Sub-periosteal peri-implant augmented layer technique for horizontal bone augmentation at implant placement
Prosthetically-driven implant placement in a reduced horizontal bone dimension often results in a peri-implant bone dehiscence or fenestration. Even in presence of an intact but thin buccal cortical bone plate (BCBP), trauma and consequent bone remodeling following surgical procedure for implant placement may result in a vertical bone loss with the exposure of the coronal part of the implant at uncovering,1 leading to a buccal bone dehiscence which may cause a mucosal recession on the long-term.
Different techniques for grafting buccal plates without barrier membranes for horizontal augmentation have been reported in the literature. Recently, a novel bone augmentation procedure based on the utilization of the periosteum as a barrier membrane and space-making “device” has been successfully used to enhance the horizontal bone dimension prior to implant placement. Consistently, the use of a laparoscopic approach to deliver a growth factor/xenograft combination into a subperiosteal pouch resulted in predictable and consistent bone regeneration. The biologic rationale for the use of the “periosteum layer” was recently confirmed by an experimental study where the peri-implant osteogenic potential of the periosteum in determining vertical bone augmentation around implants has been histologically reported. Collectively, these findings indicate that pre- and peri-implant tissue augmentation may be enhanced by surgical procedures where the periosteum is left intact although detached from the underlying bone, and a secluded space between the periosteum and the bone or implant is warranted.
The purpose of the present case report is to illustrate a simplified soft tissue management technique, namely the sub-periosteal peri-implant augmented layer (SPAL). The SPAL technique is proposed to increase hard and soft tissue dimensions at the most coronal portion of an implant.
SPAL technique application
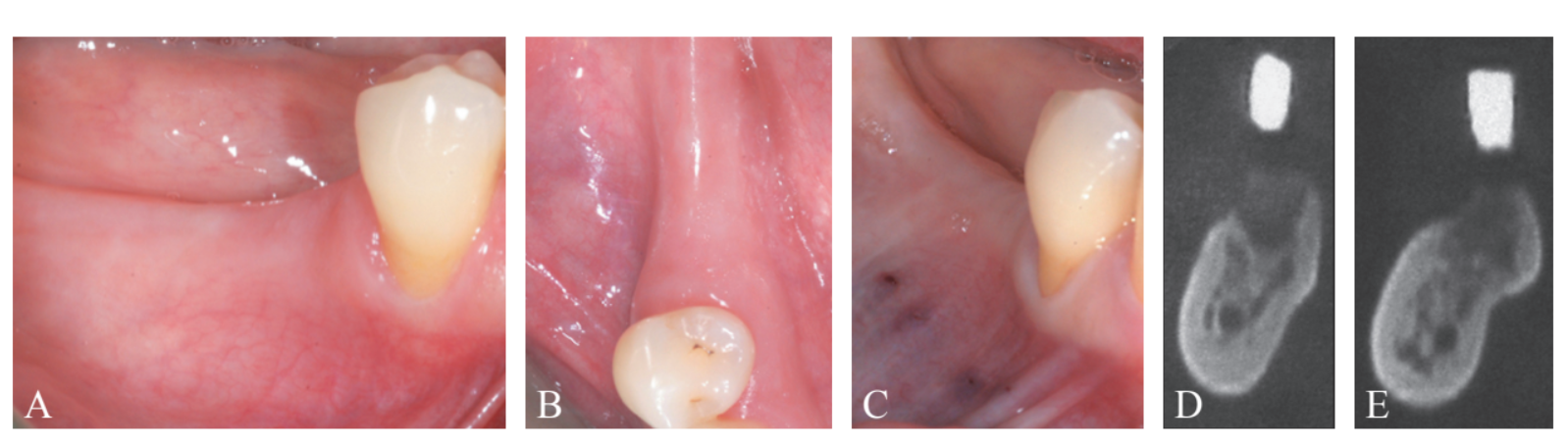
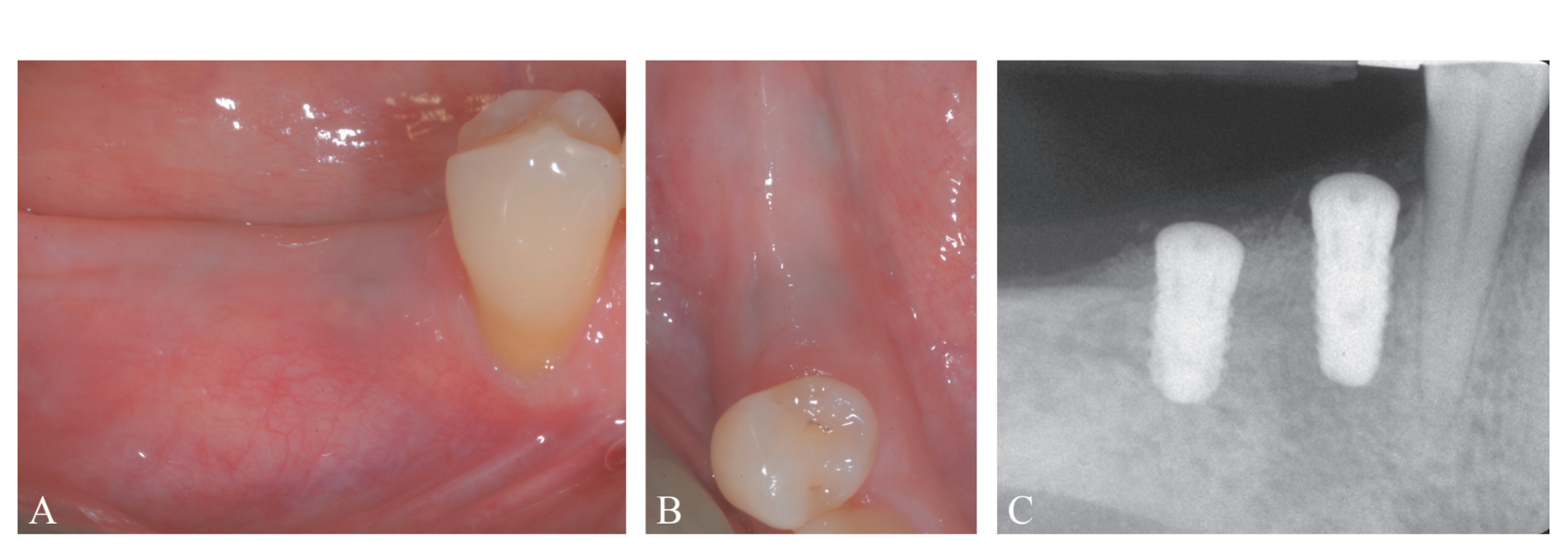
A systemically healthy, non-smoker, 61-year-old woman, was referred for implant-supported rehabilitation at the Research Center for the Study of Periodontal and Peri-implant Diseases, University of Ferrara. Prior to implant placement, the patient had undergone active periodontal therapy for the treatment of severe periodontitis and was enrolled in a professional secondary prevention program. Treatment plan was based on 3D radiographic assessment, and a surgical stent was fabricated on diagnostic wax-up. The clinical and radiographic evaluation revealed a deficient horizontal bone dimension in the area related to teeth #4.5 and 4.6, where implants were planned to be positioned (Figure 1A-E). Treatment plan was carefully explained to the patient, who gave her informed consent to the proposed surgical procedure.
At implant placement, the patient was ad- ministered 2 g of amoxicillin + clavulanic acid (Augmentin, GlaxoSmithKline, Verona, Italy) one hour prior to surgery. Local anesthesia was attained using articaine with 1:100,000 epinephrine administered by local infiltration. Attention was paid not to infiltrate the area where the split-thickness flap has to be performed.
The surgical steps of SPAL technique are illustrated in Table I.
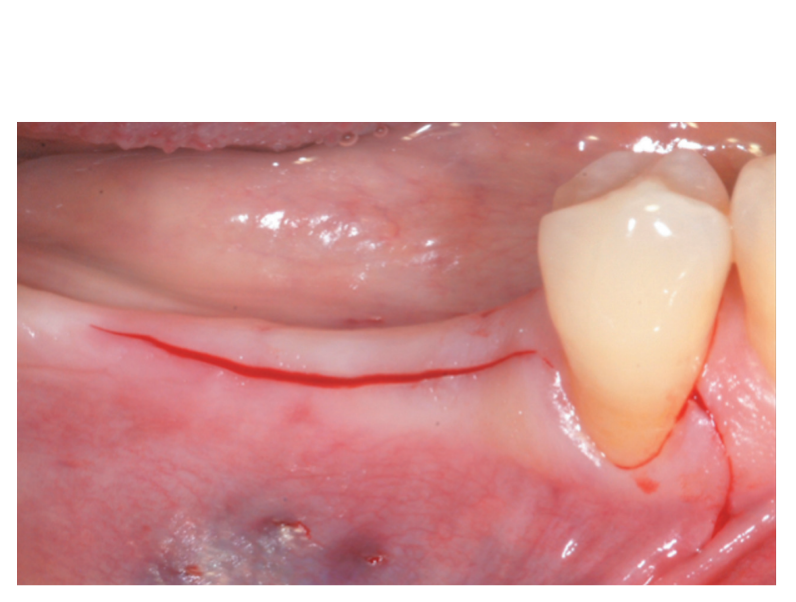
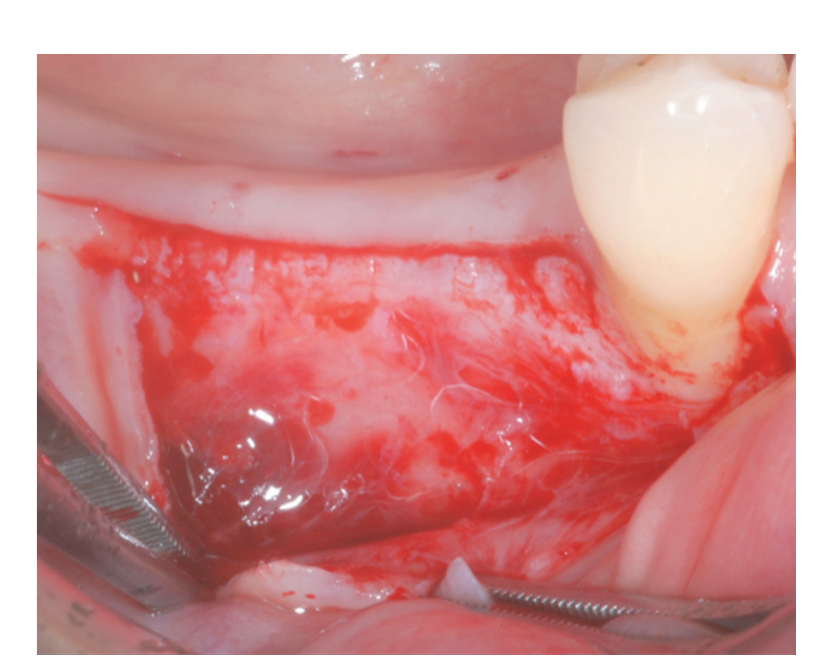
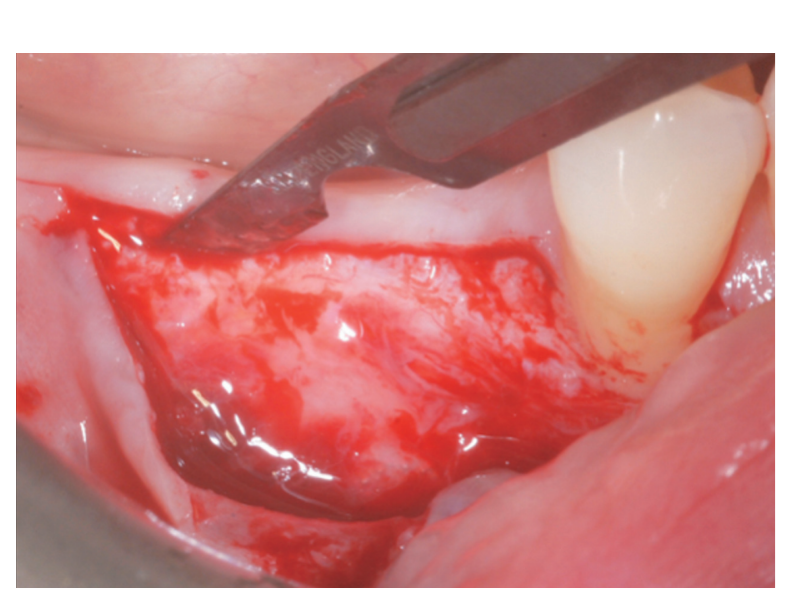
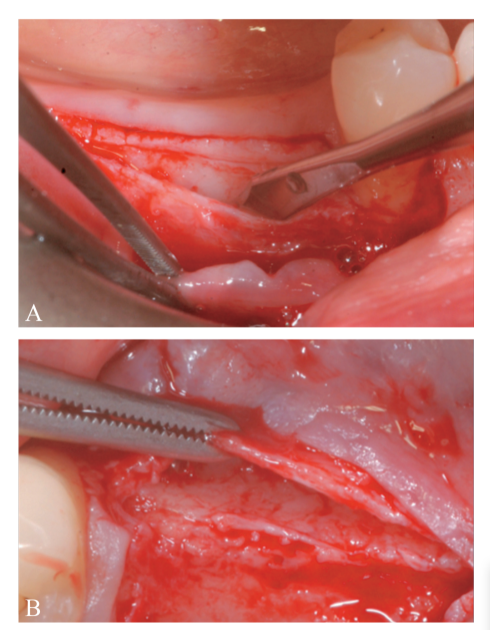
A partial thickness crestal incision was made by a 15C blade. The incision was extended intra-sulcularly on tooth #4.4 and a mesial partial-thickness releasing incisions was made to obtain better visibility and avoid tension on the flap (Figure 2). A split-thickness flap was raised on the buccal aspect by sharp dissection (namely, the mucosal layer) and leaving the periosteal layer on the edentulous ridge intact (Figure 3). After separation between the mucosal and periosteal layer to allow for a tension-free, coronal advancement of the mucosal layer, a second crestal incision reaching the bone crest was performed (Figure 4). The periosteal layer was elevated from the bone crest to create a secluded pocket that could accommodate an adequate extension and volume of the bone graft material (Figure 5A, B). A full-thickness flap was elevated on the oral (lingual/ palatal) aspect (Figure 6).
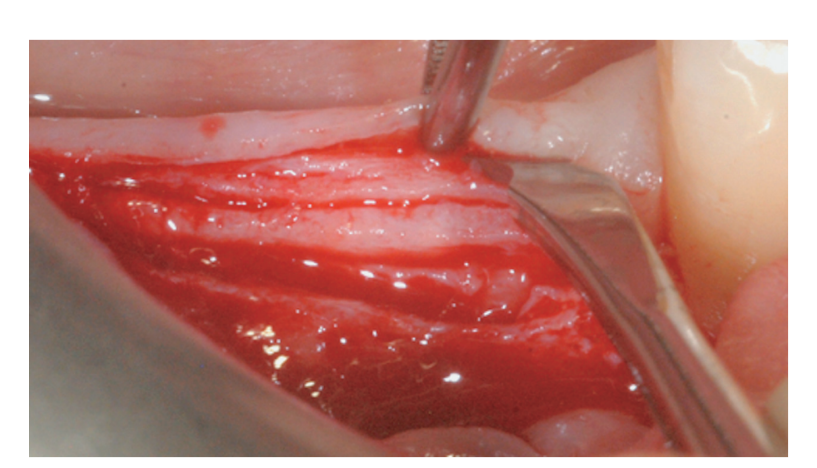
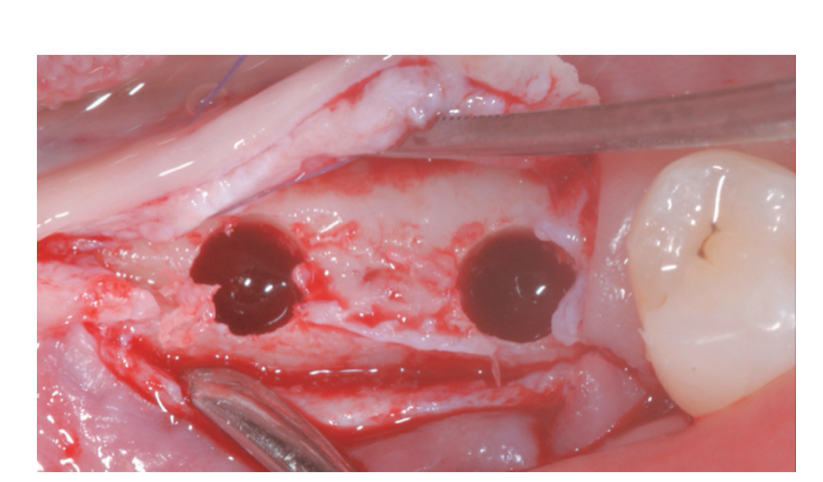
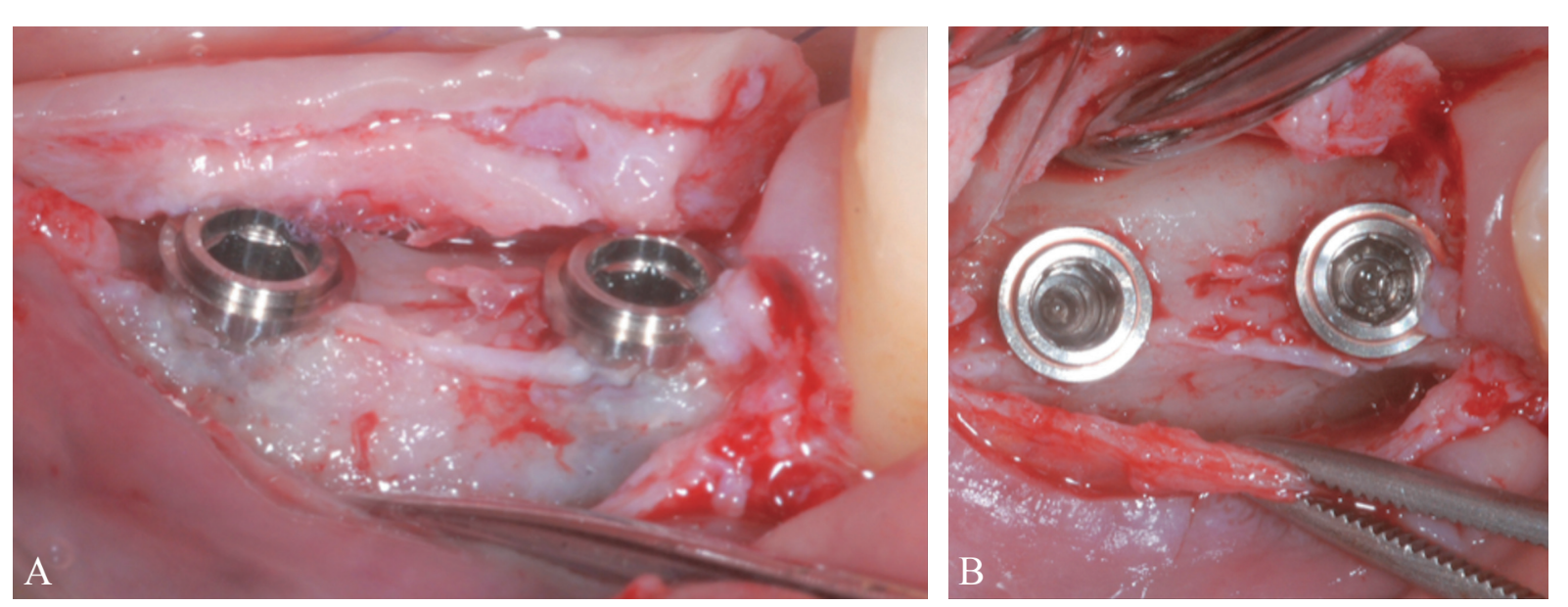
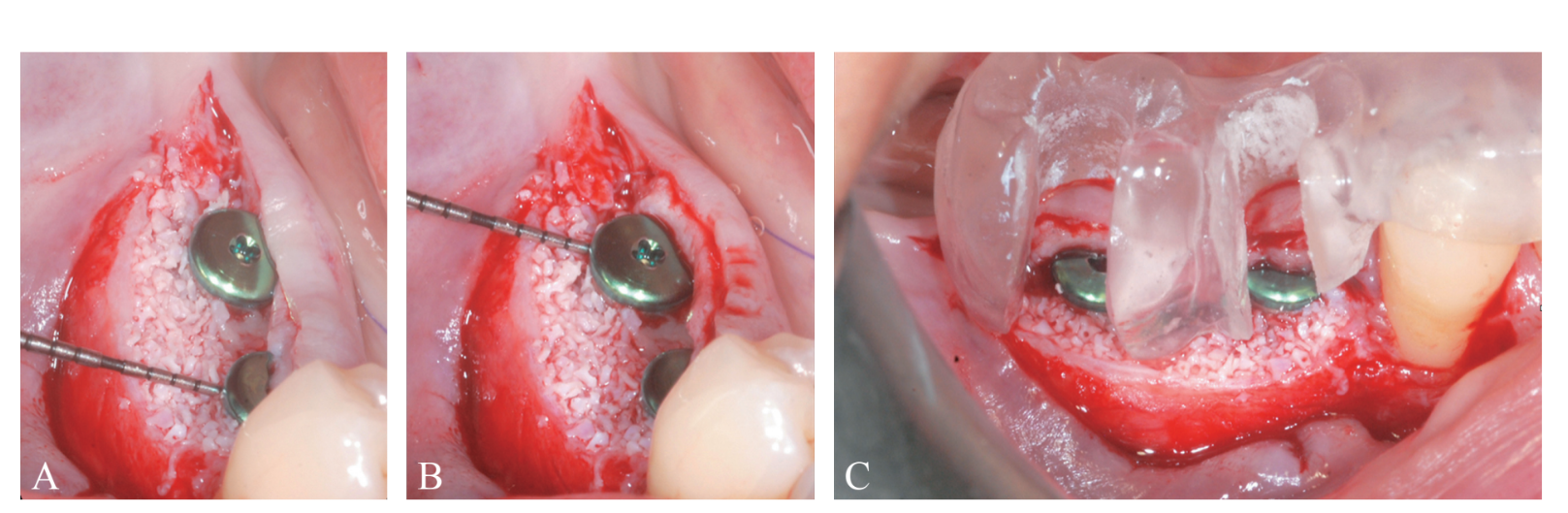
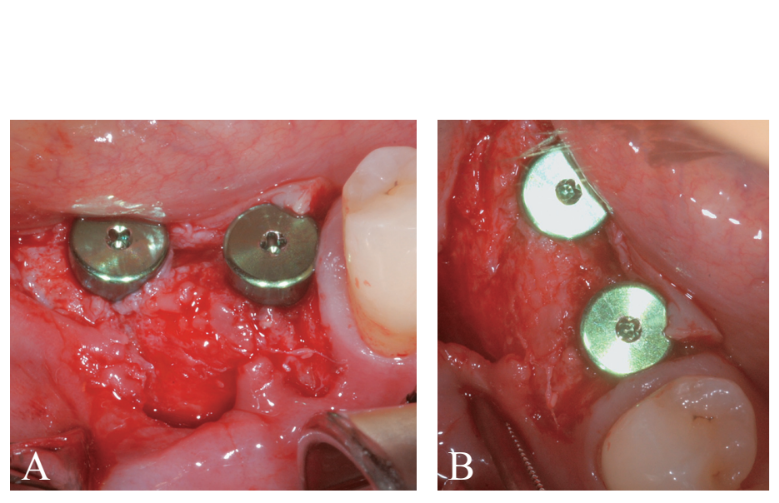
Prosthetically-driven implant site preparation resulted in a thin BCBP (Figure 7). Tissue-level implants (Thommen Medical, Grenchen, Switzerland) were placed with a 1-mm polished collar above the bone crest (Figure 8A, B). Healing caps were used on both implants. Bio-Oss® xenograft spongiosa granules (particle size 0.25-1.0 mm; Geistlich Pharma, AG, Wolhusen, Switzerland) were used as a space-making device to fill the surgically-created space between the periosteal layer and the BCBP up to the implant polished collar (Figure 9A-C).
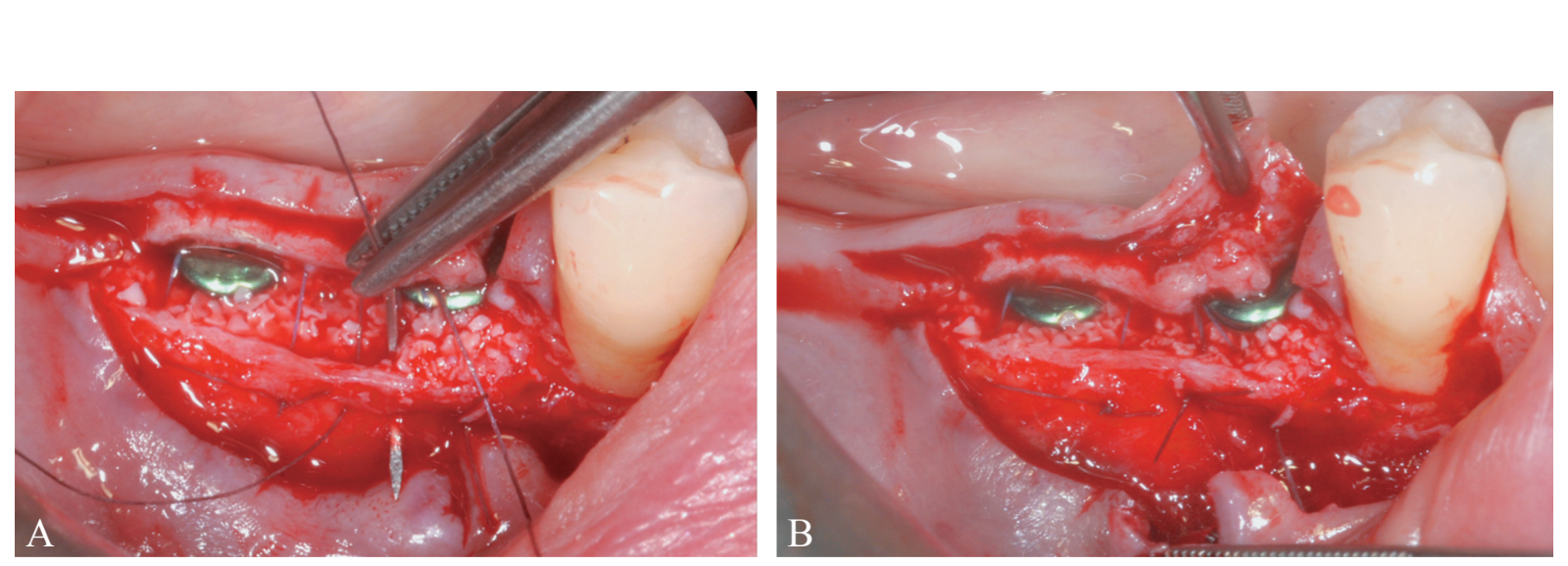
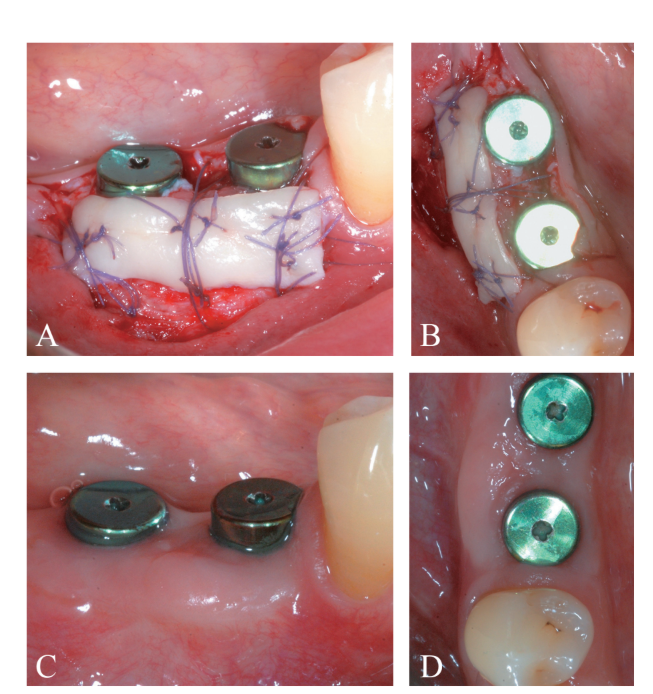
The periosteal layer was sutured to the oral mucoperiosteal flap by means of internal mattress sutures (Figure 10A, B). The mucosal layer was coronally advanced and sutured tension-free by horizontal internal mattress and interrupted sutures to submerge both the graft and the implants (Figure 11). For both mucosal and peri-osteal layers, a resorbable 6/0 suture (Vicryl 6/0, Ethicon, Somerville, NJ, USA) was used.
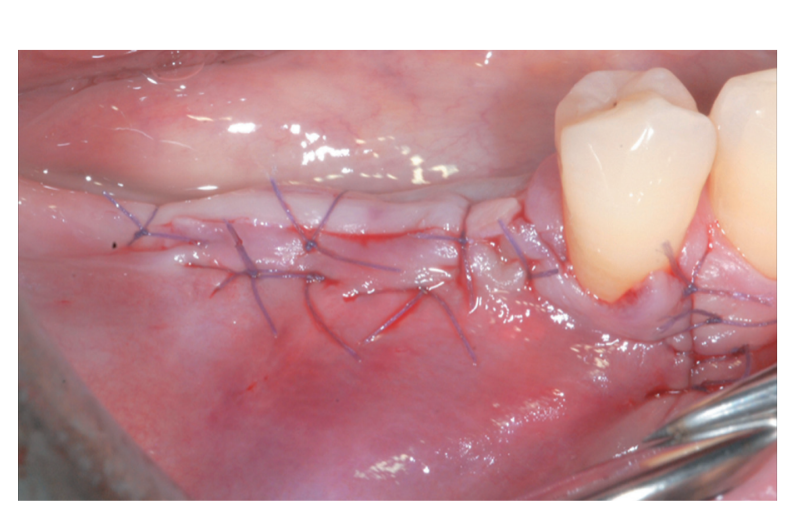
The patient did not wear any removable prostheses to avoid compression onto the surgical site and was asked not to chew or brush in the treated area for approximately 2 weeks. The home use of a 0.12% chlorhexidine solution (Curasept ADS Trattamento Rigenerante®, Curaden Healthcare, Saronno, Italy) was prescribed for chemical plaque control (1-minute rinse b.i.d. for 3 weeks). Sutures were removed 2 weeks after surgery.
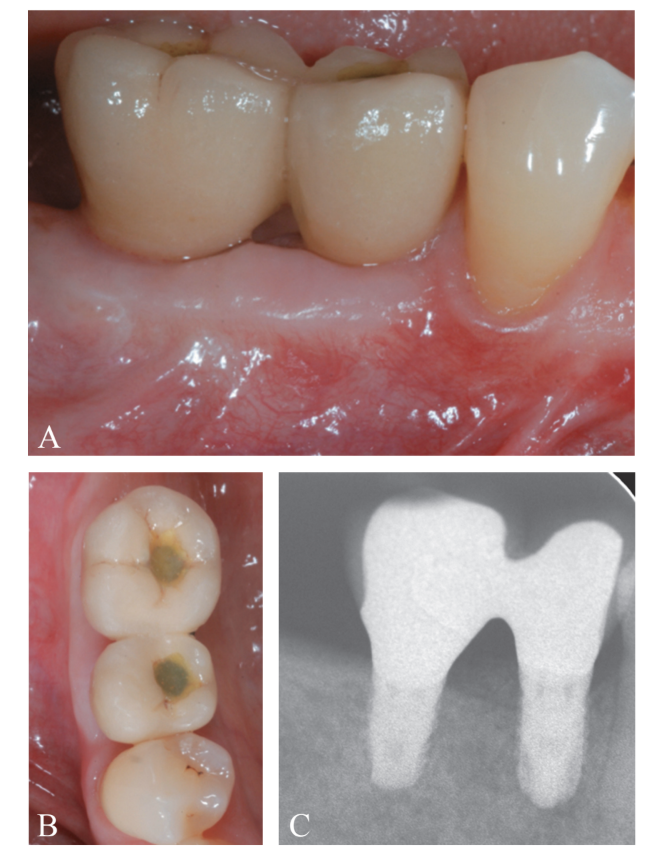
At 4 months following implant placement, a re-entry procedure for implant exposure was performed (Figure 12A-C). A buccal split-thickness flap was performed to position the healing abutment, attention was paid to leave a substantial thickness of tissue to protect the coronal part of the implant (Figure 13A, B). An increase in the thickness (>2 mm) of the buccal peri-implant tis- sues was observed. In this particular case, since the coronal advancement of the flap during the first surgical procedure resulted in absence of an adequate amount of buccal peri-implant mucosa, a free epithelialized gingival graft was positioned at the most coronal portion of the implants (Figure 14A-D). Final prosthetic rehabilitation is illustrated in Figure 15A-C.
Discussion
SPAL technique originates from the Double Flap incision as originally proposed by Hur et al. and modified by Steigmann et al. This design includes a partial-thickness flap elevation leaving the periosteal layer on the edentulous ridge and separation of the mucosal layer of the flap. The periosteal layer is then used to stabilize the regenerative site using periosteal sutures. Various regenerative devices, such as non-resorb- able/resorbable membrane and titanium mesh with different size and locations, were used for both horizontal and vertical implant site development. More recently, a similar flap design was reported to perform GBR for horizontal bone augmentation prior to implant placement. A periosteal pocket was created by splitting a mucosal from a periosteal layer, and the created sub-periosteal space was then filled with bone substitute materials and covered by a resorbable membrane. At 6 months, a substantial increase of horizontal bone crest dimensions was recorded. SPAL technique benefits by the advantages of the two previous techniques, in terms of flap design and creation of a sub-periosteal space aimed at receiving an osteoconductive scaffold.8 However, our proposal is characterized by two major technical differences that simplify the procedure while leading to predictable results: 1) the increase in BCBP is performed at the time of implant placement; 2) enhanced horizontal dimension merely relies on the osteogenetic potential inherent to the periosteum without use of membranes.
SPAL technique is based on the so-called PASS principle that includes primary wound coverage, angiogenesis, space creation, and wound stability.
Space is needed for the osteogenic cells to creep into the wound site, differentiate into osteoblasts, and form woven bone. Stability of the secluded space containing the graft biomaterial acting as scaffold for blood clot formation and maturation has been repeatedly emphasized for bone regeneration. In our technique, space provision was warranted by: 1) creating a sub-periosteal pocket which is open only in its coronal portion, while not detached by underlying bone on the mesial, apical and distal aspects; and 2) the use of slow-resorption xenograft inserted and stabilized with limited dispersion and minimal micromovements.
Deproteinized bovine bone particles may have created a suitable osteoconductive scaffold for new bone formation while mechanically maintaining the periosteal layer elevated from the overlying implant and bone surface. Previous studies have shown that periosteal elevation by different devices resulted in induction of supracortical peri-implant bone formation. The periosteum layer may not only represent a source of osteogenetic cells, but also effectively contributes angiogenesis, which is a prerequisite for new bone formation. Although the presented case did not allow any information on the nature of the augmented tissues, human histology derived form a similar procedure where a subperiosteal pouch was surgically created revealed xenograft particles surrounded by newly formed bone.
Our flap design allows for soft tissue mobilization leading to tension-free primary closure, a condition which is regarded as essential to achieve undisturbed bone regeneration. Primary closure was facilitated by two factors: 1) the amount of horizontal bone resorption is positively correlated with soft tissue thickness; 2) grafting was limited to the most coronal portion of the implant.
Conclusions
In conclusion, SPAL technique may represent a valuable surgical option in the horizontal augmentation of peri-implant tissue thickness. Further longitudinal studies are needed to evaluate whether and to what extent this procedure may be used in the treatment of peri-implant bone dehiscence and is capable to contribute long-term stability of the buccal mucosal profile and healthy conditions of peri-implant tissues.
Leonardo Trombelli, Mattia Severi, Mattia Pramstraller, Roberto Farina
References
- Spray JR, Black CG, Morris HF, Ochi S. The influence of bone thickness on facial marginal bone response: stage 1 placement through stage 2 uncovering. Ann Periodontol 2000;5:119–28.
- Le B, Burstein J. Esthetic grafting for small volume hard and soft tissue contour defects for implant site development. Implant Dent 2008;17:136–41.
- Block MS, Degen M. Horizontal ridge augmentation using human mineralized particulate bone: preliminary results. J Oral Maxillofac Surg 2004;62(Suppl 2):67–72.
- Block MS. Horizontal ridge augmentation using particulate bone. Atlas Oral Maxillofac Surg Clin North Am 2006;14:27–38.
- Le B, Burstein J, Sedghizadeh PP. Cortical tenting grafting technique in the severely atrophic alveolar ridge for implant site preparation. Implant Dent 2008;17:40–50.
- Park SH, Lee KW, Oh TJ, Misch CE, Shotwell J, Wang HL. Effect of absorbable membranes on sandwich bone augmentation. Clin Oral Implants Res 2008;19:32–41.
- Cortes AR, Cortes DN, Arita ES. Correction of buccal dehiscence at the time of implant placement without barrier membranes: a retrospective cone beam computed tomographic study. Int J Oral Maxillofac Implants 2013;28:1564–9.
- Steigmann M, Salama M, Wang HL. Periosteal pocket flap for horizontal bone regeneration: a case series. Int J Periodontics Restorative Dent 2012;32:311–20.
- Lee EA. Subperiosteal minimally invasive aesthetic ridge augmentation technique (SMART): A new standard for bone reconstruction of the jaws. Int J Periodontics Restorative Dent 2017;37:165–73.
- Lutz R, Sendlbeck C, Wahabzada H, Tudor C, Prechtl C, Schlegel KA. Periosteal elevation induces supracortical peri-implant bone formation. J Craniomaxillofac Surg 2017;45:1170–8.
- Hur Y, Tsukiyama T, Yoon TH, Griffin T. Double flap incision design for guided bone regeneration: a novel technique and clinical considerations. J Periodontol 2010;81:945–52.
- Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent 2006;15:8–17.
- Tudor C, Bumiller L, Birkholz T, Stockmann P, Wiltfang J, Kessler P. Static and dynamic periosteal elevation: a pilot study in a pig model. Int J Oral Maxillofac Surg 2010;39:897– 903.
- Dziewiecki D, van de Loo S, Gremse F, Kloss-Brandstätter A, Kloss F, Offermanns V, et al. Osteoneogenesis due to periosteal elevation with degradable and nondegradable devices in Göttingen Minipigs. J Craniomaxillofac Surg 2016;44:318–24.
