Peri-Implant Bone Augmentation by the Sub-Periosteal Peri-Implant Augmented Layer Technique and a Bovine-Derived Bone Block: A Case Report
Background: When used with deproteinized bovine bone mineral (DBBM) delivered as a particulate, the subperiosteal peri-implant augmented layer (SPAL) technique was effective in completely correcting up to 92% of peri-implant buccal bone dehiscences. The use of a DBBM block (bDBBM), however, may result in an improvement of the peri-implant bone dehiscence as well as a relevant lateral bone augmentation since its mechanical properties may ensure a better dimensional stability at flap manipulation than particulate DBBM. The aim of the present a proof-of-principle case report is to investigate if SPAL may be successfully used to obtain bone augmentation at peri-implant dehiscence sites when used with bDBBM.
Case presentation: Lateral bone augmentation was performed using the SPAL technique at two implants showing a buccal peri-implant bone dehiscence immediately after their placement. A partial-thickness flap was elevated, leaving the periosteal layer on the buccal cortical bone plate. The periosteal layer was, in turn, elevated to create a pouch, which was used to stabilize a bDBBM graft at the peri-implant buccal bone dehiscences. At re-entry, exposed implant surfaces were completely covered by new thick hard tissue up to their most coronal portion. A free epithelial-connective tissue graft was used to augment the peri-implant soft tissue phenotype.
Conclusion: When used to accommodate bDBBM over the most coronal portion of an exposed implant, SPAL may successfully lead to an increase in peri-implant buccal tissue thickness. Clin Adv Periodontics 2022;12:39–43.
Background
A simplified technique to augment the osseous component of the peri-implant phenotype at implant placement, namely, the sub-periosteal peri-implant augmented layer (SPAL), was recently proposed. SPAL is based on the separation of the buccal flap into two layers: a periosteal layer, which creates an “osteogenic” protected space to stabilize a graft at a thin or deficient peri-implant buccal bone plate, and a mucosal layer, which is mobilized to provide primary intention healing. Previous studies have shown that SPAL may result in a substantial dehiscence correction, thus providing conditions for peri-implant health at the treated implants. Also, SPAL has been shown effective in treating dehiscence-type peri-implantitis defects associated with either an interproximal or a circumferential component.
In all cases treated with SPAL, a deproteinized bovine bone mineral (DBBM)–delivered as a particulate–has been accommodated between the periosteal layer and the exposed implant surface. Although proved effective as a space-making osteoconductive scaffold in obtaining a complete coverage of peri-implant bone dehiscences, particulate DBBM might be associated with a limited increase in thickness of the buccal bone plate.
Preclinical and clinical studies seem to suggest that guided bone regeneration (GBR) may result in an improvement of the peri-implant bone dehiscence as well as a relevant lateral bone augmentation when used with a DBBM block (bDBBM). The present study consists a proof-of-principle case report aimed at investigating if SPAL may be successfully used to obtain bone augmentation at peri-implant dehiscence sites when used with bDBBM.
Ethical aspects
The present report was approved by the Ethical Committee of Area Vasta Emilia Centro, Italy (protocol n°637/2018/Oss/UniFe, date of approval 12.12.2018). The patient provided a written informed consent prior to surgical treatment. All the clinical procedures have been performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines.
Clinical Presentation, Case Management, and Clinical Outcomes
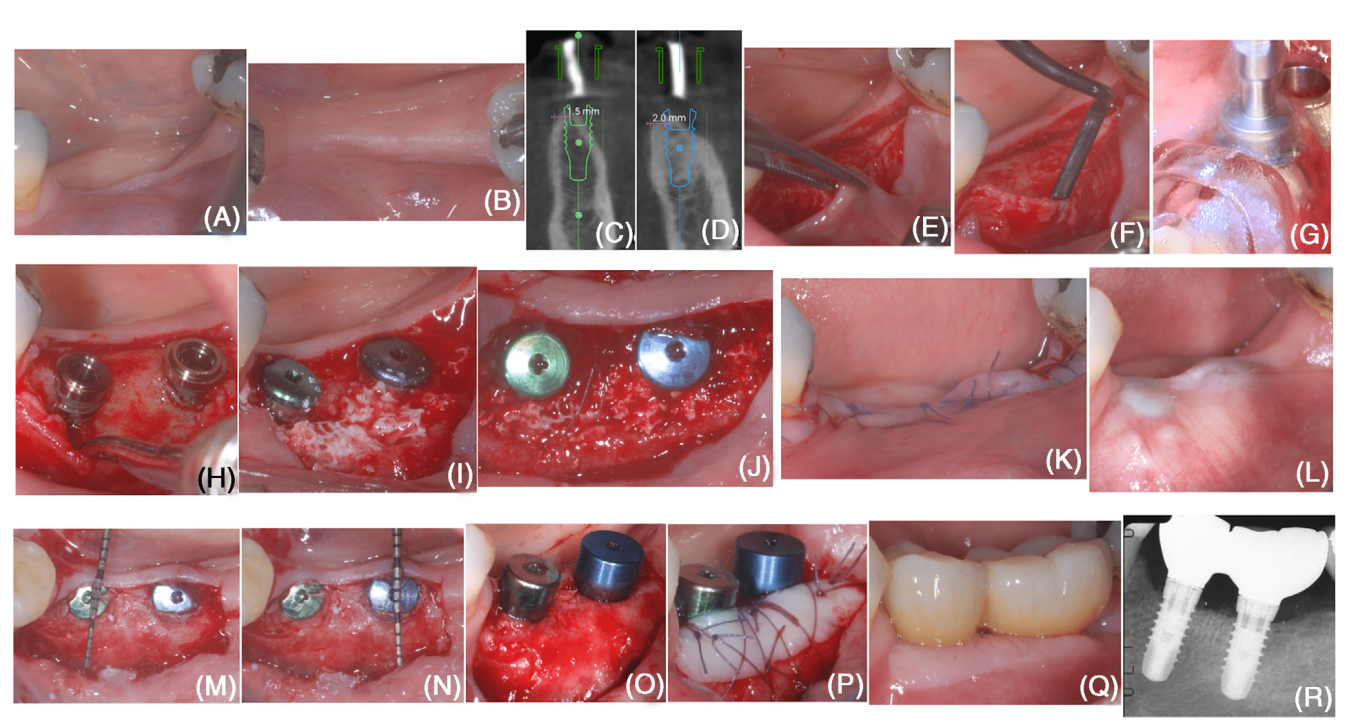
In October 2020, a 50-year-old, non-smoker, systemically healthy female patient presented for the rehabilitation of an edentulous area (#18 and #19; Figures 1A,B). After treatment for stage III periodontitis, the patient presented a bleeding on probing score <10% and no sites with probing depth ≥ 5 mm and was enrolled in a supportive
periodontal care program. An implant-supported rehabilitation was programmed in the #18 and #19 area.
Implant position was planned digitally on a cone beam computed tomography exam, and a surgical guide was fabricated (Figure). Digital planning previewed the formation of a buccal dehiscence at placement of both implants, suggesting the need for horizontal bone augmentation procedure (Figures 1C,D).
The patient was administered 2 g of amoxicillin + clavulanic acid 1 h prior to surgery. Local anesthesia was attained using articaine with 1:100,000 epinephrine administered by local infiltration.
At the buccal aspect, a split-thickness flap (creating the “mucosal layer”) was raised, leaving the periosteal layer on the edentulous ridge intact (Figure 1E) Then, the periosteal layer was elevated from the bone crest by means of a microsurgical periosteal elevator and tunneling knives with varying angulated sharp edges (Figure 1F). At the lingual aspect, a full-thickness flap was elevated. The elevation was extended in an apical direction to detach the superficial fibers of the mylohyoid muscle and obtain a tension-free lingual flap.
Implant sites were prepared using the computer-aided surgical guide (Figure 1G), and two tissue-level implants were positioned (Figure 1H). Implants presented a buccal dehiscence (measured as the distance between the apical margin of the implant polished collar and the bone crest at the buccal aspect of the implant) of 3 mm at #19 and 2 mm at #18. Cortical perforations were performed using a carbide bur.
bDBBM was trimmed using a high-speed diamond bur in order to obtain a homogeneous thickness of 3– 4 mm and was adapted beneath the periosteal layer to completely cover the exposed implant surface (Figure 1I). Using a resorbable 6/0 suture, the periosteal layer was stabilized to the lingual flap by means of internal mattress sutures (Figure 1J). The mucosal layer was coronally advanced to achieve primary closure of the wound (Figure 1K).
The patient was instructed to avoid any compression of the surgical site and not to chew or brush in the treated area for 2 weeks. A 0.12% chlorhexidine mouthrinse was prescribed (1-min rinse b.i.d. for 3 weeks). Sutures were removed at 2-week post-surgery.
At 6 months following implant placement (Figure 1L), a re-entry procedure for implant exposure was performed using a buccal split-thickness flap. Previously exposed implant surfaces were completely covered by new tissue that was at least 3 mm thick at the most coronal portion of both implants (Figures 1M–O). The reconstructed tissue offered a clear resistance to penetration with a periodon- tal probe when the latter was used at a probing force of approximately 50 N by the trained operator. A free epithelial-connective tissue graft was used to augment the peri-implant soft tissue phenotype (Figure 1P).
A digital impression was taken 4 weeks after implant exposure. The shape and emergency profile of the final restoration were digitally planned. Two splinted crowns were milled from a zirconia monoblock and cemented on the titanium inserts according to manufacturer’s instructions. Final restoration was screwed at 4 weeks after impression.
Peri-implant tissue conditions appeared adequate at both clinical and radiographic examination (Figures 1Q–R).
Discussion
The present case report suggests that bDBBM may represent a suitable alternative to particulate DBBM when applying the SPAL technique for bone augmentation at peri-implant dehiscence sites.
In the SPAL technique, graft stabilization into the sub-periosteal space is ensured by suturing its most coronal part (thus limiting the detrimental action of mechanical disrupting forces on the clot/graft blend and favoring the intimate contact of the latter with the highly vascularized structure of the periosteum) and subsequent coverage by the mucosal layer. One of the potential drawbacks when using particulate DBBM is the potential displacement and/or compression of the graft particles around the coronal portion of the implant at suturing. This consideration is well substantiated in the literature for the association of particulate DBBM and GBR. In contrast, bDBBM may act as an efficacious osteoconductive scaffold while its mechanical properties would ensure a better dimensional stability at flap manipulation. Previous preclinical and clinical seems to corroborate our findings. On the other hand, the stiffness of the block graft calls for an ideal passive adaptation of the mucosal layer to minimize the risk for flap perforation and graft exposure.
Implant uncovering was performed through a split-thickness flap, thus preventing direct inspection of the reconstructed tissues under the periosteum. Together with the lack of an histological analysis and/or a postoperative tridimensional radiographic evaluation of the peri-implant space prevents the possibility to draw any conclusion on the nature of the reconstructed tissues at the previously exposed buccal aspect of the implants. Although tissue thickness (≥ 3 mm) and its high resistance to probe penetration at re-entry could be suggestive of the presence of a mineralized component at an advanced maturation stage, any consideration on the quality of the subperiosteal tissues remains speculative, being based exclusively on the mechanical perception of the operator during the probing inspection. Available data stemming from experimental studies and 6-month human histologic samples where bDBBM was used for lateral bone augmentation showed a more limited new bone formation, mainly located at the native bone-bDBBM interface, compared to particulate DBBM. These observations seem to reinforce the need to improve the osteogenic conditions of the wound (e.g., cortical perforations, use of additional growth factors) and/or wait for longer maturation time prior to implant uncovering when a bDBBM is used. Clinical observations stemming from the present case report cumulate with previous evidence from case reports and retrospective case series on the SPAL technique. Overall, data demonstrate that SPAL can be successfully used for peri-implant bone augmentation with a bovine-derived xenograft in a variety of clinical scenarios in terms of implant topography (i.e., anterior/posterior sextant and maxillary/mandibular arch) as well as conditions of the buccal bone plate at the time of implant placement. In particular, clinically relevant augmentation was reported for implants with either a buccal bone dehiscence or an intact but thin buccal bone plate at placement, Within implants with bone dehiscence, defect depths up to 6 mm were successfully corrected with SPAL. Unfortunately, although encouraging, the available data is not sufficient to either clearly delineate the local indications/contraindications of the SPAL technique or clarify which factors may influence the clinical outcomes of the procedure.
Conclusion
The present proof-of-principle case report indicates that the SPAL technique may be successfully used to accommodate bDBBM over the most coronal portion of an exposed implant and achieve an increase in peri-implant buccal tissue thickness.
Leonardo Trombelli, Mattia Severi, Luca Ortensi and Roberto Farina
References
- Avila-Ortiz G, Gonzalez-Martin O, Couso-Queiruga E, Wang HL. The peri-implant phenotype. J Periodontol. 2020;91:283-288.
- Trombelli L, Severi M, Pramstraller M, Farina R. Sub-periosteal peri-implant augmented layer technique for horizontal bone augmentation at implant placement. Minerva Stomatol. 2018;67:217-224.
- Trombelli L, Severi M, Pramstraller M, Farina R. A simplified soft tissue management for peri-implant bone augmentation. Int J Oral Maxillofac Implants. 2019;34:197-204.
- Trombelli L, Pramstraller M, Severi M, Simonelli A, Farina R. Peri-implant tissue conditions at implants treated with Sub-periosteal Peri-implant Augmented Layer technique: a retrospective case series. Clin Oral Impl Res. 2020;31:992-1001.
- Trombelli L, Severi M, Farina R, Simonelli A. Sub-Periosteal Peri-implant Augmented Layer technique to treat peri-implantitis lesions. Clin Adv Periodontics. 2020;10:169-174.
- Benic GI, Thoma DS, Munoz F, Martin IS, Jung RE, Hämmerle CHF. Guided bone regeneration of peri-implant defects with particulated and block xenogeneic bone substitutes. Clin Oral Impl Res. 2016;27:567- 576.
- Benic GI, Thoma DS, Sanz-Martin I, et al. Guided bone regeneration at zirconia and titanium dental implants: a pilot histological investigation. Clin Oral Impl Res. 2017;00:1-9.
- Benic GI, Eisner BM, Jung RE, Basler T, Schneider D, Hämmerle CHF. Hard tissue changes after guided bone regeneration of peri-implant defects comparing block versus particulate bone substitutes: 6-month results of a randomized controlled clinical trial. Clin Oral Impl Res. 2019;30:1016-1026.
- Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229-235.
- Mir-Mari J, Wui H, Jung RE, Hämmerle CHF, Benic GI. Influence of blinded wound closure on the volume stability of different GBR materials: an in vitro cone beam computed tomographic examination. Clin Oral Impl Res. 2015;27:258-265.
- Naenni N, Berner T, Waller T, Huesler J, Hammerle CHF, Thoma DS. Influence of wound closure on volume stability with the application of different GBR materials: an in vitro cone-beam computed tomographic study. J Periodontal Implant Sci. 2019;49:14-24.
- Laass A, Hämmerle CHF, Jung RE, Thoma DS, Benic GI. Histologic outcomes after guided bone regeneration of peri-implant defects com paring individually shaped block versus particulate bone substitutes. Int J Periodontics Restorative Dent. 2020;40:519-527.
- Araujo MG, Sonohara M, Hayacibara R, Cardaropoli G, Lindhe J. Lateral ridge augmentation by the use of grafts comprised of autologous bone or a biomaterial. An experiment in the dog. J Clin Periodontol. 2002;29:1122-1131.
- Oda A, Kinoshita K, Ueda M. Effects of cortical bone perforation on periosteal distraction: an experimental study in the rabbit mandible. J Oral Maxillofac Surg. 2009;67:1478-1485.
- Schwarz F, Rothamel D, Herten M, Ferrari D, Sager M, Becker J. Lateral ridge augmentation using particulated or block bone substitutes biocoated with rhGDF-5 and rhBMP-2: an immunohistochemical study in dogs. Clin Oral Impl Res. 2008;19:642-652.
