental implant.
Today, there are many concerns associated with the etiology and the disease entity described as peri-implantitis. In particular, there is still a need of uniformity in the definitions of peri-implantitis, leading to different results that are not easily comparable and may complicate the decision-making process to prevent/treat the peri-implantitis. There are several classifications of peri-implantitis in the medical international literature, but there is still a lack of a standard classification system highlighting diagnosis and treatment of peri-implant pathologies. Accepted classifications of peri-implantitis are based on clinical bleeding on probing (BOP), probing pocket depth (PPD), and radiographic parameters without describing the etiology of the disease. These criteria describe the extent of the disease and eventually the progression over time. In addition, they should be affected by several confounding factors.
In the effort to re-evaluate the clinical approach to peri-implant diseases, the aim of this narrative review was to evaluate the existing evidence in identifying risk factors in the etiology of peri-implantitis. An additional aim was to highlight various classifications for peri-implantitis, and an attempt is made to propose a classification of implant defects. A new evidence-based etiology-driven classification of peri-implantitis is proposed to accurately and fully describe the etiology of peri-implant disease. This classification has different subcategories, and it should allow easier documentation and better communication among clinicians, researchers, and authors, to better understand the diagnostic approach to the peri-implant pathologic bone loss.
Materials and methods
The present narrative review was conducted following a previously developed systematic review on the definition of peri-implantitis. The focused question was: classification of peri-implantitis with clinical diagnosis.
Literature Search
A search strategy encompassing the literature in English from 1967 up to June 2017 was performed to identify relevant studies meeting the inclusion criteria. The PubMed database of the U.S. National Library of Medicine has been consulted using a combination of Boolean keywords including MeSH (Medical Subject Headings) and free text terms with the following combination: ((periimplantitis) OR peri-implantitis[MeSH]) AND “classification”. Screening was performed independently by two expert examiners (M.T. and S.M.M.).
Eligibility Criteria
The following inclusion criteria was defined for the selection of articles:
- Papers written in the English language
- Studies with a clinical examination of human patients
- Randomized controlled clinical trials (RCTs) of implants of ≥ 1 year in function
- Prospective and retrospective observational studies of implants of ≥ 1 year in function
- Cross-sectional studies of ≥ 1 year in function
- Systematic reviews, meta-analysis, narrative reviews, consensus
Articles were excluded if they were:
- Animal and in vitro studies
- Reports of locally or systemically compromised sites and/or conditions
- Reports with < 15 cases
- Studies on retrograde implantitis
- Mini implants, one-piece implants, blades
- Reports of implant outcomes < 1 year in function
Full-text papers were obtained for all abstracts and titles that appeared to meet the inclusion criteria and were assessed for inclusion by the same two reviewers. Reference lists of the selected studies were screened for additional papers that could meet the eligibility criteria of the study. Additionally, hand searches of the bibliographies of selected papers were conducted. A final reviewer (L.C.) evaluated possible inconsistencies between the two reviewers. No methodologic and reporting quality of selected full-text articles was performed in order to collect the greatest number of articles. All the full texts of the selected papers were stored in shared folders accessible to all the reviewers.
Results
A total of 122 articles were found according to the search criteria. After filter activation (clinical trial, meta-analysis, observational study, randomized controlled trial, review, systematic reviews), 91 articles were excluded, resulting in 31 articles. After abstract evaluation and duplicate removal, 16 articles were deemed useful for the aim of the present review. A manual search using personal contact and references of published works (13 papers) and contributions by the authors (3 papers) included another 16 articles, resulting in a total of 32 articles. Finally, after full-text article selection and reading, 15 articles were included, and the relevant information from each article was extracted.
Six authors classified peri-implantitis based on radiologic data alone. Five authors suggested a classification of peri-implantitis based on clinical parameters (BOP, Plaque Index, suppuration, and radiologic signs). Four of these studies proposed related prognosis and treatment criteria. One study proposed a new classification system for the peri-implant disease in association with natural teeth, while another study proposed a histologic classification of peri-implant mucositis and peri-implantitis. Finally, two authors proposed classifying peri-implantitis based on its causative etiology, while Nguyen-Hieu et al introduced a differential diagnosis of peri-implant mucositis, occlusal overload, retrograde peri-implantitis, and inflammatory implant periapical lesions, suggesting the appropriate treatment in each case, and highlighting the concept of prevention based on early detection and regular maintenance.
Schwarz et al classified peri-implant bony defect based on its anatomy: Class I (intraosseous defect) and Class II (supra-alveolar defect in the crestal implant insertion area). No clinical interpretation of the defects was provided. Spiekermann classified the bone resorption pattern of peri-implant defect into five categories: Class I (horizontal); Class II (hay-shaped); Class III a (funnel-shaped); Class III b (gap-like); and Class IV (horizontal-circular form). No clinical criteria for each class was given here. Nishimura et al classified the amount of horizontal bone loss along with other associated types of bony defects in four classes, ranging from slight to advanced horizontal bone loss. No clinical signs, treatment modality, and prognosis were highlighted. Vanden Bogaerde highlighted the importance of the bony defect in the progression of the regenerative process, dividing into closed (with the maintenance of intact surrounding bone walls) and open defects (lack of one or more bone walls). The open defect group was then divided into several subgroups that include the majority of situations encountered in clinical practice. Zhang et al analyzed peri-implant bone defects on the basis of panoramic radiographic shapes in patients with mandibular implant-supported overdentures, classifying into decreasing order of occurrence: saucer-shaped defects, wedge-shaped defects, flat defects, undercut defects, and slit-like defects. Ramanauskaite and Juodzbalys suggested a peri-implantitis classification based on soft tissue conditions and the amount of pathologic bone loss.
Ata-Ali et al suggested a combined (peri-implant mucositis and peri-implantitis) classification of peri-implantitis, based on its severity, giving valuable information for clinical status of implants, but lacking prognosis and treatment criteria. Lang et al, in consensus statements and recommended clinical procedures regarding implant survival and complications, proposed treatment options for peri-implantitis based on five classes of severity. Clinical and radiologic signs were used to define different severities of peri-implantitis. Froum and Rosen proposed a classification of peri-implantitis into early, moderate, and advanced, based on the onset time of clinical and radiologic signs. Decker et al and Passi et al reported prognostic systems, based on added clinical and detailed radiologic parameters, for peri-implant diseases that can be used as a tool for clinicians for prognosis and to develop a treatment plan for all stages of peri-implant disease.
Kadkhodazadeh and Amid introduced a new classification system for the peri-implant disease based on the origin of the defects. The main aim was to clarify the different pathologic situations that can be detected around the dental implant. Furthermore, this classification system can help the clinician to improve diagnosis, comparison, and subsequent selection of the best treatment option.
Kaplan et al highlighted the importance of early microscopic examination of lesions presenting clinically as peri-implantitis, a step toward more accurate diagnosis and improved treatment of peri-implantitis and lesions mimicking peri-implantitis. Lesions presenting erythema, swelling, BOP, and pocket formation with evidence of bone loss of at least 2 mm were diagnosed as peri-implantitis.
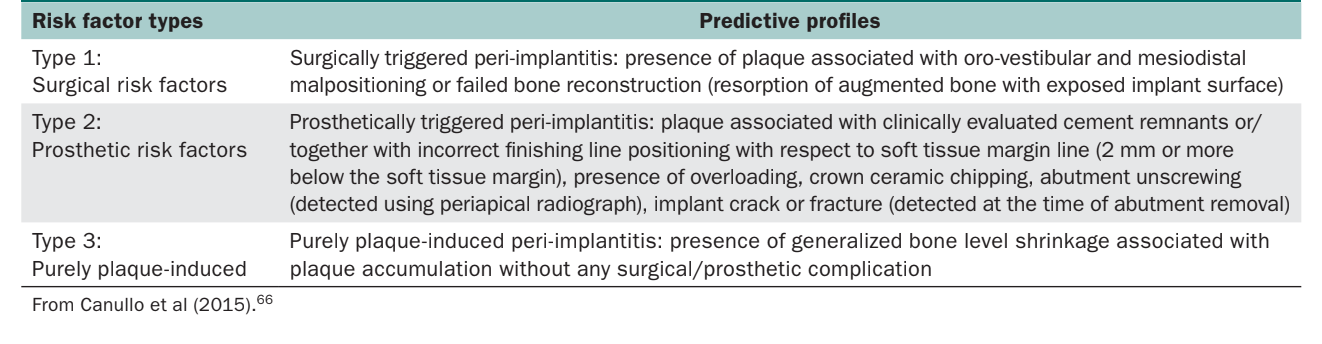
Canullo et al and Sarmiento et al proposed classifying peri-implantitis based on its etiology. Canullo et al proposed three peri-implantitis subtypes: plaque-induced and prosthetically and surgically triggered peri-implantitis. The authors proposed an advanced data mining model that could be a promising tool for diagnostics of peri-implantitis subtypes. According to this model, plaque-induced and prosthetically and surgically triggered peri-implantitis are three different entities associated with distinguishing predictive profiles; hence, the appropriate causal treatment approach remains necessary. Sarmiento et al proposed a peri-implantitis etiology classification including five possible origins (bacteria, exogenous irritants, iatrogenic factors, extrinsic pathology, and absence of keratin tissue).
Discussion
Peri-implantitis is the main cause of dental implant failure, and it is generally considered to share common characteristics and risk factors with periodontitis.
Most existing studies used radiographic findings alone or in combination with clinical parameters to define peri-implantitis. This classification describes the extent of the disease and the progression over time, without describing the etiology of the disease. Furthermore, clinical parameters such as BOP and PPD around implants are less predictable, since they are influenced by more confounding factors compared with natural dentition. Peri-implantitis is today considered as a multifactorial disease with several risk factors. Any factor that facilitates plaque formation (eg, poor oral hygiene) or host defense capability (eg, smoking habit, excessive alcohol consumption, genetic traits, history of periodontitis, or use of bisphosphonates) might contribute to the development of peri-implantitis. A wide array of factors may potentially contribute to the extent of early bone loss. Hence, radiographic control at baseline (after prosthetic rehabilitation) and rigorous periodic assessment must be followed in the routine implant practice to prevent peri-implant bone loss or to arrest peri-implant progressive bone loss when detected early. This must be supplemented with a detailed clinical examination, which includes the presence of BOP, presence of suppuration, and PPD under light probing. The lack of actual criteria for classification of the stages of peri-implantitis and recognized differences in disease severity make it difficult for proper communication among peers to establish diagnosis, prognosis, or even treatment procedures. An etiology-driven classification is proposed to better lead the clinician in a diagnostic-based, decision-making workflow, in order to diagnose, prevent, and treat the pathologic marginal bone loss around an implant. Ac- cording to the proposed classification, peri-implantitis can be defined as a “group of inflammatory disorders around an implant in function with concurrent bone loss beyond the initial bone remodeling, and it may be considered as a consequence of several pathological conditions that act primarily as predictive profiles or as co-factors for further bacterial contamination”. This classification partially concurs with previous proposed classifications of the AAP and the 7th Consensus of the European Federation of Periodontology, where knowing and understanding bone remodeling represents the main diagnostic factor to identify the presence of progressive bone loss.
Six etiology-driven categories of diagnostic criteria (DC) involved in the estimation of the implant pathologic bone loss around an implant in function were classified as follows:
- DC-1: Extent of the pathologic bone loss: clinical and radiographic signs (Table 1)
- DC-2: Risk factors associated with pathologic bone loss (Table 2)
- DC-3: Onset of the pathologic bone loss (Table 3)
- DC-4: Progression of the pathologic bone loss (Table 3)
- DC-5: Patient’s (host) characteristics (classification and analysis of patient’s risk profiles)
- DC-6: Implant’s characteristics (surface, connection, crestal module)
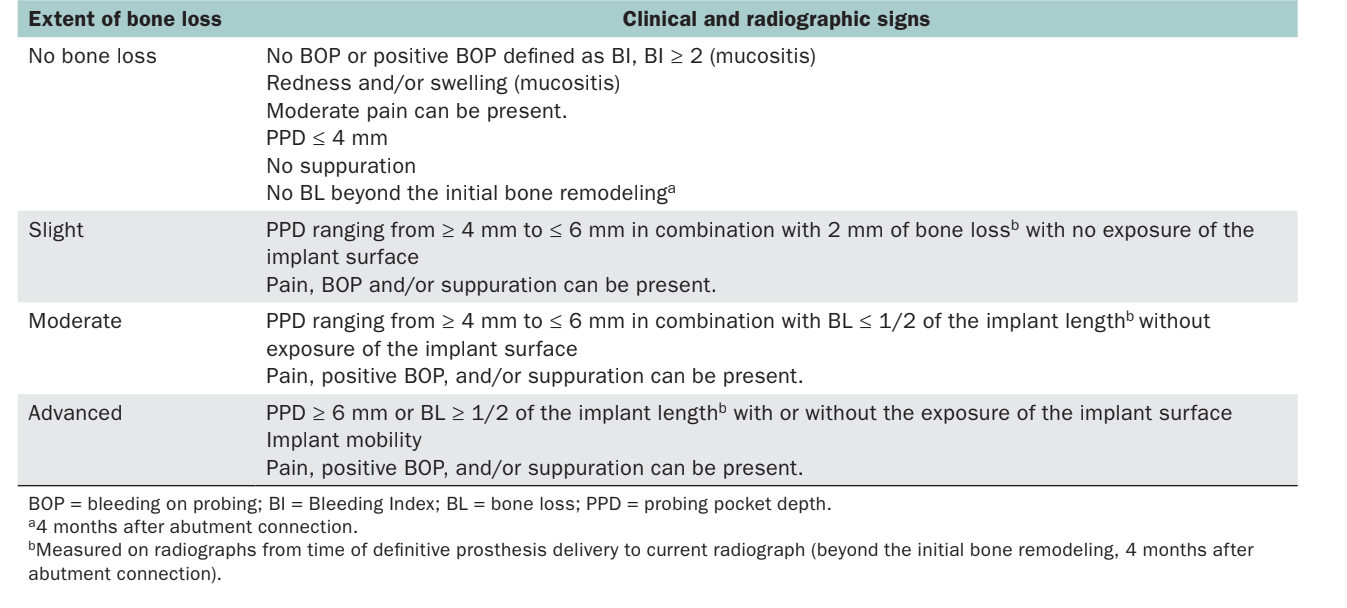

DC-1: Extent of the Implant Pathologic Bone Loss: Clinical and Radiographic Signs
This section essentially describes the extent of the pathologic bone loss without describing the etiology. The scale takes into consideration four categories (no bone loss, slight, moderate, and advanced), directly correlated with clinical and radiographic parameters (Table 1). Compared with further follow-up, this section may be useful to monitor the progression of the disease over time. In the preparation of this classification, the initial bone remodeling was complete at 4 months after abutment connection.
Severity of peri-implant disease may be based on the amount of peri-implant marginal bone loss. However, even in studies that have defined the entity of marginal bone loss, various diagnostic criteria have been applied. In combination with radiographs, PPD provides information regarding the morphology of peri-implant soft and hard tissues. Peri-implant probing depth also allows the observation of changes in the attachment level over time. However, the presence of bone loss and PPD alone may not be enough to formulate a diagnosis of peri-implantitis. In fact, probing around implants may be affected by some confounding factors, such as the size of the probe, the probing force, the direction of the probe, accessibility of the probe, depth of implant placement, the health and the thickness of the peri-implant soft tissue, and the design of the implant neck as well as the supra-structure. BOP and/or suppuration was used in all of the analyzed studies. In most of the studies, the combination of clinical and radiologic measurements was used for case definition. At the 7th European Workshop on Periodontology, peri-implantitis was defined with positive BOP and/or suppuration, in combination with radiographic bone loss ≥ 2 mm. Since peri-implantitis involves the presence of radiographic bone loss among its diagnostic signs, early marginal bone loss must be identified in order to prevent progressive bone loss (ie, peri-implantitis). Interestingly, the 8th Consensus of the European Federation of Periodontology, as well as the Position Paper of the American Academy of Periodontology agreed upon the likely initial bone remodeling after implant restoration to accommodate biologic width. Nonetheless, these papers further highlighted the lack of evidence to standardize early bone remodeling.
DC-2: Clinically Induced Triggering Factors Associated with Implant Pathologic Bone Loss
According to previous reports by consensus conferences and observational studies, numerous etiologic factors are known to be particularly associated with peri-implant bone loss. Hence, this section essentially describes specific predictive profiles associated with different subtypes of peri-implantitis, and as such, can also represent predictive factors for progressive marginal bone loss. Implant position (arch and region) was also described in this section.
According to Canullo et al, different predictive profiles are associated with different subtypes of peri-implantitis. The same authors proposed an advanced data mining model or diagnostics of peri-implantitis subtypes. Accordingly, the proposed evidence-based etiology-driven classification of peri-implant disease is based on clinical subtypes of peri-implantitis, classified as follows: purely plaque-induced and prosthetically and surgically triggered peri-implantitis (Table 2). Plaque-induced and prosthetically and surgically related peri-implantitis are different entities associated with distinguishing predictive profiles; hence, an appropriate causal treatment approach remains necessary. It must also be highlighted that multiple risk factors could act synergistically with one of the clinical scenarios, making the identification of the leading risk factors more difficult.
DC-3-4: Onset and Progression of the Implant Pathologic Bone Loss
To date, there have been no standardized parameters to clinically differentiate the various stages and severity of peri-implantitis. However, differences in disease severity have been used referring to a chronic periodontal disease. Although peri-implantitis has been described as the early, established, and advanced lesion, this peri-implantitis staging pertained to a histologic differentiation. In order to increase consistency of reporting, a classification system was proposed by Froum and Rosen to categorize the severity and thresholds of peri-implantitis.
The diagnosis as well as progression of peri-implantitis may be characterized by increased clinical indices (PPD, BOP, and suppuration), marginal bone loss, and microbiology. Progression of the disease through the advanced stages is one of the fundamental reasons why early diagnosis, prognosis assessment, and effective therapies are paramount in the long-term success of implant therapy. The 2011 EFP consensus highlighted the importance of routinely collecting the clinical and radiographic data starting from prosthesis placement, in order to establish a baseline for the diagnosis of peri-implantitis during maintenance of implant patients, and to evaluate possible evolution in the long-term follow-up. In fact, a meta-analysis by Derks and Tomasi clearly showed a positive relationship between the prevalence of peri-implantitis and functional time.
This section essentially describes the initiation and development of the pathologic bone loss around an implant in function (Table 3). The etiology-driven “diagnostic manual” was designed to take into account all stages of peri-implant diseases, from reversible inflammatory lesions limited to the peri-implant soft tissue, to nonreversible inflammatory lesions characterized by crestal bone loss and inflammation surrounding an implant beyond the initial biologic bone remodeling. The onset of the pathologic bone loss was classified in early- or late-staged basing of its initiation, which was set before or after the initial bone remodeling (4 months after abutment connection), respectively. According to previously published works, early crestal bone resorption is observed after the connection of the abutment and delivery of the definitive prosthesis in two-piece implants.
Then, pathologic bone loss may appear as progressive or chronic, which is characterized by a slow to moderate rate of progressive pathologic peri-implant bone loss; or acute, which is defined by a very rapid peri-implant bone destruction occurring within a few weeks or months.
Finally, pathologic bone loss has been further classified as localized (peri-implantitis to one implant); focalized (peri-implantitis localized in one sextant/quadrant); or generalized (peri-implantitis > 2 implants in different quadrants).
DC-5: Patient’s (Host) Susceptibility (Classification and Analysis of Patient’s Risk Profiles).
This section essentially describes patients’ characteristics (age, sex, implant site, number/status of residual teeth) and related risk factors (history of periodontitis, smoking, oral hygiene, systemic diseases, gene expression) that may increase the likelihood of peri-implant disease development, as well as increasing the severity and speed at which it may occur. Patients are classified as susceptible or not susceptible. The individual risk factors may act in one of two ways: increasing biofilm levels or impairing the body’s ability to respond to the bacterial challenge. In some cases, both mechanisms are operating.
Papantonopoulos et al suggested two implant “phenotypes”, one susceptible and one resistant to peri-implantitis. The authors found that fewer teeth, younger age, and placement mainly in the mandible characterize the susceptible implant “phenotype,” while the resistant “phenotype” was predominantly found in the maxilla.
Long-term prospective cohort studies and systematic reviews reported that patients with a history of periodontitis had a significantly higher incidence of peri-implantitis and peri-implant marginal bone loss, compared with periodontally healthy patients. Moreover, it is worthy to mention the emerging investigations on epigenetics in peri-implantitis–susceptible subjects, especially for those with a history of periodontal disease, as a consequence of regulatory pathways of genes involved in maintaining chronic inflammation. Changes in gene expression (chemical alterations in DNA and its associated proteins) due to the immune response to oral bacteria might be persistent after a tooth is lost, thus affecting peri-implant tissue stability.
The relationship between smoking and peri-implantitis remains controversial. Several studies have failed to find statistically significant differences in incidence of peri-implantitis between smokers and nonsmokers. Conversely, a long-term report by Roos-Jansåker et al46 and Swierkot et al reported statistical differences between smokers and nonsmokers. Likewise, Rinke et al, in a retrospective study, found that smoking had an odds ratio of 31.58 (P > .001) in developing peri-implantitis. The same authors reported an overall patient-level peri-implantitis rate of 11.2%, which was as high as 53% in patients who were smokers with a periodontal history, compared with 2.8% for patients who were nonsmokers. These results were also in agreement with a recent review of the literature by Heitz-Mayfield and Huynh-Ba.
Poor oral hygiene (including missing checkups and/or faulty dentistry) may increase biofilm levels. As foreseen by the Consensus of the 7th European Workshop on Periodontology, peri-implantitis is always caused by plaque and its byproducts. Plaque accumulation adjacent to dental implants has been associated with the development of peri-implant mucositis. Similarly, Roos-Jansåker et al, in a cross-sectional study, reported an association between plaque and peri-implant mucositis, but not with peri-implantitis. Monje et al, in a recent systematic review, showed that peri-implant mucositis and peri-implantitis could be significantly prevented with the implementation of peri-implant maintenance therapy and a minimum recall interval of 5 to 6 months. Schrott et al observed that in patients with good hygiene, the presence of at least 2 mm of keratinized mucosa was associated with lower BOP and plaque accumulation on the lingual surface and soft tissue recession on the buccal surface of functioning dental implants. However, other impediments to plaque control may include prosthesis design, adjacent restoration contour and margins, and/or loose or broken restorative components, which inter- fere with oral hygiene. Some of these problems may be avoided by designing removable superstructures, such as screw-retained crowns.
The association between periodontal diseases and systemic diseases (eg, uncontrolled diabetes mellitus, cardiovascular disease, immunosuppression) has been widely investigated during the last decade. The EFP/ AAP workshop on periodontitis and systemic diseases concluded that there is consistent and strong epidemiologic evidence that periodontitis may increase risk for future cardiovascular disease and diabetes. Conversely, there are still limited data available regarding possible associations between systemic diseases and peri-implantitis. Poor glycemic control is associated with peri-implant disease. Furthermore, prognosis of dental implants is improved in patients with glycosylated hemoglobin levels below 7 (normal range: 4% to 5.7%). However, in a review on risk factors for peri-implantitis, Heitz-Mayfield and Huynh-Ba highlighted that evidence showed that an association between peri-implant diseases and systemic diseases remains weak. Recently, Oates et al presented a randomized clinical trial on implants in diabetic patients with different HbA1c levels failing to find any difference between groups.
Even though it is well-known that patients with a history of periodontal disease have a higher risk of being affected by peri-implant diseases, controversies exist in the literature about the possible impact of Il-1 gene polymorphisms and peri-implantitis. A genetic disorder characterized by IL-1 gene polymorphism has been suggested as a risk factor for peri-implantitis. However, based on a systematic review of 27 relevant articles, no definitive conclusion can be drawn. In contradistinction, those with chronic inflammatory diseases, such as rheumatoid arthritis, do appear to have a higher risk of having peri-implantitis. A determination of the odds ratios through meta-analytic studies and systematic reviews is currently underway.
DC-6: Implant and Prosthetic Design (Analysis of Implant’s Macro- and Micro-design).
This section essentially describes implant characteristics. Two-piece implants unavoidably present a microgap between the implant and the abutment that might influence bacterial activity levels qualitatively and quantitatively, especially inside the implant connection. Canullo et al, in a 5-year cross-sectional study on microbiologic assessment of the implant-abutment interface with different connections, concluded that all the analyzed connections were contaminated after 5 years of functional loading. Recently, a statistically significantly higher bacterial count was reported for periodonto-pathogenic bacteria inside the implant-abutment interface of implants affected by peri-implantitis compared with those surrounded by healthy peri-implant tissues. Hence, development of implant designs has emerged to minimize crestal bone level changes.
Recent concerns over the influence of surface roughness (microdesign) on the long-term success of osseointegrated dental implants have been raised in the literature. Preclinical studies have provided further insight on peri-implantitis progression on different implant surfaces. Albouy et al, in a ligature-induced peri-implantitis model, showed that although turned and rough surfaces respond with rapid bone loss within 18 weeks due to plaque accumulation, after ligature removal there is a remission in disease progression. Furthermore, implant surfaces such as titanium anodization may progress further within a limited period of 36 weeks. Interestingly, this has been recently confirmed in a systematic review, where the results demonstrated that such a surface might be more exposed to peri-implant diseases and implant failure. On the other hand, different studies do not present statistically significant differences in terms of incidence of peri-implantitis when comparing surfaces. Zetterqvist et al compared the incidence of peri-implantitis in fully acid-etched implants with hybrid implants (implants with only the apical and the mid-third portions acid-etched). After a 5-year-follow-up time, overall peri-implantitis prevalence was 0.37%, and no significant difference was found for peri-implantitis prevalence between the fully acid-etched group and the hybrid group. Renvert et al, in a review of the literature, concluded that the reported data were not significant enough to support the evidence that rough-surfaced implants were more prone to having peri-implantitis than implants with a smooth surface.
The present review has aimed at proposing a classification that can assist the clinician/researchers in properly detecting and classifying etiology-based peri-implantitis. The present classification may also act as a “check list” for how clinicians should evaluate and record pathologic peri-implant bone loss for each implant on each visit. Then, they could compare the evaluations on subsequent visits to see if they are changing or staying the same. Although it needs validation, this classification may also support the assignment of prognosis, and if needed, therapy to arrest/prevent peri-implantitis. The present classification does not refute previous classifications of peri-implantitis, since the authors agree on the role of plaque in peri-implantitis. Nevertheless, co-factors that contribute to the initiation of initial and late crestal bone loss should also be properly addressed.
Conclusions
There is lack of a standard classification system to differentiate the various degrees of peri-implantitis. The proposed evidence-based etiology-driven classification of peri-implant disease intends to act as a “diagnostic manual” designed to work in conjunction with previously published manuscripts reporting different subtypes of peri-implantitis. It could assist the clinician in properly detecting and classifying etiology-based peri-implantitis. This classification may also support the assignment of prognosis, and if needed, therapy to arrest/prevent peri-implantitis.
Marco Tallarico, Luigi Canullo, Hom-Lay Wang, David L. Cochran, Silvio Mario Meloni
References
- Lindhe J, Meyle J; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 2008;35:282–285.
- Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000 1998;17:63–76.
- Peri-implant mucositis and peri-implantitis: A current understanding of their diagnoses and clinical implications. J Periodontol 2013;84:436–443.
- Lang NP, Berglundh T; Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: Where are we now?—Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 2011;38:178–181.
- Mombelli A. Etiology, diagnosis, and treatment considerations in peri-implantitis. Curr Opin Periodontol 1997;4:127–136.
- Salvi GE, Zitzmann NJ. The effects of anti-infective preventive measures on the occurrence of biologic implant complications and implant loss: A systematic review. Int J Oral Maxillofac Implants 2014;29(suppl):s292–s307.
- Becker ST, Beck-Broichsitter BE, Graetz C, Dörfer CE, Wiltfang J, Häsler R. Peri-implantitis versus periodontitis: Functional differences indicated by transcriptome profiling. Clin Implant Dent Relat Res 2012;16:401–411.
- Canullo L, Schlee M, Wagner W, Covani U; Montegrotto Group for the Study of Peri-implant Disease. International Brainstorming Meeting on Etiologic and Risk Factors of Peri-implantitis, Montegrotto (Padua, Italy), August 2014. Int J Oral Maxillofac Implants 2015;30:1093–1104.
- Pesce P, Canullo L, Grusovin MG, de Bruyn H, Cosyn J, Pera P. Systematic review of some prosthetic risk factors for periimplantitis. J Prosthet Dent 2015;114:346–350.
- Canullo L, Tallarico M, Radovanovic S, Delibasic B, Covani U, Rakic M. Distinguishing predictive profiles for patient-based risk assessment and diagnostics of plaque induced, surgically and prosthetically triggered peri-implantitis. Clin Oral Implants Res 2016;27:1243–1250.
- Canullo L, Schlee M, Covani U, Wagner W. Consensus meeting on periimplantitis, Galzignano 2014. Int J Oral Maxillofac Implants 2015;30:1093–1104.
- Zarb GA, Koka S. Osseointegration: Promise and platitudes. Int J Prosthodontont 2012;25:11–12.
- Albrektsson T, Dahlin C, Jemt T, Sennerby L, Turri A, Wennerberg A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin Implant Dent Relat Res 2014;16:155–165.
- Albrektsson T, Canullo L, Cochran D, De Bruyn H. “Peri-implantitis”: A complication of a foreign body or a man-made “disease”. Facts and fiction. Clin Implant Dent Relat Res 2016;18:840–849.
- Tallarico M, Monje A, Wang HL, Galindo Moreno P, Xhanari E, Canullo L. A systematic review on the definition of periimplantitis: Limits related to the various diagnoses proposed. J Oral Science Rehabilitation 2016;2:42–53.
- Spiekermann H. Implantologie. Stuttgart: Thieme, 1984.
- Schwarz F, Sahm N, Becker J. Aktuelle Aspekte zur Therapie periimplantärer Entzündungen. Quintessenz 2008;59:00.
- Nishimura K, Itoh T, Takaki K, Hosokawa R, Naito T, Yokota M. Periodontal parameters of osseointegrated dental implants. A 4-year controlled follow-up study. Clin Oral Implants Res 1997;8:272–278.
- Ramanauskaite A, Juodzbalys G. Diagnostic principles of peri-implantitis: A systematic review and guidelines for peri-implantitis diagnosis proposal. J Oral Maxillofac Res 2016;7:e8.
- Vanden Bogaerde L. A proposal for the classification of bony defects adjacent to dental implants. Int J Periodontics Restorative Dent 2004;24:264–271.
- Zhang L, Geraets W, Zhou Y, Wu W, Wismeijer D. A new classification of peri-implant bone morphology: A radiographic study of patients with lower implant-supported mandibular overdentures. Clin Oral Implants Res 2014;25:905–909.
- Ata-Ali J, Ata-Ali F, Bagán L. A classification proposal for peri-implant mucositis and peri-implantitis: A critical update. Open Dent J 2015;9:393–395.
- Decker AM, Sheridan R, Lin GH, Sutthiboonyapan P, Carroll W, Wang HL. A prognosis system for periimplant diseases. Implant Dent 2015;24:416–421.