A safe and predictable treatment option for experts and beginners
Guided implant surgery is growing in popularity due to a higher transfer accuracy of the virtual plan to the surgical site, compared to freehand placement. It has increased the accuracy of placement and improved patient satisfaction.
The introduction of new digital technologies, open-source software, and simplified protocols have reduced the cost of most systems for surgical guide fabrication and increased its popularity with novice users. But accurate guided implant placement must be based on a stringent workflow. Small errors at any step of the process can contribute to relevant deviation from the planned implant position. Therefore, knowledge of the potential maximum implant deviation of these systems is highly relevant to daily clinical practice. The Proceedings of the 5th Consensus Conference of the International Team for Implantology on guided implant surgery report a mean inaccuracy of 1.12 mm at emergence level and 1.39 mm at the apex, with maximum values of 4.5 mm and 7.1 mm, respectively. Individual errors, from data acquisition to errors during template placement and movement of the template during drilling, including human error, can also affect accuracy.
The aim of the present prospective study is to compare the virtual planning accuracy and template-related complications between expert and novice users of guided implant placement. The null hypothesis was that there would be no differences between groups.
Materials and methods
This study was designed as a comparative study aimed to evaluate implants placed by expert clinicians and novice users. This study was conducted at one centre between September 2017 and May 2018. The study protocol was approved by the Institutional Review Board of Aldent University (Tirana, Albania) (2/2017).
This trial is reported in accordance with the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines. Any partially edentulous patient, aged 18 years or older and able to sign an informed consent, in need of an implant-supported fixed restoration was considered eligible for this study and consecutively enrolled. Patients were divided into two groups. The first group included private patients treated by a clinician with expertise in guided implant placement (MT). The second group included private patients treated by clinicians without expertise in guided implant placement (first procedures) during a practical course, at the same dental clinic, assisted by an ex- pert clinician (MT).
Any implant position based on indi- vidual patient requirements was considered eligible for the present trial. Patients were not admitted to the study if any of the following exclusion criteria were present: general medical contraindications to oral surgery (ASA class III or IV), irradiation in the head and neck area less than one year before implantation, psychiatric problems, alcohol or drug abuse, pregnancy or lactation, untreated periodontitis, need for bone reconstruction, severe bruxism or clenching, un- controlled diabetes, poor oral hygiene/motivation or inability to complete the follow-up. The investigation was conducted according to the principles embodied in the Helsinki Declaration of 1975 for biomedical research involving human subjects, as revised in 2013. All patients were informed about the nature of the treatment and their written consent was obtained. Data collection was designed to preserve patient anonymity.
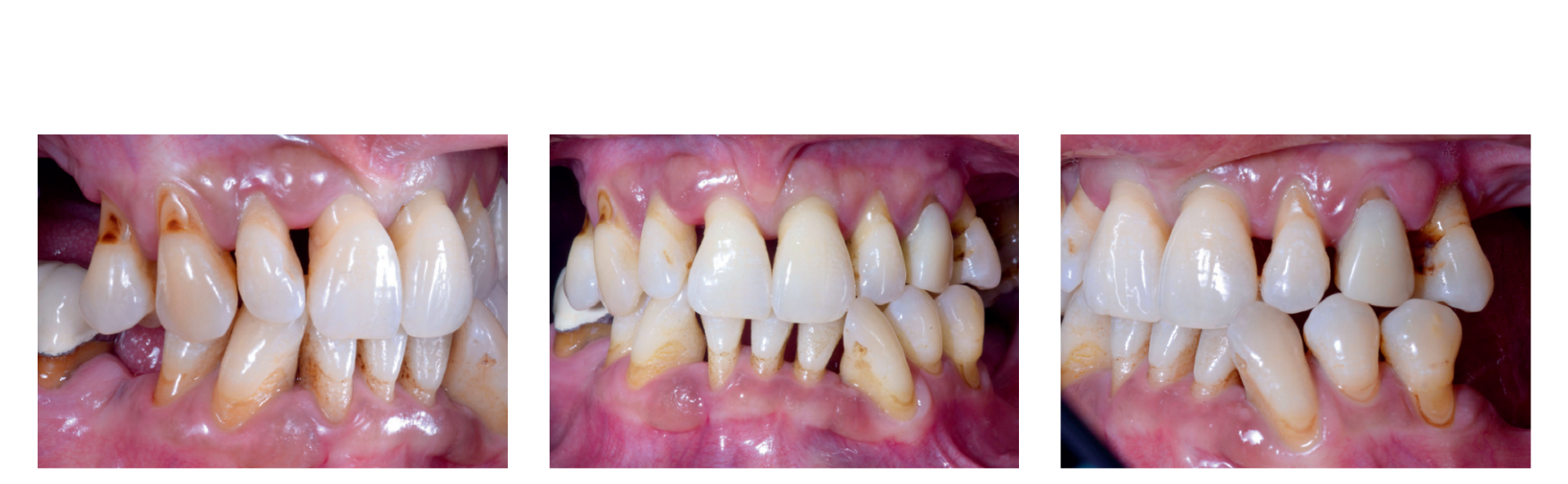
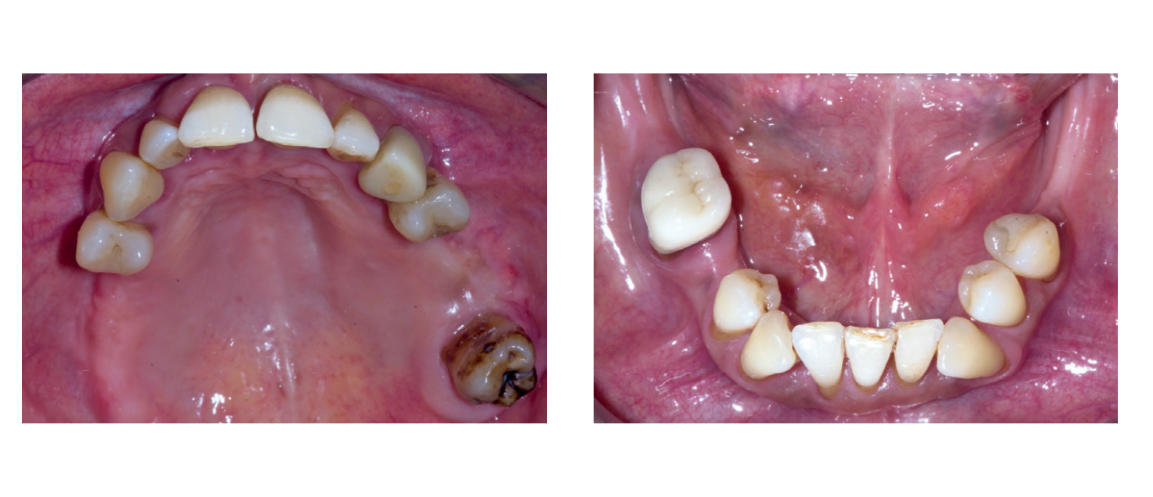
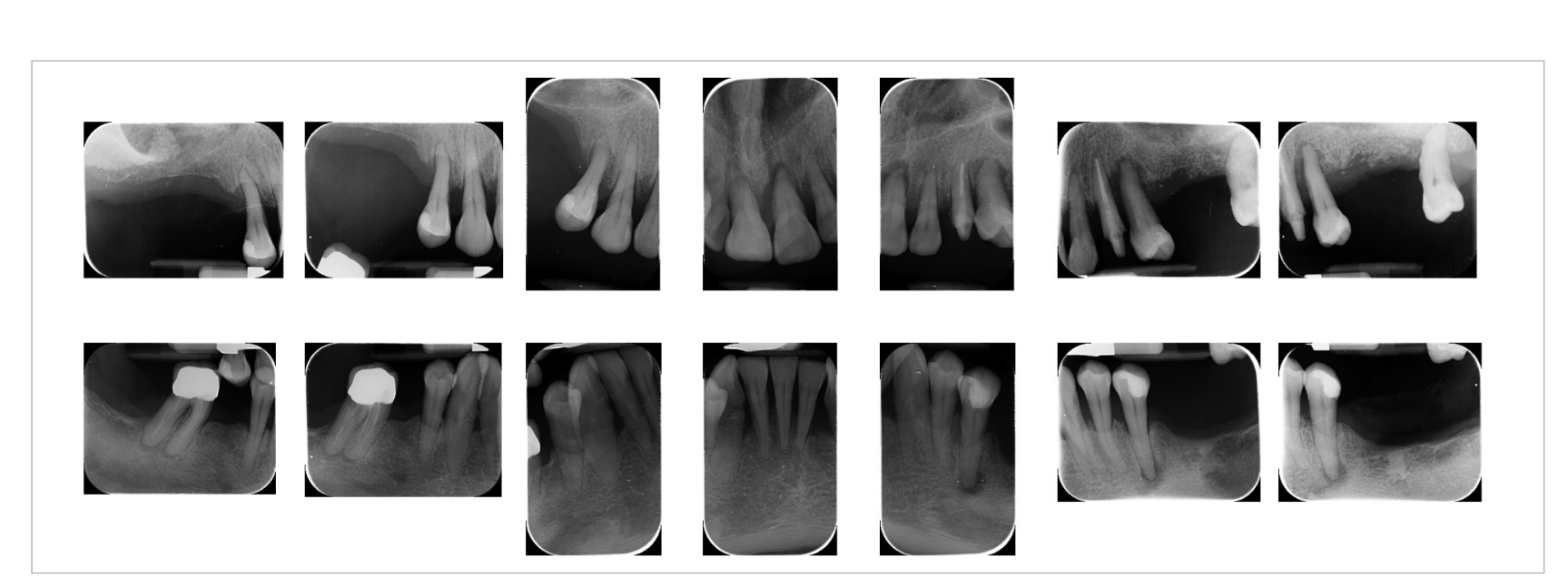
Preoperative photographs, periapical radiographs or panoramic x-rays of all patients were taken for initial screening and evaluation (Figs. 1a to 3).
The prosthetically driven planning workflow started by taking a cone-beam computed tomography (CBCT) scan (Cranex 3Dx; Soredex, Tuusula, Finland) of the patient, using a wax bite to separate the dental arches. Then a digital impression was taken using a 3M True Definition Scanner (3M Italia, Pioltello, Milano). The digital data in STL (Standard Tessellation Language) interface format was imported into a 3D design software (exocad DentalCAD; Exocad, Darmstadt, Germany) to create a virtual wax-up meeting the functional and aesthetic requirements.
The STL and DICOM (Digital Imaging and Communications in Medicine) data were imported into a 3D planning software (3Diagnosys ver. 4.2; 3DIEMME, Cantù, Italy). The reprocessed surface extrapolated from the DICOM data using a Hounsfield scale filter and the surface generated by scanning the master cast or by intraoral scanning were merged using the best-fit repositioning tools of the software (3Diagnosys ver. 4.2; 3Diemme).
At this point, prosthetically driven implants/abutments size and location were planned, taking into account bone quality and quantity, soft-tissue thickness, anatomical landmarks as well as the type, volume and shape of the final restoration. After careful functional and aesthetic evaluation and final verification, the prosthetically driven plan was approved (Figs. 4a and b).
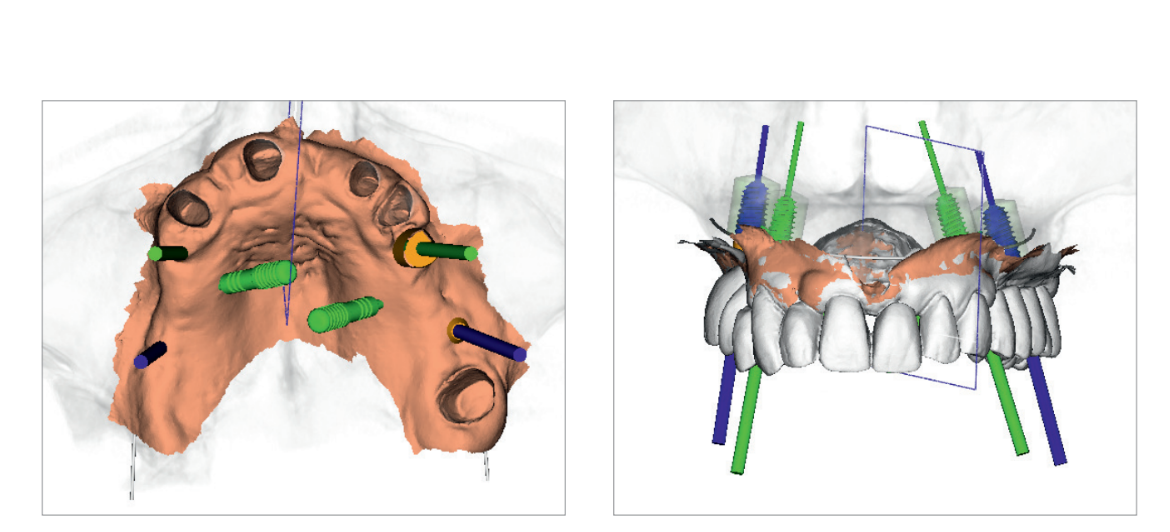
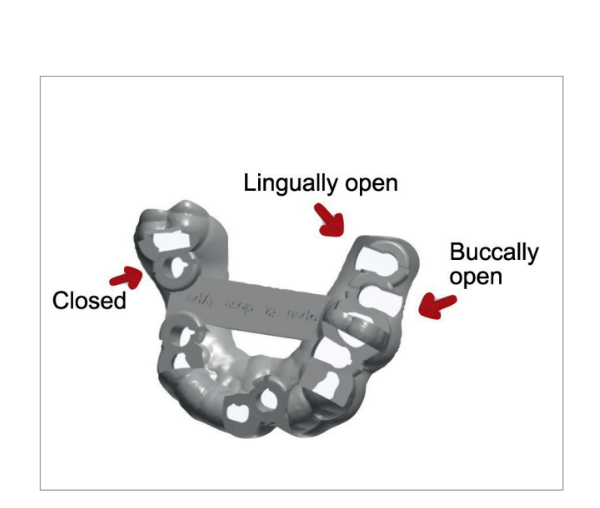
A stereolithographic surgical template was designed (Fig. 5) and fabricated using a more recent rapid-prototyping technology (New Ancorvis, Bargellino, Italy) (Fig. 6). All surgical templates were designed without metallic sleeves using biocompatible dental resin (Dental LT Clear; Formlabs, Somerville, MA, USA) with open windows to accommodate any implants to be placed in restricted occlusal space situations (premolar/molar area). A laterally open outer sleeve was used in the posterior area (again for space reasons), allowing fully guided implant placement.
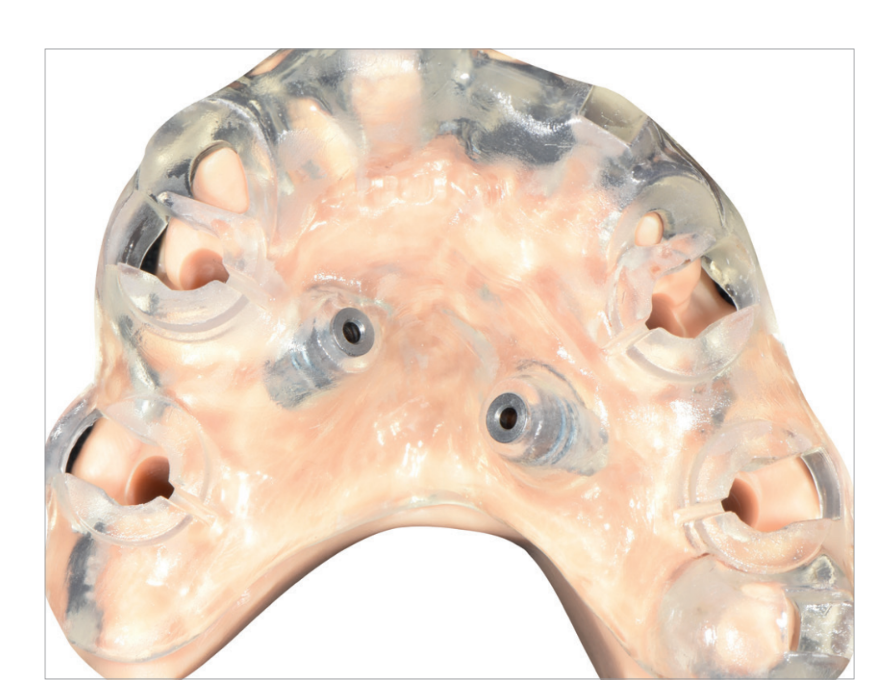
One hour before implant placement, all patients underwent professional oral tooth cleaning, a prophylactic antiseptic (Curasept, 0.2 % chlorhexidine digluconate; Curaden Healthcare, Saronno, Italy) for one minute, and prophylactic antibiotic therapy (amoxicillin 2 g, or clindamycin 600 mg if allergic to penicillin). The fit of the surgical template was tested directly in the mouth to achieve a stable fit (Fit Checker; GC, Tokyo, Japan). All patients were treated under local articaine anaesthesia with adrenaline 1 : 100,000 administered 20 minutes before surgery. The surgical templates were stabilized in relation to the opposing arch using a rigid surgical index derived from the virtual treatment plan with two to four pre-planned anchor pins. Planned implants (Osstem TSIII; Osstem Implant, Seoul, South Korea) were placed flaplessly or with a minimally invasive flap using dedicated drills (OsstemGuide Kit [Taper], Osstem) (Fig. 7).
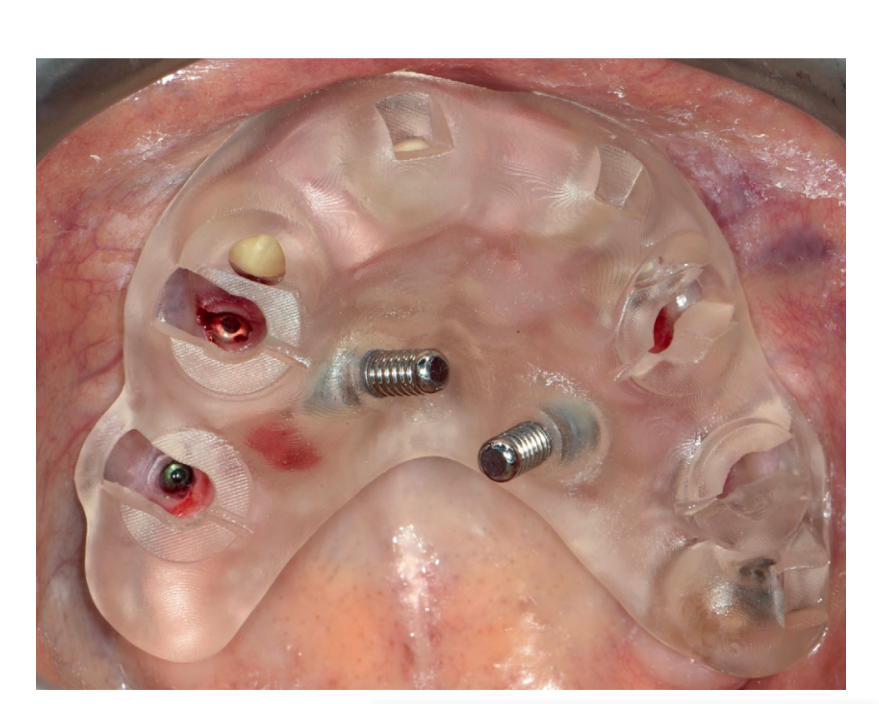
The implant site or sites were prepared based on the bone density evaluated by the surgeon at the first drilling. Any flaps were sutured with Vicryl 4.0 sutures (Vicryl; Ethicon J&J International, Sint-Stevens-Woluwe, Belgium). Immediately after implant placement, digital impressions (3M True Definition Scanner; 3M Italia) were taken at implant level in both groups, using dedicated abutments (Scanbody type AQ; New Ancorvis srl), to check the position of the implants placed. Hopeless teeth were extracted at the end of the intervention to improve the stability of the surgical template and to provide more reference points in the postoperative STL files for implant position measurements.
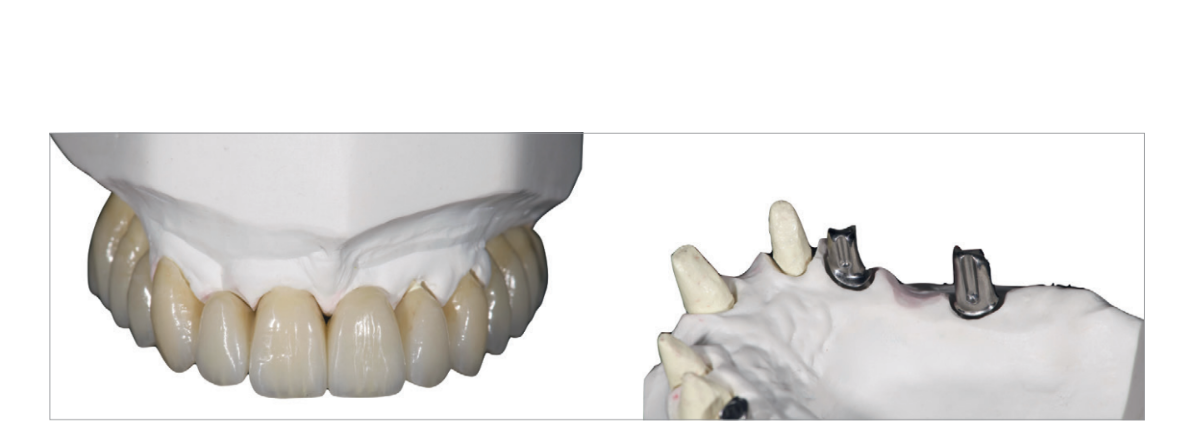
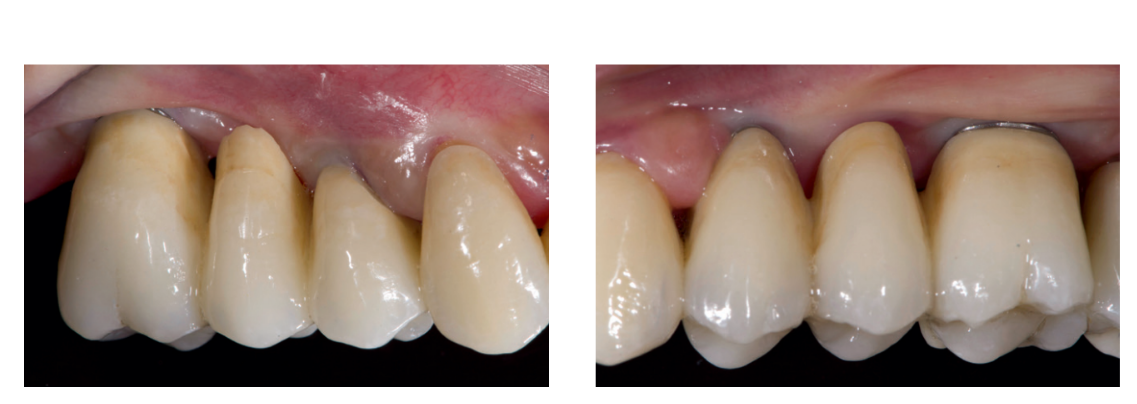
Following implant placement, all patients received oral and written recommendations about medication, oral hygiene and diet. Any sutures were removed 10 to 14 days later after local cleaning using an antiseptic (Curasept, 0.2 % chlorhexidine digluconate; Curaden Healthcare). Four months after implant placement, definitive impressions were taken using a customized open tray (Elite LC tray; Zhermac, Badia Polesine, Rovigo, Italy). Definitive screw-retained restorations were delivered a month later. The occlusion was adjusted to eliminate any premature contacts (Figs. 8 to 10b). Patients were followed every six months for hygiene maintenance and occlusion controls.
Outcome metrics
- Failures: An implant was considered a failure if it had to be removed due to a lack of stability, implant mobility, progressive marginal bone loss or infection or mechanical complications (for example, implant fracture) rendering the implant unusable. The stability of individual implants was assessed during the delivery of the definitive crowns by tightening the abutment screw with a torque of 20 Ncm, and then by percussion testing one year after implant placement.
- Complications: Early surgical and template-related complications (limited access in posterior areas, buccal bone dehiscence due to a mismatched surgical template, insertion of a different implant than planned, and fracture of the surgical template) were recorded. All complications were recorded by the expert clinician (MT) during the follow-up.
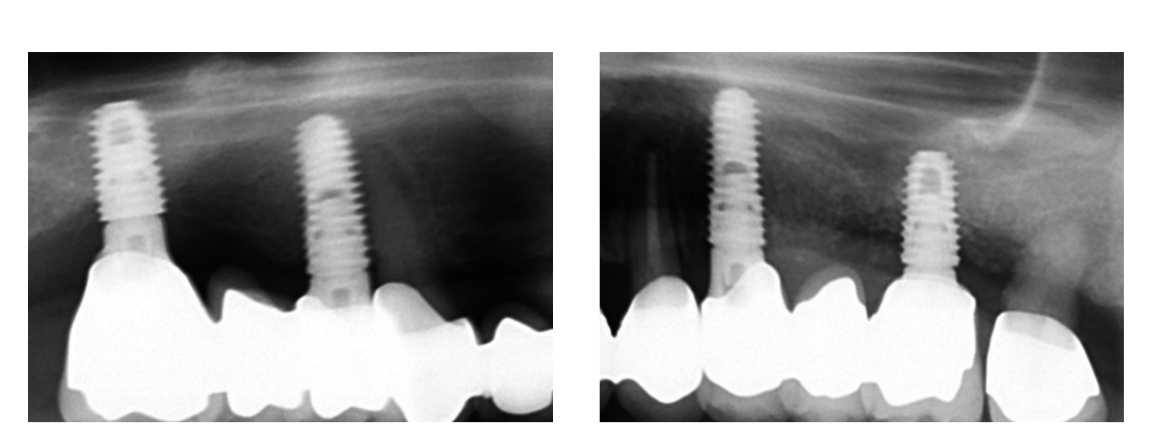
- Accuracy: Three deviation parameters (horizontal, vertical, angular) were defined and calculated between the planned and placed implant positions. The postoperative STL file, derived from the intraoral scan, was geometrically aligned with the files exported from the planning software by automated image registration (Dental Scan, ver. 6; Open Technologies, Brescia, Italy) (Fig. 11). The horizontal (lateral), vertical (depth) and angular deviation between virtual and placed implants were calculated along the long axis of each implant. An expert blinded mechanical engineer (FC) performed all the measurements.

Statistical analysis
Patient data was collected in a Numbers spreadsheet (Version 3.6.1 for Mac OS X 10.11.4). A biostatistician with expertise in dentistry analyzed the data using SPSS software for Mac OS X (version 22.0; SPSS., Chicago, IL, USA). A descriptive analysis was performed for numeric parameters using means ± standard deviations and medians with confidence intervals (95 % CI). Complications were compared between the two groups using Fisher’s exact probability test.
The mean differences of the overall deviation in clinical outcomes from the plan were compared between groups using the non-parametric Mann-Whitney U test. All statistical comparisons were conducted at a 0.05 level of significance.
Results
A total of 38 patients were evaluated but seven patients were excluded for specific reasons: two due to a need for bone reconstruction, four due to inability to complete the follow-up and one refused to participate in the research.
Preoperative data were collected by the same expert clinician (MT) who also performed the implant planning. In all, 18 patients (13 women, 5 men; mean age, 51.2 years) with 48 implants were treated by the expert clinician and 13 patients (7 women, 6 men; mean age, 49.6 years) with 28 implants were treated by novice clinicians. The expert clinician assisted the novices during the procedures without directly interfering with implant placement. Before surgery, the novice clinicians received intensive theoretical training on guided surgery instruments.
All implants were inserted in healed sites according to the manufacturer’s instructions, with insertion torques ranging between 35 and 45 Ncm. By the end of the study, no patients had dropped out, no implants had failed and no complications had occurred.
The final accuracy tests revealed a total mean error in angulation of 2.96° ± 2.28° (range, 0.2°–6.8°; 95 % CI, 1.46°–3.94°) for the expert and 3.61° ± 3.0° (range, 0.2°–11.8°; 95 % CI, 0.97°–4.23°) for the novice clinicians. The difference was not statistically significant (p = 0.5383).
The mean error in the horizontal (mesiodistal) plane was 0.64 ± 0.32 mm (range, 0.2–1.5 mm; 95 % CI, 0.43–0.77 mm) for the expert clinician and 0.97 ± 0.55 mm (range, 0.44–2.53 mm; 95 % CI, 0.59–1.19 mm) for the novice clinicians. The difference was not statistically significant (p = 0.0820).
The mean error in the vertical (apicocoronal) plane was 0.38 ± 0.32 mm (range, 0.08–1.0 mm; 95 % CI, 0.13–0.47 mm) for the expert clinician and 0.40 ± 0.41 mm (range, 0.0–1.3 mm; 95 % CI, 0.0–1.44 mm) for the novice clinicians. The difference was not statistically significant (p = 0.9026).
Subgroup comparison of implant accuracy between expert and novice clinicians revealed no statistically significant differences between both open and closed windows.
Discussion
Several works have been published in recent years on the accuracy of the digital guided implant surgery to scientifically assess these techniques. The present prospective comparative study was conducted to examine the mean error in accuracy between virtual planning and actual implant position for guided implant placement performed by novice clinicians. Both expert and novice clinicians achieved successful results, and no statistically significant differences were observed regarding early implant failure, template-related complications, or implant accuracy.
To the best of our knowledge, at the time of this writing, there were no published similar RCTs or comparative studies. This makes it difficult to evaluate to what extent the present results were consistent with other comparable studies.
The main limitation of the present study was that no a-priori sample size calculation was performed; the limited power of the analysis, due to a limited number of participants, may have hidden some differences between groups.
Nevertheless, the present study is one of the first to evaluate in vivo the virtual planning accuracy for implants placed in guided surgery. All procedures were conducted in a real clinical situation, allowing the results of the present study to be generalized to a larger population with similar characteristics and under similar conditions.
Patients today expect top quality and outstanding aesthetic results, with a minimum number of appointments. To meet these expectations, collaboration within the treatment team and network is crucial.
In the present study, although there was a trend towards higher accuracy achieved by the expert clinician, no statistically significant differences were found. This could be due to careful case planning, an easy-to-use surgical kit and a simplified protocol.
Furthermore, the discrepancy between virtual planning and final implant position as achieved by novice users was found to be within the safety limits of the software for implant planning and comparable with previously published results. In fact, a recent published meta-analysis of in-vitro and in-vivo studies revealed a total mean error of 1.12 mm at the emergence level and 1.39 mm at the apex.
The accuracy of guided implant placement depends on several factors, from dataset acquisition to the surgical procedure. Hands-on surgical training is the most important part of implant dentistry, allowing novice clinicians to develop the surgical skills required to accomplish good clinical results and to acquire knowledge about dedicated instrumentation.
The authors would like to underscore that modern computer-assisted template-based implant placement is a safe and predicable treatment option, both for expert and novice clinicians. It is necessary for the novice clinician to pay attention to the learning curve inherent in following the step-by-step protocol. The assistance of an expert clinician in the initial phase could be a valuable step to success.
Conclusions
With the limitations of the present study, novice users can achieve similarly successful results to expert clinicians with computer-guided template-assisted implant placement in combination with the newly developed sleeveless templates and dedicated drills.

