Peri-implant tissue conditions at implants treated with Sub-periosteal Peri-implant Augmented Layer technique: A retrospective case series
Abstract
Objectives: To assess peri-implant tissue conditions on the short term in patients receiving the Sub-periosteal Peri-implant Augmented Layer (SPAL) technique and in patients with adequate thickness (≥2 mm) of the peri-implant buccal bone plate (PBBP) at placement.
Methods: Patients where either a dehiscence defect or thin PBBP at implant placement was corrected by SPAL technique (SPALdehiscence and SPALthin groups, respectively) and patients presenting a residual PBBP thickness ≥2 mm at implant placement (control group) were retrospectively selected. The number of peri-implant sites positive to bleeding on probing (BoP) at 6 months following prosthetic loading was the primary outcome. Also, height of keratinized mucosa, marginal soft tissue level, Plaque Index, peri-implant probing depth, suppuration on probing, and interproximal radiographic bone level (RBL) were evaluated.
Results: Thirty-four patients (11 in the SPALdehiscence group, 11 in the SPALthin group, and 12 in the control group) were included. In each SPAL group, 10 patients (90.9%) showed peri-implant tissue thickness ≥2 mm at the most coronal portion of the im- plant at uncovering. The prevalence (number) of BoP-positive sites was 2, 1, and 0 in the SPALdehiscence , SPALthin, and control groups, respectively. RBL amounted to 0.3 mm in the SPALdehiscence group, 0.2 mm in the SPALthin group, and 0 mm in the control group.
Conclusion: After 6 months of prosthetic loading, patients treated with SPAL technique show limited peri-implant mucosal inflammation in association with shallow PD and adequate KM. At implants receiving SPAL technique, however, interproximal RBL was found apical to its ideal position.
Introduction
Prosthetically driven implant placement in a reduced horizontal bone dimension often results in a peri-implant bone dehiscence or fenestration. Even in the presence of an intact but thin buccal cortical bone plate, surgical trauma and consequent bone remodeling following implant placement may lead to a vertical bone loss with the exposure of the coronal part of the implant at uncovering (Merheb et al., 2017, Monje, et al., 2019, Spray, Black, Morris, 2000). Although the amount of bone remodeling following implant insertion was shown to be similar at both thin and thick buccal bone plates (Merheb et al., 2017), such remodeling may have a different impact on the integrity of peri-implant buccal bone plate (PBBP). In this respect, an increased risk of esthetic and biological complications following implant placement at sites with either a dehiscence defect or a thin PBBP compared to thick PBBP has been shown in preclinical (Monje, et al., 2019) and clinical (Schwarz, Sahm, Becker, 2012, Jung et al., 2017) studies. Collectively, these findings underline the relevance of the integrity and thickness of PBBP at implant placement in favoring stable, healthy conditions of peri-implant tissues over time (Sanz-Sánchez et al., 2018).
The most documented and efficacious procedure to surgically correct a dehiscence-type defect is based on the use of barrier membranes combined with bone replacement grafts according to guided bone regeneration (GBR) principles (Sanz-Sánchez, Ortiz-Vigón, Sanz-Martín, Figuero, Sanz, 2015). The reduction or resolution of peri-implant bone dehiscence reported following GBR (Thoma, Bienz, Figuero, Jung, Sanz-Martín, 2019) seems to positively impact on long-term implant conditions, in terms of implant survival rate and peri-implant tissue stability (Sanz-Sánchez et al., 2018). Unfortunately, whether and to what extent an increased amount of peri-implant bone thickness associated with complete coverage of the exposed implant surface may support peri-implant health has not been entirely elucidated.
Recently, a simplified bone augmentation procedure, namely the Sub-periosteal Peri-implant Augmented Layer (SPAL) technique, based on the use of the periosteum as a barrier membrane and a graft as space-making “device” for bone augmentation concomitant to implant placement, has been described (Trombelli, Severi, Pramstraller, Farina, 2018). The effectiveness of this technique to correct a peri-implant bone dehiscence and/or to augment the thickness of peri-implant bone was previously reported (Trombelli, Severi, Pramstraller, Farina, 2019), and its application has also been explored in the treatment of peri-implantitis defects (Trombelli et al. 2020). The aim of the present retrospective case series was to assess peri-implant tissue conditions on the short term in patients receiving SPAL technique compared to patients with adequate thickness (≥2 mm) of PBBP at implant placement.
Material and methods
2.1 Study design and ethical aspects
The present study was designed in accordance with the STROBE guideline Appendix S1. The protocol was approved by the Ethical Committee of Area Vasta Emilia Centro, Italy (protocol no. 637/2018/ Oss/UniFe, date of approval 12.12.2018). Each patient had provided a written informed consent prior to surgical treatment. All the clinical procedures had been performed in accordance with the Declaration of Helsinki and the Good Clinical Practice (GCP) Guidelines.
2.2 Study population
The record charts of patients undergone implant-supported prosthetic rehabilitation in the period December 2015–July 2018 at the Research Centre for the Study of Periodontal and Peri-implant Diseases, University of Ferrara, and one private dental office in Ferrara were screened to determine patient eligibility for the study. Patient inclusion into the study was subordinated to the following criteria:
- Non-smokers or smokers ≤10 cigarettes/day at the time of surgery;
- Non-diabetics or well-controlled diabetics (HbA1c ≤ 7%) at the time of surgery;
- Availability of clinical parameters and radiographic examinations for the study (see “Study parameters” for details).
- Not taking drugs influencing osseous metabolism (e.g., bisphosphonates, corticosteroids);
- Undergone implant placement entirely in native bone (with a residual PBBP thickness ≥2 mm after implant insertion) or concomitantly with SPAL technique.
Implant inclusion into the study was subordinated to the following criteria:
- Placement in healed ridge (type IV implants, Hämmerle, Chen, Wilson, 2004);
- Primary stability, as assessed by insertion torque.
Based on the conditions of PBBP at the time of implant placement and on its clinical management, patients were categorized into three groups:
- Patients with implant/s presenting a residual PBBP thickness ≥2 mm after implant insertion (control group);
- Patients with implant/s treated with SPAL technique for correcting a peri-implant bone dehiscence ≥3 mm concomitantly with implant placement (SPALdehiscence group);
- Patients with implant/s treated with SPAL technique for augmenting a thin (≤1 mm) PBBP concomitantly with implant placement (SPALthin group).
2.3 Clinical procedures
Prior to implant placement, all patients had undergone active therapy for treating carious lesions and periodontal diseases and had been enrolled in a professional maintenance with frequency of recalls scheduled according to the PerioRisk assessment tool (Trombelli, Farina, Ferrari, Pasetti, Calura, 2009, Trombelli et al., 2017).
All the surgical procedures were performed by two experienced periodontists (L.T. and M.P.). Patients were administered 2 g of amoxicillin + clavulanic acid (Augmentin, GlaxoSmithKline) one hour prior to surgery. Local anesthesia was attained using articaine with 1:100,000 epinephrine administered by local infiltration.
2.3.1 Surgical procedures—SPAL groups
In patients where either a dehiscence defect or thin PBBP at placement was corrected by SPAL technique (Figures 1 and 2, respectively), surgical access to the bone crest was performed as previously described (Trombelli, Severi, Pramstraller, & Farina, 2018). Briefly, a mucosal layer was raised on the buccal aspect by split-thickness dissection with a 15C blade as well as tunneling knives (KPAX, TKN1X, and TKN2X, Hu-Friedy) with varying angulated sharp edges according to the anatomical location. Then, the periosteal layer was elevated from the bone with a periosteal elevator (PTROM, Hu-Friedy), creating a pouch that could accommodate a graft. A full-thickness flap was elevated on the oral (lingual/palatal) aspect.
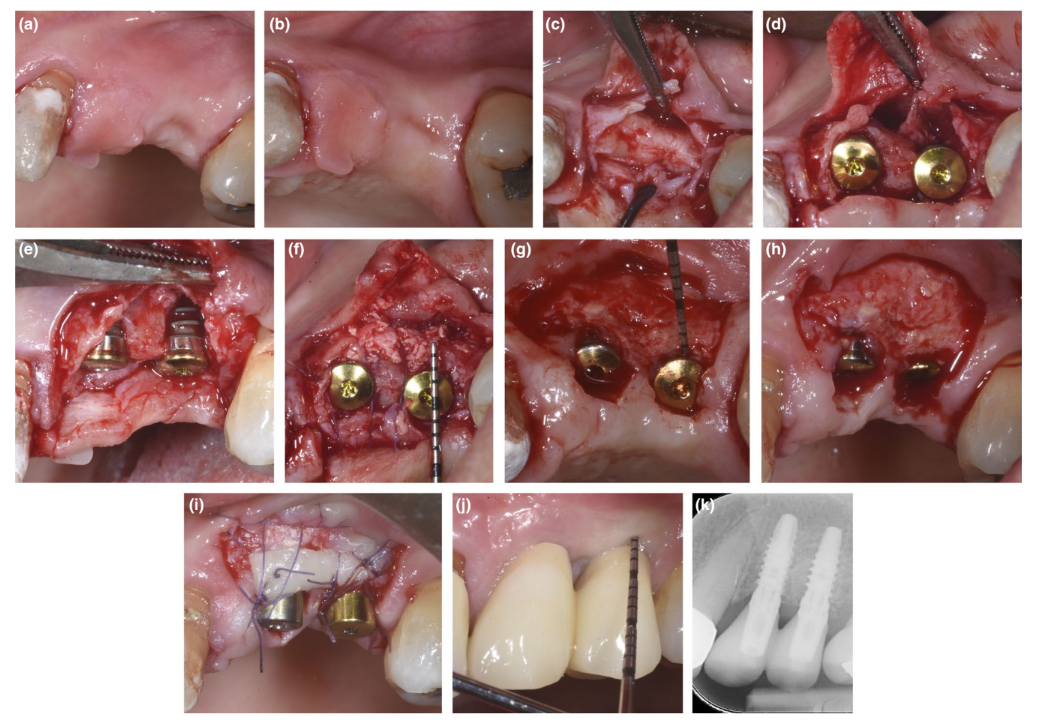
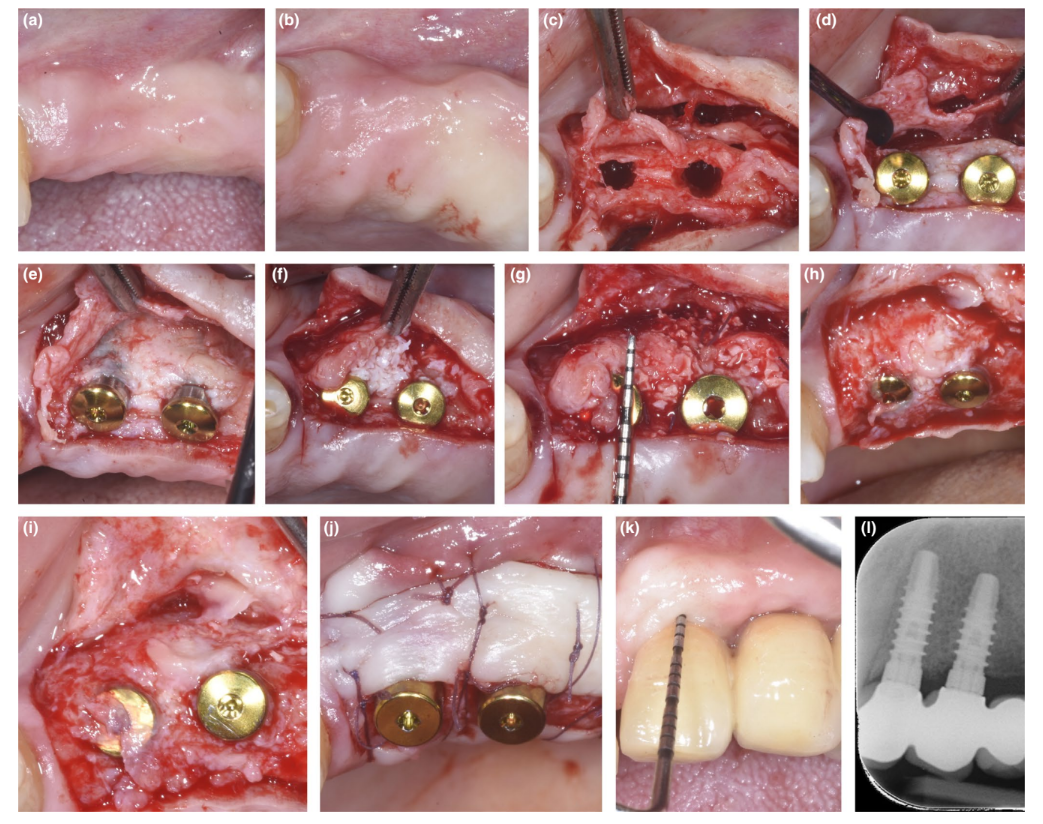
Tissue-level implants (SPI Element™; Thommen Medical) were inserted. A bovine-derived xenograft (Bio-Oss® spongiosa gran- ules, particle size 0.25–1.0 mm; Geistlich Pharma, AG) was used alone or in combination with autogenous cortical bone particles to fill the surgically created space between the periosteal layer and either thin buccal bone plate or exposed implant surface. In the presence of a dehiscence, grafting was performed to completely correct the peri-implant defect up to the polished collar. In all cases, the sub-periosteal graft provided at least 2 mm of thickness at the most coronal portion of the implant.
The coronal portion of the periosteal layer was stabilized to the oral mucoperiosteal flap by means of resorbable internal mattress sutures (Vicryl 6/0, Ethicon). The mucosal layer was then coronally advanced and sutured tension-free by horizontal internal mattress and interrupted sutures to submerge both graft and implants.
At re-entry procedure for implant uncovering, a buccal split-thickness flap was dissected to position the healing abutment. To provide adequate dimensions of keratinized peri-implant mucosa, either an apically positioned flap or a free gingival graft was performed (Trombelli, Severi, Pramstraller, & Farina, 2019).
2.3.2 Surgical procedures—control group
A buccal and lingual/palatal full-thickness flap was raised to expose the bone crest. The implant site was prepared according to the manufacturer's instructions, and tissue-level implants (SPI Element™; Thommen Medical) were inserted. Due to the presence of a residual PBBP thickness ≥2 mm, no bone augmentation procedure was performed. In all cases, the flap was trimmed and positioned around the healing abutment by resorbable sutures (Vicryl 6/0, Ethicon). Flap design and manipulation as well as suture technique were performed to ensure adequate dimensions (height, thickness) of keratinized peri-implant mucosa.
2.3.3 Postoperative procedures
Patients were instructed not to wear any removable prostheses to avoid compression onto the surgical site for at least 4 weeks and not to chew or brush in the treated area for approximately 2 weeks.
The home use of a 0.12% chlorhexidine solution (Curasept ADS Trattamento Rigenerante®; Curaden Healthcare) was prescribed for chemical plaque control (1-min rinse b.i.d. for 3 weeks). Sutures were removed at 2 weeks post-surgery.
2.3.4 Timing of prosthetic rehabilitation
Prosthetic rehabilitation was started at 3–4 months after implant placement in the control group whereas at least 4 weeks following implant uncovering in the SPAL groups.
2.4 Study parameters
2.4.1 Clinical parameters
After 6 months of prosthetic loading, a trained examiner (M.S.) who had been involved in previous studies on the SPAL technique (Trombelli, Severi, Pramstraller, Farina, 2019) performed the following clinical measurements with a UNC-15 periodontal probe in the following chronological sequence:
- Height of keratinized mucosa (KM): measured at the mid-buccal aspect of the implant as the distance between the buccal peri-implant mucosal margin and the mucogingival junction, and recorded to the nearest millimeter;
- Marginal soft tissue level (MSTL) (Zitzmann, Schärer, Marinello, 2001): measured at the mid-buccal aspect of the implant as the distance between the buccal peri-implant mucosal margin and the implant-abutment junction, and recorded to the nearest millimeter. MSTL was recorded as positive or negative when the abutment margin was located above or below the mucosal margin, respectively;
- Plaque Index (PlI; O'Leary, Drake, Naylor, 1972): recorded at the mesiobuccal, mid-buccal, distobuccal, mid-lingual/palatal implant aspects as supragingival plaque present or absent after exploring the juxtagingival prosthetic margin with the probe tip;
- Probing depth (PD): measured from mucosal margin to deepest probe penetration at six sites (mesiobuccal, mid-buccal, distobuccal, disto-lingual, mid-lingual, and mesiolingual) using a force of 0.2–0.3 N, and recorded to the nearest millimeter;
- Bleeding on probing (BoP; Ainamo and Bay, 1975): recorded as present or absent at PD assessment;
- Suppuration on probing (SoP): recorded as present or absent at PD assessment.
2.4.2 Radiographic bone level
Non-standardized periapical radiographs taken with the long-cone parallel technique at 6 months after prosthetic loading were digitized and analyzed using a specifically designed software (NIS elements v4.2; Nikon Instruments, Campi Bisenzio). Radiographic bone level (RBL) was measured as the distance (approximated to the nearest 0.1 mm) between the apical margin of the implant polished collar and the bone crest at the mesial (mRBL) and distal (dRBL) aspect of each implant using a 10x–15x magnification. A reference mark 1-mm high present on digital radiograph was used for calibration.
One examiner (A.S.) performed the radiographic measurements. The examiner was involved in a calibration session on a sample of radiographs obtained from patients not selected for the present study. The calibration session consisted of two sessions of RBL measurements, performed at a 7-day interval, and allowed for reaching an excellent intra-examiner agreement (k score = 0.89), with a mean difference between paired measurements of 0.04 ± 0.15 mm.
2.5 Statistical analysis
The patient was regarded as the statistical unit. If two or more implants in the same patient were eligible for the study, only one implant was randomly included for analysis. Data were described using mean and standard deviation (SD), median and interquartile range (IR), minimum–maximum values for quantitative variables, and frequency and percentage for categorical variables.
The median number of BoP-positive sites as assessed at 6 months following implant loading was the primary outcome variable of the study. Median values of PD, KM, MSTL, RBL, number of PlI-positive sites, and number of SoP-positive sites were secondary outcome variables.
Due to the limited sample size, no inferential statistics were performed and the results were reported with a narrative approach. However, effect size (ES) was computed for each outcome variable according to non-parametric Kruskal–Wallis test. ES was classified as small (d = 0.1–0.3), medium (d = 0.3–0.5), or large (d ≥ 0.5) (Cohen, 1988).
Results
3.1 Study population
Thirty-four patients with 34 implants (11 in the SPALdehiscence group, 11 in the SPALthin group, and 12 in the control group) were included for analysis. The vast majority of the patients were non-smokers (90.9% in the SPALdehiscence group, 90.9% in the SPALthin group, and 75% in the control group). Implants in the SPALdehiscence group were predominantly located in the mandible, whereas implants in the SPALthin and control group were predominantly placed in the maxilla (Table 1). No patients or implants were lost during the follow-up period.
In the SPALdehiscence group, 1 patient revealed wound dehiscence after 2 weeks, with partial exposure of the implant threads. The patient was seen monthly until re-entry, and the site was locally disinfected with a 0.12% chlorhexidine solution at each recall visit.
In both the SPALdehiscence and SPALthin groups, re-entry was performed at 3–6 months after implant placement (median: 4.0 months in both groups; p = 1; Table 1). Thickness of peri-implant bone and height and width of the peri-implant bone dehiscence recorded for the SPALdehiscence and SPALthin groups are reported in Tables 2 and 3, respectively. In each SPAL group, 10 patients (90.9%) showed absence of peri-implant dehiscence combined with peri-implant bone thickness ≥2 mm (Tables 2 and 3). One patient in the SPALdehiscence group presented a residual dehiscence of 2 mm (Table 2), which was covered with a free gingival graft. One patient in the SPALthin group presented a peri-implant bone thickness of 1 mm without dehiscence (Table 3).
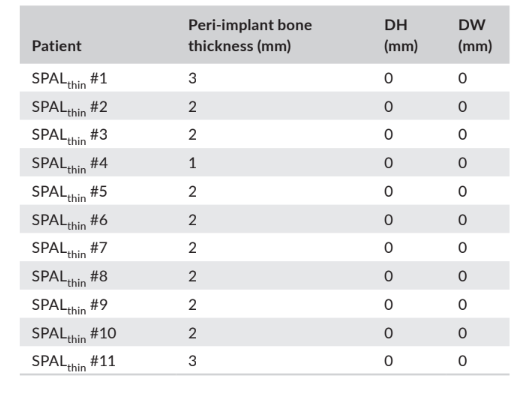
and width (DH and DW, respectively) as assessed at re-entry for
implant uncovering in each patient of the SPALthin group
In the SPALdehiscence group, 8 implants supported a fixed partial prosthesis, 2 implants were restored with a single crown, and 1 implant was part of an overdenture. In the SPALthin group, 9 implants supported a fixed partial prosthesis, 2 implants were restored with a single crown, and 1 was part of an overdenture. In the control group, 4 implants were part of a fixed partial prosthesis and 8 implants were restored with a single crown.
3.2 Study outcomes
Data related to clinical outcomes (i.e., PD, BoP, SoP, PlI, MSTL, and KM) and RBL as assessed at 6 months following implant loading are reported in Table 4.
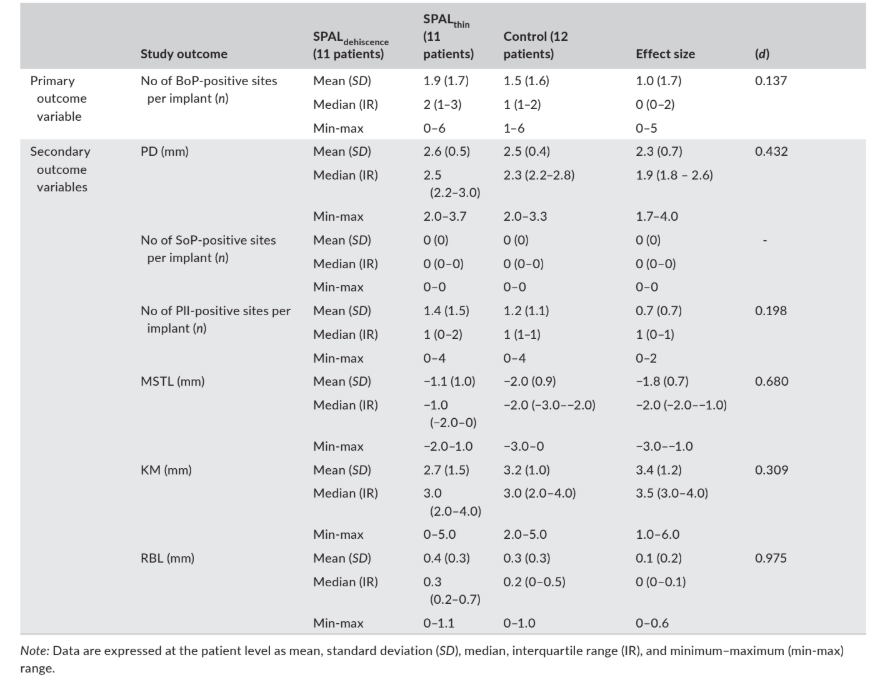
soft tissue level, MSTL; and width of keratinized mucosa, KM) and radiographic bone level (RBL) as assessed at 6 months following implant loading.
The median prevalence (number) of BoP-positive sites was 2, 1, and 0 in the SPALdehiscence, SPALthin, and control groups, respectively.
The median number of PlI-positive sites was 1 in all groups. SoP was negative at all implant sites.
The mucosal margin was located 1 mm (SPALdehiscence group) or 2 mm (SPALthin and control groups) above the implant–abutment junction in all groups, and study groups presented a median KM of at least 3 mm.
Radiographic bone level amounted to 0.3 mm in the SPALdehiscence group, 0.2 mm in the SPALthin group, and 0 mm in the control group.
ES was small for the number of BoP + sites (d = 0.137) and PlI (d = 0.198), medium for KM (d = 0.309), PD (d = 0.432) and MSTL (d = 0.680), and large for RBL (d = 0.975) (Table 4).
Discussion
The aim of the present retrospective case series was to assess peri-implant tissue conditions on the short term at patients receiving SPAL technique and in patients with adequate thickness (≥2 mm) of PBBP at implant placement. The results indicated that patients treated with SPAL technique showed low number of peri-implant inflamed sites and shallow PD (<4 mm) at 6 months of prosthetic loading. Also, the interproximal bone level was found apical (although to a limited extent) to the implant polished collar only in SPAL groups.
Bleeding on probing was selected as primary outcome since (a) the assessment of BoP is currently identified as the clinical measure to distinguish between peri-implant health and disease, being an invariable diagnostic element of peri-implant mucositis and peri-implantitis (Renvert, Persson, Pirih, Camargo, 2018, Berglundh et al., 2018), and (b) its absence is associated with stability of peri-implant tissue conditions (Jepsen, Rühling, Jepsen, Ohlenbusch, Albers, 1996, Luterbacher, Mayfield, Brägger, Lang, 2000). The proportion of inflamed peri-implant sites as recorded in the study groups compares with previous findings evaluating BoP prevalence on 289 implants (Farina, Filippi, Brazzioli, Tomasi, Trombelli, 2017). Also, similar peri-implant inflammatory conditions were reported at 18 months following GBR (Jung et al., 2017).
In our study, a low frequency of inflamed peri-implant mucosal sites was observed in all study groups. This may be partly due to simi- lar characteristics for factors shown to influence BoP around implants, such as low presence of juxtagingival plaque (Pontoriero et al., 1994; Salvi et al., 2012), shallow PD (Farina, Filippi, Brazzioli, Tomasi, & Trombelli, 2017), and adequate amount of KM (Chung, Oh, Shotwell, Misch, Wang, 2006, Perussolo et al., 2018). Our findings are consistent with those stemming from a recent systematic review on biological complications of dental implants placed either in pristine or in augmented sites. Meta-analysis showed a similar prevalence of peri-implant mucositis at patient either receiving (19.6%; 95% CI: 0%–40%) or not receiving (22.4%; 95% CI: 6%–38%) procedures for alveolar ridge preservation and/or vertical/lateral ridge augmentation (Salvi, Monje, Tomasi, 2018). Also, similar inflammatory conditions were reported at implants placed in native bone compared to implants placed concomitantly with a GBR procedure (Benic, Jung, Siegenthaler, Hammerle, 2009; Benic, Bernasconi, Jung, Hammerle, 2017).
It is noteworthy to consider that at re-entry, the great majority of patients receiving SPAL technique showed a peri-implant bone thickness ≥2 mm at the most coronal portion of the implant. Although the measurement of PBBP was not available at re-entry for the control group (one-stage procedure), the integrity of PBBP following post-insertion peri-implant bone remodeling may be assumed based on preclinical (Monje, et al., 2019) and clinical (Spray, Black, Morris, & Ochi, 2000) data on critical dimensions of buccal bone plate. Collectively, available data seem to suggest that adequate vertical and horizontal dimensions of peri-implant tissues achieved by means of augmentation procedures may favor conditions to limit peri-implant tissue inflammation. However, the association of the integrity of PBBP up to the most coronal portion of the implant and the severity of peri-implant mucosal inflammation is not entirely clear (Jung et al., 2017).
At 6 months of prosthetic loading, a different position of the interproximal peri-implant bone level was observed among groups, with a more apical RBL in the SPAL groups. Noteworthy, in the SPAL groups tissue-level implants were positioned slightly subcrestally (Figures 1 and 2). Although it may have facilitated the grafting of the periosteal pouch up to the most coronal part of the implant as well as primary intention closure, subcrestal positioning might also have contributed interproximal bone remodeling (Saleh et al., 2018). Moreover, since implants receiving SPAL technique underwent additional surgery for uncovering including an apically positioned flap or a free gingival graft, interproximal bone remodeling in the SPAL groups may be also partly ascribed to the detrimental effect of flap elevation on local blood supply. Consistently, marginal, peri-implant bone loss has been reported between re-entry for uncovering and final prosthesis delivery by other authors (Cardaropoli, Lekholm, Wennstrom, 2006, Nader et al., 2016). It should also be considered that, in some patients of the SPALdehiscence group, grafting was extended to the mesial and/or distal implant aspects due to an interproximal extension of the peri-implant bone defect. In the SPALdehiscence group, therefore, the extent of graft remodeling at interproximal sites may have negatively impacted on RBL values. Recent data have shown that even slowly resorbable graft biomaterials, such as DBBM, are associated with a substantial reduction of the grafted area at 12-month radiographic evaluation following endosinusal augmentation procedures (Franceschetti et al., 2019). However, the magnitude of RBL observed in the present study is lim- ited compared to that reported for implants placed with concomitant GBR or in native bone (Urban et al., 2019) and implants presenting an untreated buccal dehiscence (Jung et al., 2017).
A slightly lower KM and MSTL was observed for the SPALdehiscence group. This occurred despite peri-implant soft tissue manipulation was adequately performed to provide adequate dimensions of keratinized peri-implant mucosa and a subgingival position of the prosthetic margins. This finding may be somewhat correlated with the increased bone remodeling (RBL) observed in the SPALdehiscence group, which may also have involved the regenerated buccal bone plate. A recent systematic review correlated the remodeling of the buccal bone with the occurrence of peri-implant soft tissue recession (Aizcorbe-Vicente, Peñarrocha-Oltra, Canullo, Soto-Peñaloza, & Peñarrocha-Diago, 2020). In the SPALdehiscence group, 1 patient (9.1%) experienced a wound dehiscence at 2 weeks that lead to partial exposure of the implant threads at re-entry. This finding compares with incidence of wound dehiscence and consequent membrane exposure following GBR procedures to correct peri-implant bone dehiscence at placement, as reported in a recent meta-analysis conducted on both prospective and retrospective studies (Garcia et al., 2018). In particular, membrane exposure occurred with an incidence ranging from 16.7% (Tawil, El-Ghoule, & Mawla, 2001) to 62.8% (Gher, Quintero, Assad, Monaco, & Richardson, 1994), and was associated with a significantly lower dehiscence coverage (Garcia et al., 2018). The limitations of this preliminary report include the retrospective design, small sample size, and short follow-up time of 6 months after restoration of the implants. Also, the impact of patient-related factors (e.g., soft tissue thickness at edentulous area, smoking habit, diabetes) and surgery-related complications (e.g., perforations of the periosteal and/or mucosal layer) on clinical outcomes has not been comprehensively analyzed. Moreover, specific clinical conditions (i.e., thin PBBP or peri-implant bone dehiscence of limited vertical dimension) have been selected for SPAL treatment. Further studies are needed to assess which clinical conditions/lesions may be effectively treated with SPAL technique or a more conventional treatment (e.g., GBR) should be preferred.
In conclusion, the results of the present study showed that, after 6 months of prosthetic loading, patients treated with SPAL technique show limited peri-implant mucosal inflammation in association with shallow PD and adequate KM. At implants receiving SPAL technique, however, interproximal RBL was found apical to its ideal position. Whether and to what extent the favorable short-term results observed following SPAL technique might be beneficial for long-term healthy conditions of peri-implant tissues and stability of the buccal mucosal profile needs to be assessed.
Leonardo Trombelli, Mattia Pramstraller, Mattia Severi, Anna Simonelli, Roberto Farina
References
- Ainamo, J., & Bay, I. (1975). Problems and proposals for recording gingivitis and plaque. International Dental Journal, 25, 229–235.
- Aizcorbe-Vicente, J., Peñarrocha-Oltra, D., Canullo, L., Soto-Peñaloza, D., & Peñarrocha-Diago, M. (2020). Influence of facial bone thickness after implant placement into the healed ridges on the remodeled facial bone and considering soft tissue recession: A systematic review. International Journal of Oral and Maxillofacial Implants, 35, 107–119. https://doi.org/10.11607/jomi.7259
- Benic, G. I., Bernasconi, M., Jung, R. E., & Hammerle, C. H. F. (2017). Clinical and radiographic intra-subject comparison of implants placed with or without guided bone regeneration: 15-year results. Journal of Clinical Periodontology, 44, 315–325. https://doi.org/10.1111/jcpe.12665
- Benić, G. I., Jung, R. E., Siegenthaler, D. W., & Hämmerle, C. H. F. (2009). Clinical and radiographic comparison of implants in regenerated or native bone: 5-year results. Clinical Oral Implant Research, 20, 507–513. https://doi.org/10.1111/j.1600-0501.2008.01583.x
- Berglundh, T., Armitage, G., Araujo, M. G., Avila-Ortiz, G., Blanco, J., Camargo, P. M., Zitzmann, N. (2018). Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl 20), S286–S291. https://doi.org/10.1034/j.1600-0501.1994.050409.x
- Cardaropoli, G., Lekholm, U., & Wennstrom, J. L. (2006). Tissue alterations at implant-supported single-tooth replacements: A 1-year prospective clinical study. Clinical Oral Implant Research, 17, 165–171. https://doi.org/10.1111/j.1600-0501.2005.01210.x
- Chung, D. M., Oh, T. J., Shotwell, J. L., Misch, C. E., & Wang, H. L. (2006). Significance of Keratinized mucosa in maintenance of dental implants with different surfaces. Journal of Periodontology, 77, 1410–1420. https://doi.org/10.1902/jop.2006.050393
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences, 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associated Publishers.
- Farina, R., Filippi, M., Brazzioli, J., Tomasi, C., & Trombelli, L. (2017). Bleeding on probing around dental implants: A retrospective study of associated factors. Journal of Clinical Periodontology, 44, 115–122. https://doi.org/10.1111/jcpe.12647
- Franceschetti, G., Farina, R., Minenna, L., Riccardi, O., Stacchi, C., Di Raimondo, R., … Trombelli, L. (2019). The impact of graft remodeling on peri-implant bone support at implants placed concomitantly with transcrestal sinus floor elevation: A multicenter, retrospective case series. Clinical Oral Implant Research, 31(2), 1–16. https://doi.org/10.1111/clr.13541
- Garcia, J., Dodge, A., Luepke, P., Wang, H. L., Kapila, Y., & Lin, G. H. (2018). Effect of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clinical Oral Implants Research, 29, 328–338. https://doi.org/10.1111/clr.13121
- Gher, M. E., Quintero, G., Assad, D., Monaco, E., & Richardson, A. C. (1994). Bone grafting and guided bone regeneration for immediate dental implants in humans. Journal of Periodontology, 65, 881–891. https://doi.org/10.1902/jop.1994.65.9.881
- Hämmerle, C. H., Chen, S. T., & Wilson, T. G. Jr (2004). Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. International Journal of Oral and Maxillofacial Implants, 19(suppl), 26–28.
- Jepsen, S., Rühling, A., Jepsen, K., Ohlenbusch, B., & Albers, H. K. (1996). Progressive peri-implantitis. Incidence and prediction of peri-implant attachment loss. Clinical Oral Implants Research, 7, 133–142. https://doi.org/10.1034/j.1600-0501.1996.070207.x
- Jung, R. E., Herzog, M., Wolleb, K., Ramel, C. F., Thoma, D. S., & Hammerle, C. H. F. (2017). A randomized controlled clinical trial comparing small buccal dehiscence defects around dental implants treated with guided bone regeneration or left for spontaneous healing. Clinical Oral Implants Research, 28, 348–354. https://doi.org/10.1111/clr.12806
- Luterbacher, S., Mayfield, L., Brägger, U., & Lang, N. P. (2000). Diagnostic characteristics of clinical and microbiological tests for monitoring periodontal and peri-implant mucosal tissue conditions during supportive periodontal therapy (SPT). Clinical Oral Implants Research, 11, 521–529. https://doi.org/10.1034/j.1600-0501.2000.011006521.x
- Merheb, J., Vercruyssen, M., Coucke, W., Beckers, L., Teughels, W., & Quirynen, M. (2017). The fate of buccal bone around dental implants. A 12-month postloading follow-up study. Clinical Oral Implants Research, 28, 103–108. https://doi.org/10.1111/clr.12767
- Monje, A., Chappuis, V., Monje, F., Muñoz, F., Wang, H. L., Urban, I. A., & Buser, D. (2019). The Critical Peri-implant Buccal Bone Wall Thickness Revisited: An Experimental Study in the Beagle Dog. Int J Oral Maxillofac Implants, 34, 1328–1336. https://doi.org/10.11607/jomi.7657
- Nader, N., Aboulhosn, M., Berberi, A., Manal, C., & Younes, R. (2016). Marginal bone remodeling around healing abutment vs final abutment placement at second stage surgery: A 12-month randomized clinical trial. Journal of Contemporary Dental Practice, 17, 7–15. https://doi.org/10.5005/jp-journals-10024
- O'Leary, T. J., Drake, R. B., & Naylor, J. E. (1972). The plaque control record. Journal of Periodontology, 43, 38. https://doi.org/10.1902/jop.1972.43.1.38
- Perussolo, J., Souza, A. B., Matarazzo, F., Oliveira, R. P., & Araújo, M. G. (2018). Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: A 4-year follow-up study. Clinical Oral Implant Research. Journal of Clinical Periodontology, 29, 1177–1185. https://doi.org/10.1111/clr.13381
- Pontoriero, R., Tonelli, M. P., Carnevale, G., Mombelli, A., Nyman, S. R., & Lang, N. P. (1994). Experimentally induced peri-implant mucositis. A clinical study in humans. Clinical Oral Implants Research, 5, 254–259. https://doi.org/10.1034/j.1600-0501.1994.050409.x
- Renvert, S., Persson, G. R., Pirih, F. Q., & Camargo, P. M. (2018). Peri- implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. Journal of Clinical Periodontology, 45(Suppl 20), S278–S285. https://doi.org/10.1111/jcpe.12956
- Saleh, M. H. A., Ravidà, A., Suárez-López Del Amo, F., Lin, G.-H., Asa'ad, F., & Wang, H.-L. (2018). The effect of implant-abutment junction position on crestal bone loss: A systematic review and meta-analysis. Clinical Implant Dentistry and Related Research, 20, 617–633. https://doi.org/10.1111/cid.12600
- Salvi, G. E., Aglietta, M., Eick, S., Sculean, A., Lang, N. P., & Ramseier, C. A. (2012). Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clinical Oral Implants Research, 23, 182–190. https://doi.org/10.1111/j.1600-0501.2011.02220.x
- Salvi, G. E., Monje, A., & Tomasi, C. (2018). Long-term biological complications of dental implants placed either in pristine or in augmented sites: A systematic review and meta-analysis. Clinical Oral Implant Research, 29(Suppl. 16), 294–310. https://doi.org/10.1111/clr.13123
- Sanz-Sánchez, I., Carrillo de Albornoz, A., Figuero, E., Schwarz, F., Jung, R., Sanz, M., & Thoma, D. (2018). Effects of lateral bone augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clinical Oral Implants Research, 29, 18–31. https://doi.org/10.1111/clr.13126
- Sanz-Sánchez, I., Ortiz-Vigón, A., Sanz-Martín, I., Figuero, E., & Sanz, M. (2015). Effectiveness of lateral bone augmentation on the alveolar crest dimension: A systematic review and meta-analysis. Journal of Dental Research, 94, 1s–15s. https://doi.org/10.1177/0022034515594780
- Schwarz, F., Sahm, N., & Becker, J. (2012). Impact of the outcome of guided bone regeneration in dehiscence-type defects on the long-term stability of peri-implant health: Clinical observations at 4 years. Clinical Oral Implants Research, 23, 191–196. https://doi.org/10.1111/j.1600-0501.2011.02214.x
- Spray, J. R., Black, C. G., Morris, H. F., & Ochi, S. (2000). The influence of bone thickness on facial marginal bone response: Stage 1 placement through stage 2 uncovering. Annals of Periodontology, 5, 119–128. https://doi.org/10.1902/annals.2000.5.1.119
- Tawil, G., El-Ghoule, G., & Mawla, M. (2001). Clinical evaluation of a bilayered collagen membrane (bio-gide) supported by autografts in the treatment of bone defects around implants. The International Journal of Oral & Maxillofacial Implants, 16, 857–863.
- Thoma, D. S., Bienz, S. P., Figuero, E., Jung, R. E., & Sanz-Martín, I. (2019). Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Journal Clinical Periodontology, 46(Suppl 21), 257–276. https://doi.org/10.1111/jcpe.13050
- Trombelli, L., Farina, R., Ferrari, S., Pasetti, P., & Calura, G. (2009). Comparison between two methods for periodontal risk assessment. Minerva Stomatologica, 58, 277–287.
- Trombelli, L., Minenna, L., Toselli, L., Zaetta, A., Checchi, L., Checchi, V., … Farina, R. (2017). Prognostic value of a simplified method for periodontal risk assessment during supportive periodontal therapy. Journal of Clinical Periodontology, 44, 51–57. https://doi.org/10.1111/jcpe.12645
- Trombelli, L., Severi, M., Farina, R., & Simonelli, A. (2020). Sub-periosteal Peri-implant Augmented Layer technique to treat peri-implantitis lesions. Clinical Advances in Periodontics. https://doi.org/10.1002/ cap.10107. [Epub ahead of print].
- Trombelli, L., Severi, M., Pramstraller, M., & Farina, R. (2018). Sub-Periosteal Peri-Implant Augmented Layer technique for horizontal bone augmentation at implant placement. Minerva Stomatologica, 67, 217–224. https://doi.org/10.23736/S0026-4970.18.04161-4
- Trombelli, L., Severi, M., Pramstraller, M., & Farina, R. (2019). A simplified soft tissue management for peri-implant bone augmentation. International Journal of Oral and Maxillofacial Implants, 34, 197–204. https://doi.org/10.11607/jomi.6959
- Urban, I. A., Wessing, B., Alández, N., Meloni, S., González-Martin, O., Polizzi, G., … Zechner, W. (2019). A multicenter randomized controlled trial using a novel collagen membrane for guided bone regeneration at dehisced single implant sites: Outcome at prosthetic delivery and at 1-year follow-up. Clinical Oral Implant Research, 30, 487–497. https://doi.org/10.1111/clr.13426
- Zitzmann, N. U., Schärer, P., & Marinello, C. P. (2001). Long-term results of implants treated with guided bone regeneration: A 5-year prospective study. International Journal of Oral and Maxillofacial Implants, 16, 355–366.
