Correction of Peri-Implant Buccal Bone Dehiscence Following Sub-Periosteal Peri-Implant Augmented Layer Technique With Either Block or Particulate Xenograft: A Retrospective Study
Abstract
Objective: To evaluate the effectiveness of Sub-periosteal Peri-implant Augmented Layer (SPAL) technique performed with deproteinized bovine bone mineral (DBBM), delivered either as particulate (pDBBM) or block (bDBBM), in correcting a peri implant bone dehiscence (PIBD). Implants showing a thick (≥ 2 mm) peri-implant buccal bone plate (PBBP) at placement were also examined.
Material and Methods: Patients with a PIBD ≥ 1 mm, treated with SPAL with either pDBBM (SPALparticulate) or bDBBM (SPALblock), and patients with an implant showing a PBBP ≥ 2 mm at insertion (CONTROL) were included. Re-entry was performed either at 6 months (SPAL groups) or 3 months (CONTROL). The rate of patients presenting no PIBD at re-entry was the primary outcome. Bone dehiscence height (BDH) and width (BDW), thickness of buccal tissues (BTT) and marginal bone level (MBL) were secondary outcomes.
Results: Thirty-nine implants in 39 patients (14 in SPALparticulate,14 in SPALblock and 11 in CONTROL) were analyzed. No PIBD were found in SPALparticulate whereas in SPALblock one PIBD was present. Two patients in CONTROL presented a PIBD. A reduction in both BDH and BDW was observed in both SPALparticulate (2.7 ± 1.6 mm for BDH and 3.9 ± 0.2 mm for BDW) and SPALblock (2.5 ± 1.8 mm for BDH and 3.8 ± 1.1 mm for BDW). SPALblock showed a higher BTT than SPALparticulate at re-entry (3.6 ± 1.3 mm for SPALblock and 2.6 ± 0.6 mm for SPALparticulate, p = 0.0160). All groups showed similar MBL.
Introduction
Prosthetically-driven implant placement in a reduced horizontal bone dimension often results in a peri-implant bone dehiscence (PIBD) (Bressan et al. 2017; Pramstraller et al. 2018).
Small bony dehiscence defects left for spontaneous healing revealed more vertical bone loss at the buccal aspect after im- plant insertion and also more marginal bone loss compared to sites treated with GBR (Jung et al. 2017; Monje et al. 2023). Although implants with small, non-contained buccal bone dehiscences exhibited high implant survival rates and healthy peri-implant tissues at a 7.5-year follow-up (Waller et al. 2020), the presence of an overt PIBD was associated with a higher incidence of mucositis and peri-implantitis as well as a faster progression of peri-implantitis compared to implants completely surrounded by bone (Schwarz, Sahm, and Becker 2012; Monje et al. 2019). Despite the prognostic value of a PIBD on the long-term peri-implant tissue health is still controversial, the correction of a buccal bone dehiscence at implant placement has been recently recommended to favor the stability and healthy conditions of peri-implant tissues over time (Herrera, et al. 2023; Monje et al. 2023; Song et al. 2024).
In order to treat a PIBD, either bone or soft tissue augmentation procedures were proposed. Both treatments were shown to provide stable clinical and radiographic outcomes in the medium and long-term (Jensen et al. 2023; Monje et al. 2023). Among different lateral bone augmentation procedures at simultaneous implant placement, Guided Bone Regeneration (GBR) and Sub-periosteal Peri-implant Augmented Layer technique (SPAL, Trombelli et al. 2018) have been associated with a high probability of completely correcting a PIBD (Severi et al. 2022). In particular, SPAL is based on the use of patient periosteum as a barrier membrane to contain a graft acting as a “space-making” scaffold. When performed in combination with a deproteinized bovine bone mineral (DBBM) particulate graft (pDBBM), SPAL was shown effective in completely correcting up to 90.9% of PIBDs (Trombelli et al. 2019, 2020). A recent retrospective study compared peri-implant hard and soft tissue conditions at implants presenting either a PIBD treated with SPAL or a thick (≥ 2 mm) PBBP at implant placement (Trombelli et al. 2020). Although similar healthy conditions of the peri-implant tissues were found in both groups, a more apical position of the radiographic marginal bone level (MBL) was found in SPAL group, suggesting a different remodeling pattern of the graft compared to native bone (Trombelli et al. 2020). These findings may question the relevance of the physico-chemical characteristics of the graft with respect to the stability of the reconstructive outcome following SPAL. In this respect, an anectodotal report suggested that the use of a DBBM block (bDBBM) may be a successful alternative in combination with SPAL (Trombelli et al. 2022).
Therefore, the aim of the present retrospective study was to evaluate the effectiveness of SPAL performed in combination with either pDBBM or bDBBM in correcting a PIBD. Moreover, changes in the peri-implant hard tissue occurring from implant placement to re-entry for implant uncovering were analyzed. A control group comprising of implants showing a thick (≥ 2 mm) PBBP at implant placement was also examined.
Materials and Methods
2.1 Ethical Aspects
The present retrospective study was approved by the Ethical Committee of Area Vasta Emilia Centro, Italy (protocol n° 523/2024/Oss/UniFe, date of approval 16.10.2024). Each patient provided a written informed consent prior to surgical treatment.
All the clinical procedures have been performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines (GCPs).
2.2 Study Population
The record charts of patients undergone implant-supported prosthetic rehabilitation in the period May 2020–September 2021 at the Research Centre for the Study of Periodontal and Peri-implant Diseases, University of Ferrara, and at one private dental office based in Ferrara were screened to determine patient eligibility for the study. The present retrospective study was carried on following the STROBE statement guidelines/checklist for cross-sectional studies.
Based on the conditions of buccal bone plate at the time of implant placement and surgical management, patients were categorized into 2 groups:
- Patients with at least one implant presenting a PIBD ≥ 1 mm, treated with SPAL in combination with pDBBM (SPALparticulate group);
- Patients with at least one implant presenting a PIBD ≥ 1 mm, treated with SPAL in combination with bDBBM (SPALblock group).
SPAL groups comprised of consecutively treated patients where pertinent clinical and radiographic measurements could be retrieved for data analysis. If two or more implants in the same patient were eligible for the study, only the implant presenting the PIBD with the highest bone dehiscence was selected for analysis. Moreover, patients with at least one implant presenting a residual PBBP thickness ≥ 2 mm after implant insertion were regarded as control group (CONTROL group).
All selected implants had to be placed in a healed bone crest (type 4C implants, Gallucci et al. 2018) and had to show primary stability, as assessed by insertion torque. Heavy smokers (cigarette consumption > 10 cigarette/day) and patients with diabetes mellitus at the time of surgery were excluded from the study.
2.3 Clinical Procedures
2.3.1 Pre-Operative Procedures
Prior to implant placement, all patients had undergone active therapy for treating carious lesions and periodontal diseases, and had been enrolled in a professional maintenance with frequency of recalls scheduled according to the PerioRisk assessment tool (Trombelli et al. 2009, 2017; Farina et al. 2021).
All the surgical procedures were performed by two trained operators (L.T., M.S.). Patients were administered 2 g of amoxicillin + clavulanic acid (Augmentin, GlaxoSmithKline, Verona, Italy) 1 h prior to surgery. Local anesthesia was attained using articaine with 1:100,000 epinephrine administered by local infiltration.
2.3.2 Surgical Procedures
2.3.2.1 SPAL Groups.
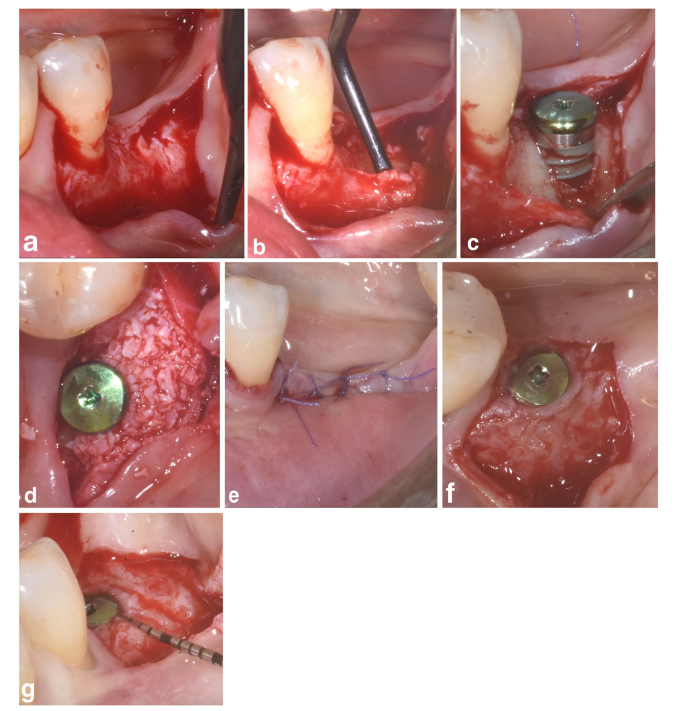
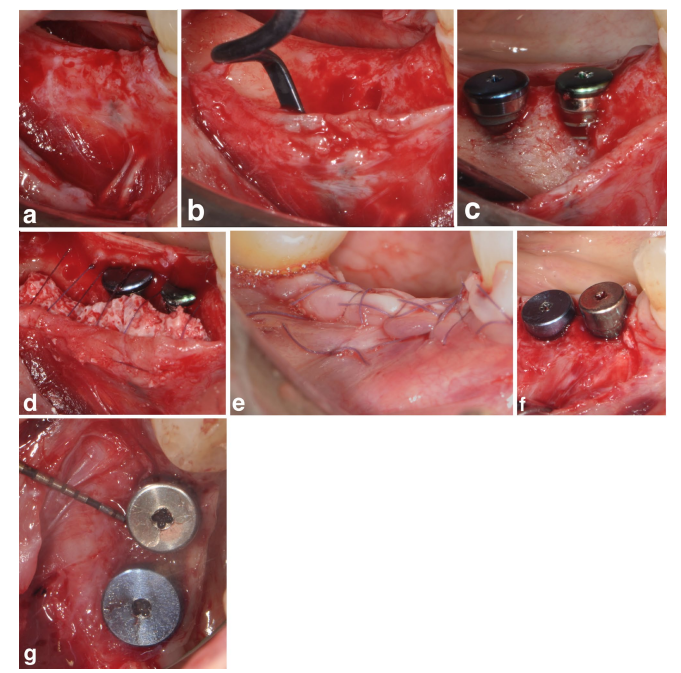
In both SPALparticulate and SPAL-block groups, surgical access to the bone crest was performed according to a previously described procedure (Trombelli et al. 2018). Briefly, a mucosal layer was raised on the buccal aspect by split-thickness dissection with a 15C blade (Figures 1a and 2a). Then, the periosteal layer was elevated from the bone with a periosteal elevator (PTROM, Hu-Friedy, Chicago, Illinois) as well as tunneling knives (KPAX, TKN1X and TKN2X, Hu Friedy, Chicago, Illinois) with varying angulated sharp edges, creating a sub-periosteal pouch that could accommodate a graft (Figures 1b and 2b). A full-thickness flap was elevated on the oral (lingual/palatal) aspect. Mylohyoid muscle fibers were detached from the lingual flap using a blunt instrument to allow for coronal advancement. Cortical perforations were performed with a calibrated cylindrical carbide bur in order to increase blood supply to the surgical area (Majzoub et al. 1999; Acar et al. 2016).
Tissue-level implants (SPI Element; Thommen Medical, Grenchen, Switzerland) were placed with the coronal margin of the 1-mm polished collar at the level of the bone crest (Figures 1c and 2c).
A bovine-derived xenograft, delivered as either particulate (Bio-Oss spongiosa granules, particle size 0.25–1.0 mm; Geistlich Pharma, AG, Wolhusen, Switzerland) (Trombelli et al. 2018) or block (Bio-Oss block; Geistlich Pharma, AG, Wolhusen, Switzerland) (Trombelli et al. 2022), was used to fill the surgically-created space between the periosteal layer and exposed implant surface. The block was fragmented into smaller pieces by means of a 15C blade or diamond bur in order to be adapted to the extent of the sub-periosteal space and reach the desired buccal plate recontouring. Grafting was performed to completely correct the PIBD up to the bone crest. In all cases, the sub-periosteal graft provided at least 2 mm of thickness at the polished collar of the implant.
The coronal portion of the periosteal layer was then secured to the oral flap by means of resorbable internal mattress sutures (Vicryl 6/0, Ethicon, Somerville NJ, USA) (Figures 1d and 2d). Subsequently, the mucosal layer was coronally advanced and sutured to the oral flap by horizontal internal mattress and interrupted sutures to provide a submerged healing for both graft and implant (Figures 1e–2e).
At 6-months surgical re-entry for implant uncovering, a buccal split-thickness flap was dissected to position the healing abutment. In order to assess the presence/absence of a residual PIBD, the presence of hard tissue around the implant surface was evaluated using a UNC-15 probe. If no probe penetration beyond the polished implant collar was present, the implant was categorized as “completely corrected dehiscence”.
To provide adequate dimensions of keratinized peri-implant mucosa (KT), either an apically positioning of the flap (APF) or a free gingival graft (FGG) was performed (Trombelli et al. 2019) (Figures 1f,g and 2f). The choice to perform either an APF or a FGG was based on the need to ensure a KT height of at least 2 mm at both buccal and lingual aspects of the implant.
2.3.2.2 CONTROL Group.
A buccal and lingual/palatal full thickness flap were raised to expose the bone crest. The implant site was prepared according to manufacturer instructions and tissue-level implants (SPI Element; Thommen Medical, Grenchen, Switzerland) were inserted with the coronal margin of the 1-mm polished collar at the level of the bone crest. No bone augmentation procedure was performed. In all cases, the flap was sutured using internal mattress and interrupted sutures (Vicryl 5/0, Ethicon, Somerville NJ, USA) to provide a submerged healing.
At 3-months surgical re-entry for implant uncovering, a buccal full-thickness flap was elevated to screw the healing abutment. Flap was then trimmed and adapted to the healing abutment to provide adequate dimensions of peri-implant keratinized mucosa.
2.3.2.3 Postoperative Procedures.
A rescue anti-inflammatory drug (i.e., ibuprofen 600 mg tablets) was prescribed immediately after surgery, then pro re nata for the following postoperative days. Patients were instructed not to wear any removable prostheses to avoid compression onto the surgical site for at least 4 weeks, and not to chew or brush in the treated area for approximately 2 weeks. The home use of a 0.12% chlorhexidine solution (Dentosan, Recor- dati, Milan, Italy) was prescribed for chemical plaque control (1-min rinse b.i.d. for 3 weeks). Sutures were removed at 2-weeks post-surgery.
2.4 Study Parameters
2.4.1 Clinical Measurements
To evaluate the effect of the treatment on PIBD, the following clinical measurements were retrieved from clinical charts:
- Bone dehiscence height (BDH): measured at the mid-buccal aspect of the implant as the distance between the apical margin of the polished collar of the implant and the first bone-to-implant contact;
- Bone dehiscence width (BDW): measured at the buccal aspect of the implant as the widest exposed portion of the rough implant surface.
In all groups, BDH and BDW had been assessed immediately after implant placement and at re-entry for implant uncovering.
Moreover, the thickness of buccal tissues (BTT) had been measured as the distance between the buccal contour of the polished collar and the outer aspect of either the grafted area (SPAL groups) or native bone ridge (CONTROL group) at the mid-buccal aspect of the implant. Baseline BTT had been recorded:
- Immediately after implant placement for the CONTROL group;
- Immediately after grafting for both SPAL groups; BTT was recorded anew at surgical re-entry for all groups.
All measurements had been performed using a UNC-15 periodontal probe and rounded at the nearest millimeter.
2.4.2 Radiographic Measurements
Periapical radiographs, taken with the long-cone parallel technique immediately after surgery and at re-entry for all groups, were digitized and analyzed using a specifically designed software (NIS elements v4.2; Nikon Instruments, Campi Bisenzio, Firenze, Italy). Marginal bone level (MBL) was measured as the distance (approximated to the nearest 0.1 mm) between the most apical margin of the polished implant collar and the bone crest at the mesial (mMBL) and distal (dMBL) aspect of each implant using a 10×–15× magnification. MBL was recorded as negative or positive when the apical margin of the polished collar was located either apically or coronally to the bone crest, respectively.
A reference mark 1-mm high present on digital radiograph was used for calibration.
Two examiners (C.F. and M.S.) performed the radiographic measurements. Examiners were involved in a calibration session on a sample of radiographs obtained from patients not selected for the present study. The calibration session consisted of two sessions of MBL measurements, performed at a 7-day interval, and allowed for reaching an excellent inter- and intra-examiner agreement (k-score 0.87).
Statistical Analysis
The rate of patients presenting the rough implant surface covered up to the apical part of the polished collar (i.e., no PIBD) at re-entry was considered as the primary outcome. Values and changes in BDH, BDW, BTT and MBL were secondary outcome variables.
Sample size was calculated using data derived from a previous study (Benic et al. 2019) where the rate of patients presenting the rough implant surface covered up to the implant shoulder at re-entry was 91.7% in DBBM block groups and 25.0% in DBBM particulate group, respectively. Z-test estimated that at least 11 patients for each of the two independent SPAL groups were required to achieve 95% statistical power with an alpha error of 0.05.
The patient was regarded as the statistical unit. Data were described using mean, standard deviation, median, inter-quartile range (IR) and minimum-maximum values for quantitative variables, and percentage proportions for categorical variables.
Intra-group comparisons for continuous/ordinal variable were performed using the Wilcoxon signed-rank test for paired data. Inter-group comparisons for continuous/ordinal variable were performed using Kruskal-Wallis ANOVA. In case of significance at Kruskal–Wallis test, multiple (post hoc) comparisons of average ranks have been computed; normal z-values were computed for each comparison, as well as post hoc probabilities (corrected for the number of comparisons) for a two-sided test of significance. Inter-group comparisons for binary variable were performed using Maximum-Likelihood Chi-square tests with Yate's correction. The change in the secondary outcome variables was statistically evaluated using Generalized Linear Models (GLZ) adjusted for the significant confounders, followed by ANOVA for repeated measures for inter-group comparisons and with post hoc unequal N HSD test.
Results
4.1 Study Population
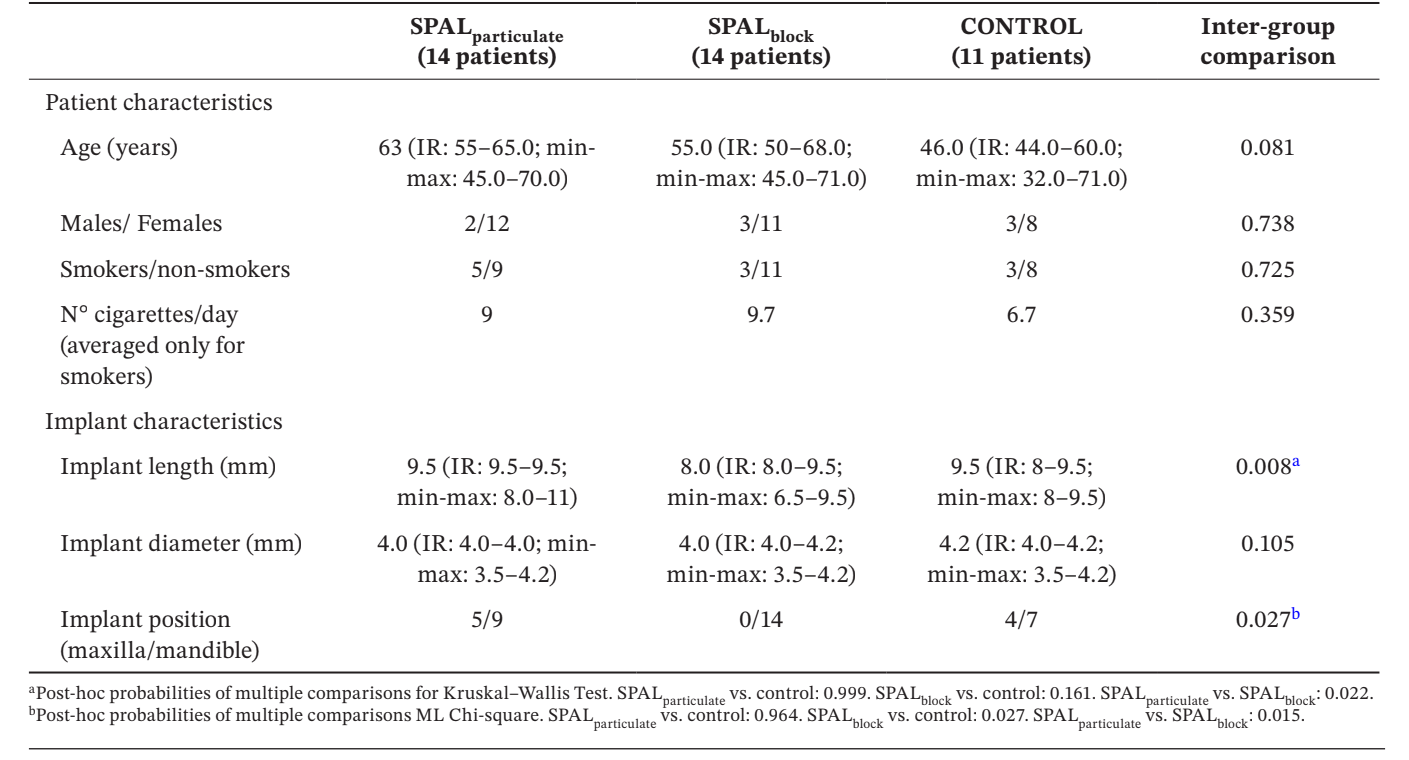
Thirty-nine implants in 39 patients (14 in SPALparticulate group, 14 in SPALblock group and 11 in CONTROL group) were selected for analysis (Table 1). No differences in age, gender and smoking status were observed among groups, the vast majority of the patients being non-smokers. In SPALparticulate and CONTROL groups one third of the implants were located in the maxilla, whereas all implants were located in the mandible in SPALblock group. This difference was statistically significant (p = 0.027). Implants placed in SPALblock group where significantly shorter than those placed in both SPALparticulate and CONTROL groups (p = 0.008).
4.1.1 Post-Operative Healing
In both SPALparticulate and CONTROL groups, early healing was uneventful in all patients.
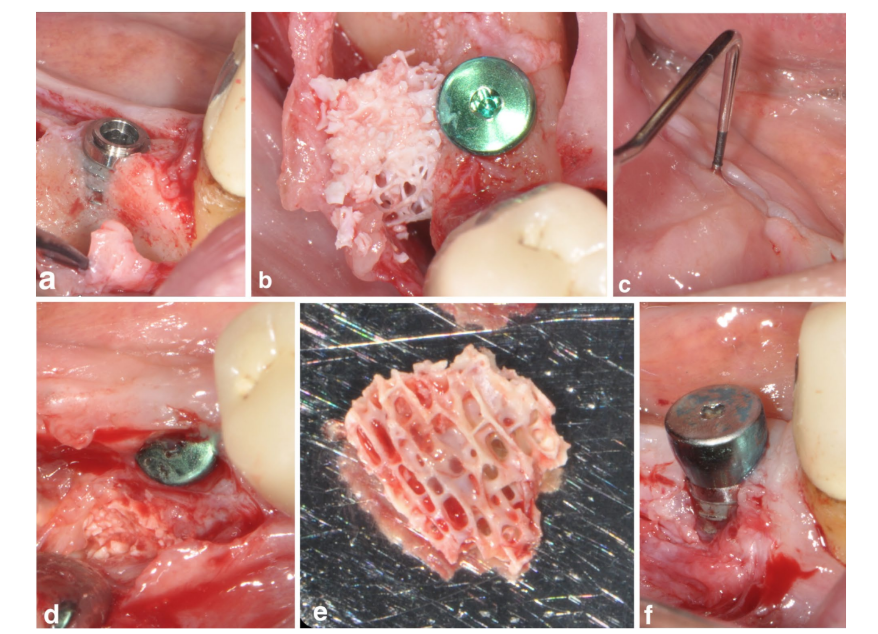
One patient in SPALblock group experienced a post-operative infection at 4 weeks after surgical intervention. The patient reported swelling and pain in the surgically treated area, and a fistula draining purulent exudate was evident (Figure 3a–f). After partial thickness flap elevation, the DBBM block graft was found embedded in granulation tissue and therefore removed.
4.2 Study Outcomes
4.2.1 Primary Outcome
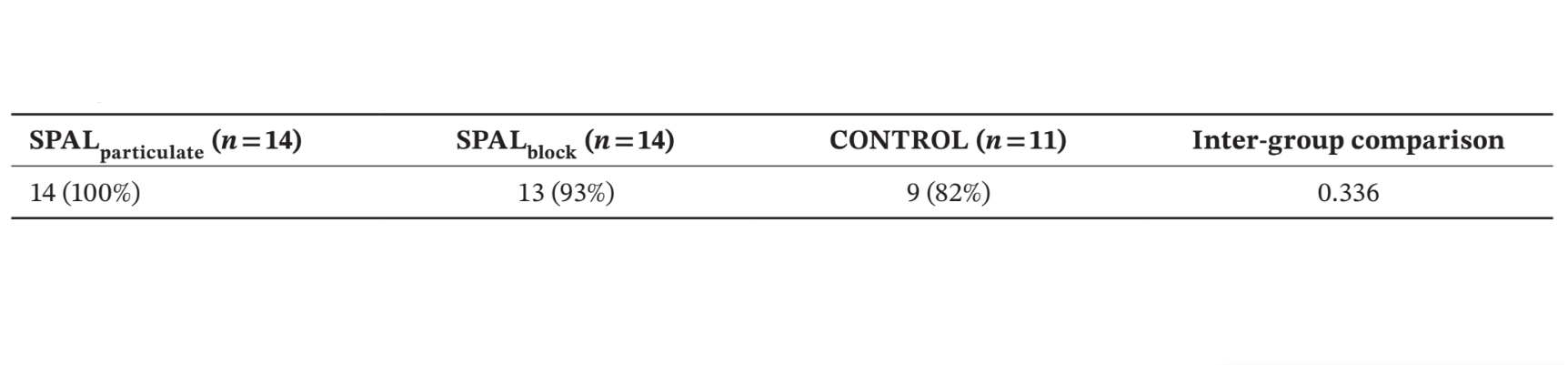
Number and % of patients presenting the rough implant surface covered up to the apical part of the polished collar at re-entry (i.e., no PIBD) is shown in Table 2. No residual defect was found in SPALparticulate group whereas in SPALblock group the patient who had experienced the post-operative graft infection presented a residual PIBD. Two patients in CONTROL group presented an incident PIBD at re-entry (Table 2). The differences among groups were not statistically significant.
4.2.2 Secondary Outcomes
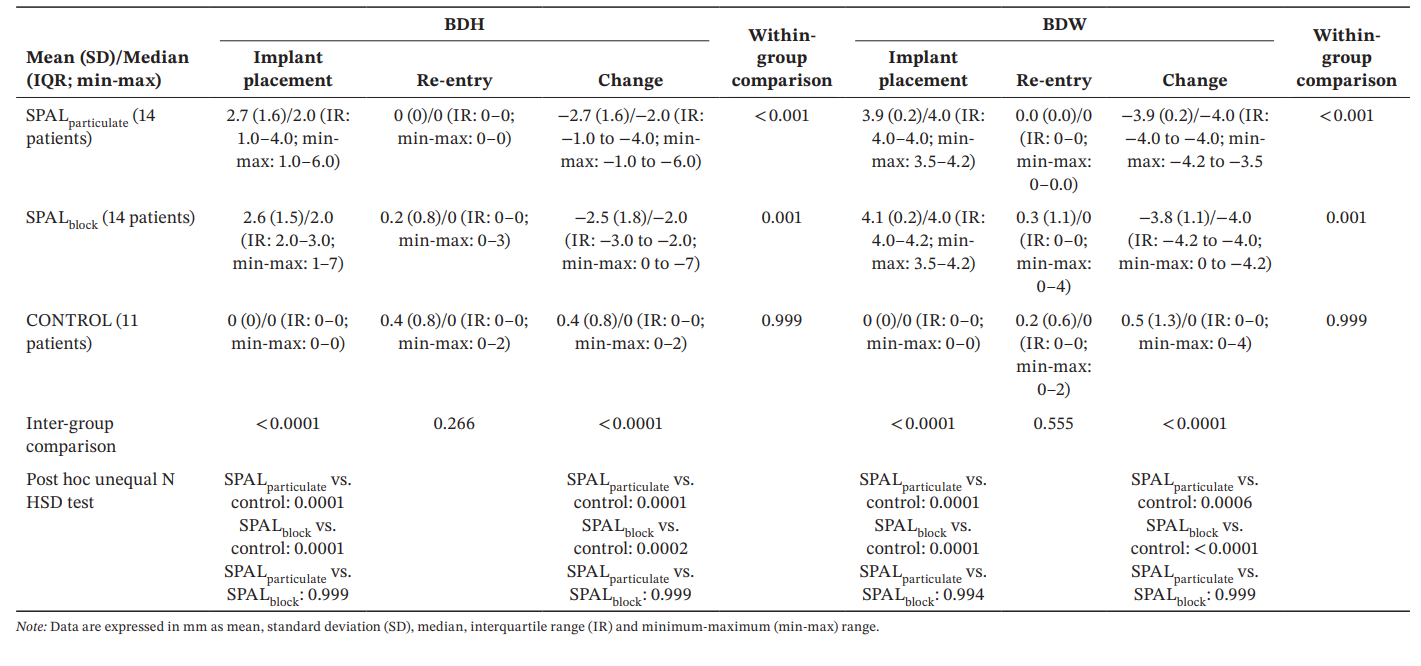
The GLZ model adjusted for the identified confounders (implant length and position) showed that both treatment and time were significant predictors for both BDH and BDW (time, p < 0.0001 for both; treatment, p < 0.001 for both). Within- and inter-group comparisons for BDH and BDW are reported in Table 3. The Wilcoxon test for paired data showed a significant reduction in time for both SPALparticulate (p < 0.001 for both BDH and BDW) and SPALblock groups (p = 0.001 for both BDH and BDW). At implant placement, SPAL groups were both significantly different from CONTROL group (p = 0.0001 for both BDH and BDW). However, no significant differences were detected among groups at re-entry for both BDH and BDW. In SPALblock group the patient with the residual PIBD showed a BDH of 3 mm and a BDW of 4 mm. In CONTROL group the two patients with an incident PIBD at re-entry showed a BDH of 2 mm and BDW of 2 mm and a BDH of 2 mm and BDW of 4 mm, respectively.
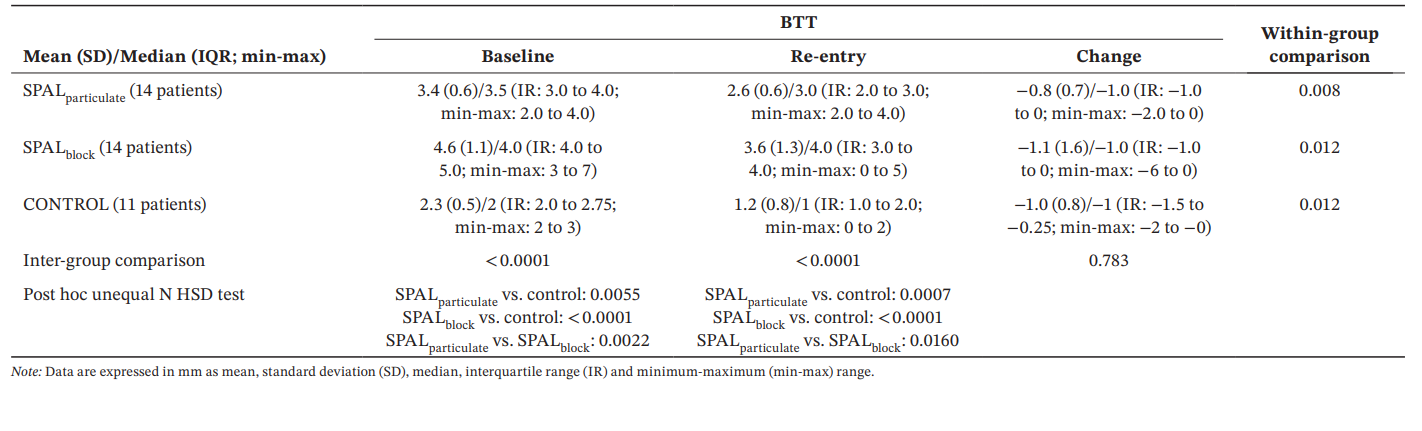
For BTT, the GLZ model adjusted for the identified confounders showed that both treatment and time were significant predictors (p < 0.0001 for both factors). Within- and inter-group comparisons for BDH and BDW are reported in Table 4. A significant difference among groups was observed either at baseline and re-entry (p < 0.0001). In particular, SPALblock group showed a significantly greater values than SPALparticulate either at baseline (p = 0.0022) and re-entry (p = 0.0160). Within-group comparisons showed a significant reduction in BTT over time for all groups, the magnitude of this reduction being similar among groups.
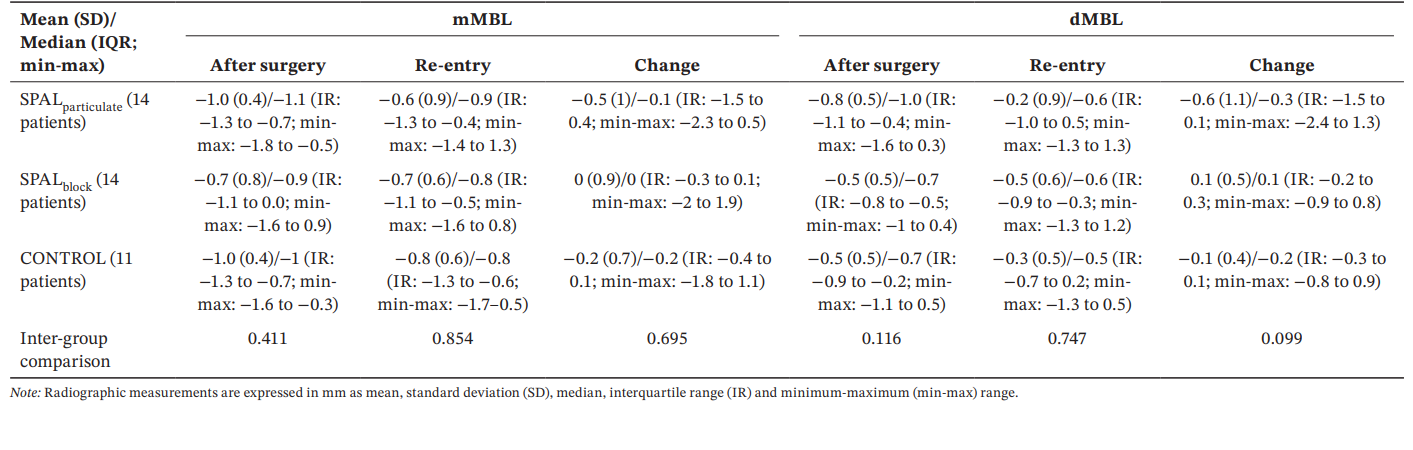
All groups showed similar mMBL and dMBL values after surgery and at re-entry as well as changes over time (Table 5).
Discussion
The aim of the present retrospective study was to evaluate the effectiveness of SPAL technique performed in combination with DBBM in form of either particulate or block to completely correct a PIBD at implant placement. SPAL groups were also compared with a group of patients showing a peri-implant buccal bone plate of at least 2 mm following implant placement. The results showed that, irrespective of DBBM form, implants treated with SPAL technique showed a similarly high rate of patients showing a complete defect correction at 6 months re-entry. SPALblock group showed a significantly greater BTT at both baseline and re-entry. However, the extent of graft/bone remodeling was similar among groups.
The choice of using the rate of complete dehiscence correction as primary outcome was due to the fact that an untreated or partially corrected PIBD may (i) favor the occurrence of a biological complication (Monje et al. 2019; Schwarz, Sahm, and Becker 2012) and (ii) lead to an increased interproximal peri-implant bone loss (Jung et al. 2017). The CONTROL group was included in order to evaluate the extent of remodeling of the two graft biomaterials compared to the native bone.
The effectiveness of SPAL performed in combination with a pDBBM for the treatment of a PIBD was consistent with that reported in previous clinical studies where the same graft particulate was used, showing a rate of complete dehiscence coverage ranging from 80% to 91% (Trombelli et al. 2019, 2020). These findings could be partly ascribed to biological and technical aspects specific to the regenerative procedure. In SPAL technique, the periosteum layer represents a source of osteogenic cells which may (i) favorably contribute new bone formation (Ceccarelli, et al., 2016) and (ii) act as a neo-angiogenic inductor thus providing the early vascularization of the DBBM graft (Nobuto et al. 2005). Furthermore, the creation of a secluded sub-periosteal space may allow for a proper graft accommodation and stabilization at the most coronal portion of the implant (Trombelli et al. 2018, 2019, 2020). Graft stability to support the osteoconductive activity of the graft is also enhanced by means of internal mattress sutures that secure the coronal portion of the periosteal layer to the oral flap. Consistently, a network meta-analysis comparing different treatment options to completely correct a PIBD showed that SPAL with a pDBBM has the highest probability of success among the included procedures (Severi et al. 2022).
Recently, the use of a bDBBM in combination with SPAL was proposed as a promising alternative to a particulate graft (Trombelli et al. 2022). bDBBM was suggested to act as an efficacious osteoconductive scaffold due to its mechanical properties that encompass a limited dislocation at flap manipulation as well as a higher dimensional stability (Benic et al. 2016; Benic et al. 2017; Mir-Mari et al. 2016). The effectiveness of bDBBM is supported by our findings which showed a 93% rate of complete PIBD correction. Moreover, the use of bDBBM resulted in a greater BTT than pDBBM at both base-line and at re-entry. These results are consistent with those stemming from a study where the combination of a bDBBM and a collagen membrane, simultaneously with implant placement, was shown to be superior to the combination of the same membrane and a pDBBM in complete defect resolution and restoration of a thick buccal plate (Benic et al. 2019). It should be, however, stressed that one patient receiving the block graft experienced a post-surgery graft infection leading to a persisting dehiscence at 6-months. Previous studies where a xenogenic bone block was used for a ridge augmentation procedure performed prior to implant placement reported a rate of graft infection and subsequent removal from 33% (Ortiz-Vigón et al. 2017) to 40% (Schwarz et al. 2021) of the treated cases. Preclinical (Benic et al. 2016; Benic et al. 2017) and clinical (Laas, et al., 2020) studies showed that bDBBM was poorly colonized by novel bone when compared to pDBBM. The observed limited bone formation was related to an impaired vascularization of the block due to its macrostructure (Laass et al. 2020). Whether and to what extent the use of a bDBBM might represent a safe and efficacious alternative to pDBBM when combined to SPAL technique needs be further evaluated.
Our study design may provide preliminary insight on the extent of graft remodeling following SPAL procedures with different graft forms compared to bone remodeling after implant placement. Despite SPALblock group showed a significantly greater BTT both after surgery and at re-entry, the magnitude of graft remodeling was similar for the two grafts and compared to native bone. BTT changes in SPALblock group were consistent with previous studies evaluating graft remodeling following GBR procedure performed using a bDBBM in combination with a collagen membrane (Benic et al. 2019). In contrast, the same study showed that the mean reduction in thickness for a pDBBM plus collagen membrane was two-fold greater (2 mm) compared to our data. Differences in BTT changes observed in the two studies may be explained by varying graft remodeling dynamics as well as extent of graft displacement consequent to the specific regenerative procedure. Collectively, these observations suggest the need to over-correct the BTT dimension to compensate the post-surgical horizontal shrinking of the graft irrespectively of the bone augmentation procedures used.
In our material CONTROL group consisted of patients where a PBBP thickness of at least 2 mm was present after implant placement. This choice was based on previous studies showing that PBBP thickness correlates with the incidence of vertical bone loss at buccal plate following implant insertion (Spray et al. 2000) and the consequent need for bone augmentation procedures (Roccuzzo, Imber, and Jensen 2021). Interestingly, a mean reduction in BTT of 1 mm was also observed in CONTROL group which led to two patients (18%) experiencing an incident PIBD at re-entry. Bone remodeling exceeded that reported in previous studies where the reduction in PBBP thickness following implant placement ranged from 0.3 (Merheb et al. 2017) to 0.4 mm (Cardaropoli, Lekholm, and Wennström 2006). Different factors, including 3D implant positioning (Nomiyama et al. 2022), methods for implant site preparation (Baggi et al. 2008; Peker Tekdal et al. 2016), implant design/surface (Linkevicius et al. 2020; Galindo-Moreno et al. 2016; Camarda et al. 2021), anatomical location (anterior/posterior, mandible/maxilla) (Ghaly et al. 2023), thickness of the peri-implant mucosa (Maia et al. 2015; Suárez-López Del Amo et al. 2016), and prosthetic connection (Linkevicius et al. 2015) may have partly accounted for this discrepant observation.
In order to facilitate primary intention closure, tissue level implants had been placed with the coronal margin of the polished collar at the level of the bone crest. In this respect, an impact of implant positioning on the amount of PIBD correction as well as the extent of peri-implant bone remodeling following implant insertion cannot be excluded (Saleh et al. 2018). Moreover, SPAL groups and CONTROL group were re-entered for implant uncovering at different post-surgical periods (6 months vs. 3 months). This difference may have variously impacted on tissue remodeling following implant insertion.
Peri-implant marginal bone loss following implant installation may result from physiological remodeling due to mechanical and thermal trauma during implant site preparation. Interestingly, in our material a limited amount of graft/bone remodeling was similarly observed among groups and was consistent with previous studies where tissue-level implants were either equicrestally placed (Saleh et al. 2018) or treated with SPAL plus pDBBM (Trombelli et al. 2020).
Some limitations of the present study should be considered when interpreting its findings. The choice of a pilot study with a retrospective design was based on the lack of specific clinical indications (i.e., patient/defect related factors) to combine SPAL technique with either DBBM particulate or DBBM block due to the limited, anecdotal evidence (Trombelli et al. 2022) supporting the use of DBBM block in combination with SPAL technique. A specific DBBM formulation was used according to the opera- tor's preference. This unbiased selection of the graft used has resulted in no significant differences in terms of patient and defect characteristics. However, some factors, such as anatomical location and length of implants, were unevenly distributed among groups and, although controlled by the statistical analysis, may have biased the observed results. Since the included patients were consecutively treated during the routine clinical activity, a UNC 15 probe was the regular/standardized tool used to take all the clinical measurements, including PIBD dimensions. Other measurement methods, such as a caliper (Roccuzzo, Imber, and Jensen 2021) or a specifically-designed device (Merheb et al. 2017), could have resulted in more appropriate recordings of dehiscence size and BBT.
In conclusion, our results seem to indicate that SPAL performed in combination with either a particulate or block DBBM graft, is similarly effective in correcting a PIBD as well as in increasing BTT at 6 months.
Mattia Severi, Franzini Chiara, Anna Simonelli, Chiara Scapoli, Leonardo Trombelli
References
- Baggi, L., I. Cappelloni, M. Di Girolamo, F. Maceri, and G. Vairo. 2008. “The Influence of Implant Diameter and Length on Stress Distribution of Osseointegrated Implants Related to Crestal Bone Geometry: A Three-Dimensional Finite Element Analysis.” Journal of Prosthetic Dentistry 100, no. 6: 422–431. https://doi.org/10.1016/S0022-3913(08)60259-0.
- Benic, G. I., B. M. Eisner, R. E. Jung, T. Basler, D. Schneider, and C. H. F. Hämmerle. 2019. “Hard Tissue Changes After Guided Bone Regeneration of Peri-Implant Defects Comparing Block Versus Particulate Bone Substitutes: 6-Month Results of a Randomized Controlled Clinical Trial.” Clinical Oral Implants Research 30: 1016– 1026. https://doi.org/10.1111/clr.13515.
- Benic, G. I., D. S. Thoma, F. Muñoz, I. S. Martin, R. E. Jung, and C. H. F. Hämmerle. 2016. “Guided Bone Regeneration of Peri-Implant Defects With Particulated and Block Xenogeneic Bone Substitutes.” Clinical Oral Implants Research 27, no. 5: 567–576. https://doi.org/10.1111/clr.12625.
- Benic, G. I., D. S. Thoma, I. Sanz-Martin, et al. 2017. “Guided Bone Regeneration at Zirconia and Titanium Dental Implants: A Pilot Histological Investigation.” Clinical Oral Implants Research 28, no. 12: 1592–1599. https://doi.org/10.1111/clr.13030.
- Bressan, E., N. Ferrarese, M. Pramstraller, D. Lops, R. Farina, and C. Tomasi. 2017. “Ridge Dimensions of the Edentulous Mandible in Posterior Sextant: An Observational Study on Cone Beam Computed Tomography Radiographs.” Implant Dentistry 26, no. 1: 66–72. https:// doi.org/10.1097/ID.0000000000000489.
- Camarda, A. J., R. Durand, M. Benkarim, P. H. Rompré, G. Guertin, and H. Ciaburro. 2021. “Prospective Randomized Clinical Trial Evaluating the Effects of Two Different Implant Collar Designs on Peri-Implant Healing and Functional Osseointegration After 25 Years.” Clinical Oral Implants Research 32: 285–296. https://doi.org/10.1111/clr.13699.
- Cardaropoli, G., U. Lekholm, and J. L. Wennström. 2006. “Tissue Alterations at Implant-Supported Single-Tooth Replacements: A 1-Year Prospective Clinical Study.” Clinical Oral Implants Research 17, no. 2: 165–171. https://doi.org/10.1111/j.1600-0501.2005.01210.x.
- Farina, R., A. Simonelli, A. Baraldi, et al. 2021. “Tooth Loss in Complying and Non-complying Periodontitis Patients With Different Periodontal Risk Levels During Supportive Periodontal Care.” Clinical Oral Investigations 25, no. 10: 5897–5906. https://doi.org/10.1007/s00784-021-03895-8.
- Galindo-Moreno, P., A. León-Cano, A. Monje, I. Ortega-Oller, F. O'Valle, and A. Catena. 2016. “Abutment Height Influences the Effect of Platform Switching on Peri-Implant Marginal Bone Loss.” Clinical Oral Implants Research 27, no. 2: 167–173. https://doi.org/10.1111/clr.12554.
- Gallucci, G. O., A. Hamilton, W. Zhou, D. Buser, and S. Chen. 2018. “Implant Placement and Loading Protocols in Partially Edentulous Patients: A Systematic Review.” Clinical Oral Implants Research 29, no. Suppl 16: 106–134. https://doi.org/10.1111/clr.13276.
- Ghaly, M., D. Tarrazzi, V. Xia, S. Tharrington, and T. R. Schoenbaum. 2023. “Changes in Peri-Implant Marginal Bone Level by Jaw Location: A Systematic Review and Meta-Analysis of 4970 Implants.” Journal of Oral Implantology 49, no. 4: 444–455. https://doi.org/10.1563/aaid-joi-D-22-00252.
- Herrera, D., T. Berglundh, F. Schwarz, et al. 2023. “Prevention and Treatment of Peri-Implant Diseases—The EFP S3 Level Clinical Practice Guideline.” Journal of Clinical Periodontology 50, no. Suppl 26: 4–76. https://doi.org/10.1111/jcpe.13823.
- Jensen, S. S., T. Aghaloo, R. E. Jung, et al. 2023. “Group 1 ITI Consensus Report: The Role of Bone Dimensions and Soft Tissue Augmentation Procedures on the Stability of Clinical, Radiographic, and Patient- Reported Outcomes of Implant Treatment.” Clinical Oral Implants Research 34, no. Suppl 26: 43–49. https://doi.org/10.1111/clr.14154.
- Jung, R. E., M. Herzog, K. Wolleb, C. F. Ramel, D. S. Thoma, and C. H. F. Hämmerle. 2017. “A Randomized Controlled Clinical Trial Comparing Small Buccal Dehiscence Defects Around Dental Implants Treated With Guided Bone Regeneration or Left for Spontaneous Healing.” Clinical Oral Implants Research 28, no. 3: 348–354. https://doi.org/10.1111/clr.12806.
- Laass, A., B. M. Eisner, C. H. F. Hämmerle, R. E. Jung, D. S. Thoma, and G. I. Benic. 2020. “Histologic Outcomes After Guided Bone Regeneration of Peri-Implant Defects Comparing Individually Shaped Block Versus Particulate Bone Substitutes.” International Journal of Periodontics and Restorative Dentistry 40, no. 4: 519–527. https://doi.org/10.11607/prd.4575.
- Linkevicius, T., A. Puisys, L. Linkeviciene, V. Peciuliene, and M. Schlee. 2015. “Crestal Bone Stability Around Implants With Horizontally Matching Connection After Soft Tissue Thickening: A Prospective Clinical Trial.” Clinical Implant Dentistry and Related Research 17, no. 3: 497–508. https://doi.org/10.1111/cid.12155.
- Linkevicius, T., A. Puisys, R. Linkevicius, J. Alkimavicius, E. Gineviciute, and L. Linkeviciene. 2020. “The Influence of Submerged Healing Abutment or Subcrestal Implant Placement on Soft Tissue Thickness and Crestal Bone Stability. A 2-Year Randomized Clinical Trial.” Clinical Implant Dentistry and Related Research 22, no. 4: 497–506. https://doi.org/10.1111/cid.12903.
- Maia, L. P., D. M. Reino, V. A. Muglia, et al. 2015. “Influence of Periodontal Tissue Thickness on Buccal Plate Remodelling on Immediate Implants With Xenograft.” Journal of Clinical Periodontology 42: 590–598. https:// doi.org/10.1111/jcpe.12405.
- Merheb, J., M. Vercruyssen, W. Coucke, L. Beckers, W. Teughels, and M. Quirynen. 2017. “The Fate of Buccal Bone Around Dental Implants. A 12-Month Postloading Follow-Up Study.” Clinical Oral Implants Research 28, no. 1: 103–108. https://doi.org/10.1111/clr.12767.
- Mir-Mari, J., H. Wui, R. E. Jung, C. H. F. Hämmerle, and G. I. Benic. 2016. “Influence of Blinded Wound Closure on the Volume Stability of Different GBR Materials: An In Vitro Cone-Beam Computed Tomographic Examination.” Clinical Oral Implants Research 27, no. 2: 258–265. https://doi.org/10.1111/clr.12590.
- Monje, A., V. Chappuis, F. Monje, et al. 2019. “The Critical Peri-Implant Buccal Bone Wall Thickness Revisited: An Experimental Study in the Beagle Dog.” International Journal of Oral & Maxillofacial Implants 34, no. 6: 1328–1336. https://doi.org/10.11607/jomi.7657.
- Monje, A., A. Roccuzzo, D. Buser, and H. L. Wang. 2023. “Influence of Buccal Bone Wall Thickness on the Peri-Implant Hard and Soft Tissue Dimensional Changes: A Systematic Review.” Clinical Oral Implants Research 34, no. 3: 157–176. https://doi.org/10.1111/clr.14029.
- Nobuto, T., F. Suwa, T. Kono, et al. 2005. “Microvascular Response in the Periosteum Following Mucoperiosteal Flap Surgery in Dogs: Angiogenesis and Bone Resorption and Formation.” Journal of Periodontology 76, no. 8: 1346–1353. https://doi.org/10.1902/jop.2005.76.8.1346.
- Nomiyama, L. M., E. K. Matumoto, M. G. Corrêa, et al. 2022. “Comparison Between Flapless-Guided and Conventional Surgery for Implant Placement: A 12-Month Randomized Clinical Trial.” Clinical Oral Investigations 27: 1665–1679. https://doi.org/10.1007/s00784-022-04793-3.
- Ortiz-Vigón, A., I. Suarez, S. Martínez-Villa, I. Sanz-Martín, J. Bollain, and M. Sanz. 2017. “Safety and Performance of a Novel Collagenated Xenogeneic Bone Block for Lateral Alveolar Crest Augmentation for Staged Implant Placement.” Clinical Oral Implants Research 29, no. 1: 36–45. https://doi.org/10.1111/clr.13036.
- Peker Tekdal, G., N. Bostanci, G. N. Belibasakis, and A. Gürkan. 2016. “The Effect of Piezoelectric Surgery Implant Osteotomy on Radiological and Molecular Parameters of Peri-Implant Crestal Bone Loss: A Randomized, Controlled, Split-Mouth Trial.” Clinical Oral Implants Research 27, no. 5: 535–544. https://doi.org/10.1111/clr.12620.
- Pramstraller, M., G. P. Schincaglia, R. Vecchiatini, R. Farina, and L. Trombelli. 2018. “Alveolar Ridge Dimensions in Mandibular Posterior Regions: A Retrospective Comparative Study of Dentate and Edentulous Sites Using Computerized Tomography Data.” Surgical and Radiologic Anatomy 40, no. 12: 1419–1428. https://doi.org/10.1007/s00276-018-2095-0.
- Roccuzzo, A., J. C. Imber, and S. S. Jensen. 2021. “Need for Lateral Bone Augmentation at Two Narrow-Diameter Implants: A Prospective, Controlled, Clinical Study.” Clinical Oral Implants Research 32, no. 4: 511–520. https://doi.org/10.1111/clr.13721.
- Saleh, M. H. A., A. Ravidà, F. Suárez-López Del Amo, G. H. Lin, F. Asa'ad, and H. L. Wang. 2018. “The Effect of Implant-Abutment Junction Position on Crestal Bone Loss: A Systematic Review and Meta-Analysis.” Clinical Implant Dentistry and Related Research 20, no. 4: 617–633. https://doi.org/10.1111/cid.12600.
- Schwarz, F., D. Sahin, S. Civale-Schweighöfer, and J. Becker. 2021. “Long-term Outcomes Following Lateral Alveolar Ridge Augmentation Using a Collagenated Xenogeneic Bone Block: A Monocenter, Prospective Single-arm Clinical Study.” International Journal of Implant Dentistry 7, no. 1: 9. https://doi.org/10.1186/s40729-021-00293-3.
- Schwarz, F., N. Sahm, and J. Becker. 2012. “Impact of the Outcome of Guided Bone Regeneration in Dehiscence-Type Defects on the Long-Term Stability of Peri-Implant Health: Clinical Observations at 4 Years.” Clinical Oral Implants Research 23, no. 2: 191–196. https://doi.org/10.1111/j.1600-0501.2011.02214.x.
