Microbial colonization at the implant-abutment interface and its possible influence on periimplantitis: A systematic review and meta-analysis
Abstract
Purpose: The aim of this systematic review and meta-analysis was to evaluate the microbial colonization at the implant-abutment interfaces (IAI) on bone-level implants and to identify possible association with peri-implant conditions.
Study selection: The focus question aimed to answer whether two-piece osseointegrated implants, in function for at least 1 year, in human, relate to higher bacterial count and the onset of periimplantitis, compared to healthy peri-implant conditions. Search strategy encompassed the on-line (MedLine, Google scholar, Cochrane library) literature from 1990 up to March 2015 published in English using combinations of MeSH (Medical Subject Headings) and search terms. Quality assessment of selected full-text articles was performed according to the ARRIVE and CONSORT statement guidelines. For data analysis, the total bacterial count of Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Prevotella intermedia, and Fusobacterium nucleatum was calculated and compared to IAI with or without peri-implant pathology.
Results: A total of 14 articles, reporting data from 1126 implants, fulfilled the inclusion criteria and subjected to quality assessment. The selected studies revealed contamination of the IAI, in patients who received two-piece implant systems. Meta-analysis indicated significant difference in total bacterial count between implants affected by periimplantitis versus healthy peri-implant tissues (0.387 0.055; 95% CI 0.279–0.496). Less bacterial counts were identified in the healthy IAI for all the investigated gram-negative bacteria except for T. forsythia.
Introduction
Microgaps at the implant-abutment interface (IAI) are typical for two-piece osseointegrated dental implant systems and seem to play a significant role in bacterial colonization at the peri-implant sulcus. This, in turn, may yield to peri-implant inflammatory reactions and subsequently loss of supporting bone. Bacterial leakage at the IAI along with the abutment screw assemblies that act as bacterial reservoir may trigger a host response with inflamed soft tissues and possible marginal peri-implant bone loss.
Bacterial colonization of the gap at the IAI has also been implicated in the physiological biologic width establishment. The major part of the marginal bone loss was reported during the first year after implant placement, whereafter, in patients with adequate levels of oral hygiene, the marginal bone levels stabilized over years. The microleakage at the gap between the implant and the abutment may allows the passage of acids, enzymes, bacteria and/or their metabolic products that directly affect the periodontal tissue, causing bleeding, swelling and odor. Nevertheless, morse taper connections are supposed to present lower levels of micro-leakage and marginal bone loss than external connection implants.
A recent review of literature described Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, founded in subgingival biofilm samples, as micro-organisms having a moderate evidence of association with the onset of periimplantitis. On the contrary, only some evidencewas found for Prevotella intermedia and Campylobacter rectus. In the anaerobic conditions, like those present inside the IAI, the microbiologic figures could be responsible for the selection, in the middle-long term, of microbiologic species able to trigger the periimplantitis process.
The objectives of this systematic review and meta-analysis were to evaluate the microbiological colonization at the implant-abutment interface on two-piece, bone level implants, independently from the configuration of the connection, and investigate whether it relates to the onset of periimplantitis.
Study selection
This systematic review conformed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prisma-statement.org). The protocol of this systematic review has been published in the international prospective register of systematic reviews (PROSPERO, http://www.crd.york.ac.uk/PROSPERO/) with registration number CRD42016037481. The focused question of the review was to identify whether there is a relationship between the presence of higher bacterial count and the onset of periimplantitis, compared to healthy peri-implant conditions in patients with two-piece osseointegrated implants after at least 1 year of function. Periimplantitis was defined by the presence of peri-implant probing depth ≥5mm associated with bleeding on probing and/or suppuration, and radiographic images of bone loss ≥3 mm, compared to initial radiographs at delivery of the prosthetic restoration.
2.1. Information sources
Articles published only in English were searched that reported on microbial colonization at the IAI and its relationship with the onset of periimplantitis, published from 1990 until March 2015 PubMed database of the US National Library of Medicine (http://www.ncbi.nlm.nih.gov/pubmed/), Google scholar (http://www.google.com) and the Cochrane Library (http://www.cochranelibrary.com/). Furthermore, the references of the included articles were checked manually in order to find additional articles.
2.2. Search strategy
Initially, PICOS question (Population (P), Intervention (I), Comparison (C), Outcomes and Study Design (O), Study type (S)) defined the search strategy, where P=Two-piece osseointegrated implants with a diagnosis of periimplantitis after at least 1 year of function; I=Microbial colonization at the IAI; C=Healthy peri-implant conditions; O=Survival rate; S=Randomized controlled clinical trials (RCT) and clinical follow-up studies.
The electronic databases were searched using a combinations of MeSH (Medical Subject Headings) terms, search terms and their combinations: “dental implants” [MeSH] AND “bacterial contamination” OR “presence of bacterium” OR “dental leakage/microbiology” [MeSH] OR “microleakage” OR “microbiological findings” OR “microbiological colonization” OR “microbiota” OR “peri-implant microflora” AND “periimplantitis” [MeSH] OR “peri-implant pathology” OR “peri-implant disease” AND “Dental Abutments*/microbiology” [MeSH]“connection, implant-abutment” OR “dental Implant- abutment design” [MeSH] OR “implant-abutment junction” OR “implant-abutment microgap” OR “inner space of dental implants” OR “inner part of dental implants”.
2.3. Study Selection and eligibility criteria
All titles and abstracts of the selected studies were first assessed for the following inclusion criteria: (1) Articles written in English; (2) studies with a clinical examination of the patients; (3) studies assessing the counts of different bacterial species (bacterial count, BC) at the IAI level in patients who received two-stage bone level implant systems, independently from the configuration of the connection; (4) Randomized controlled clinical trials (RCTs), prospective cohort studies or cross-sectional studies reporting on implants in function for at least 1year.
After evaluating the full text of the articles according to the previously defined exclusion criteria, articles with the following features, without restriction in languages, were not considered eligible: (a) Letters, narrative or historical reviews; (2) animal and in vitro studies; (3) Reports on locally or systemically compromised sites and/or conditions (i.e. major bone defect before implantation, bone pathologies, head and neck radiotherapy, treatment with bisphosphonates); (4) reports on patients who received mechanical debridement in the previous 3 months or antibiotics in the last 6 months before analysis.
2.4. Data collection process
Two calibrated reviewers (M.C. and L.C.) screened and collected the data from selected papers onto structured tables. Cohen’s Kappa values between examiners were calculated at both the first and the second stage of the research. Discrepancies were resolved by consensus and a third examiner (M.T.) was consulted.
Articles without abstracts but with titles related to the objectives of this review were selected and their full text were screened for eligibility. Reference lists of the selected articles were further screened for possible additional papers. Additionally, hand searches of the bibliographies of selected systematic reviews were conducted limited to the following journals: Clinical Implant Dentistry and Related Research; Clinical Oral Implants Research; International Journal of Oral and Maxillofacial Implants; Journal of Clinical Periodontology; Journal of Periodontology.
2.5. Assessment of quality, heterogeneity and risk of bias of individual studies
The same reviewers assessed the risk of bias in the included sample according to the guidelines provided by the CONSORT statement for the evaluation of randomized controlled trials (http://www.consort-statement.org), the STROBE statement for observational studies (http://www.strobe-statement.org), as well as the modified items from the Cochrane Collaboration Tool for assessing risk of bias (Table 1).
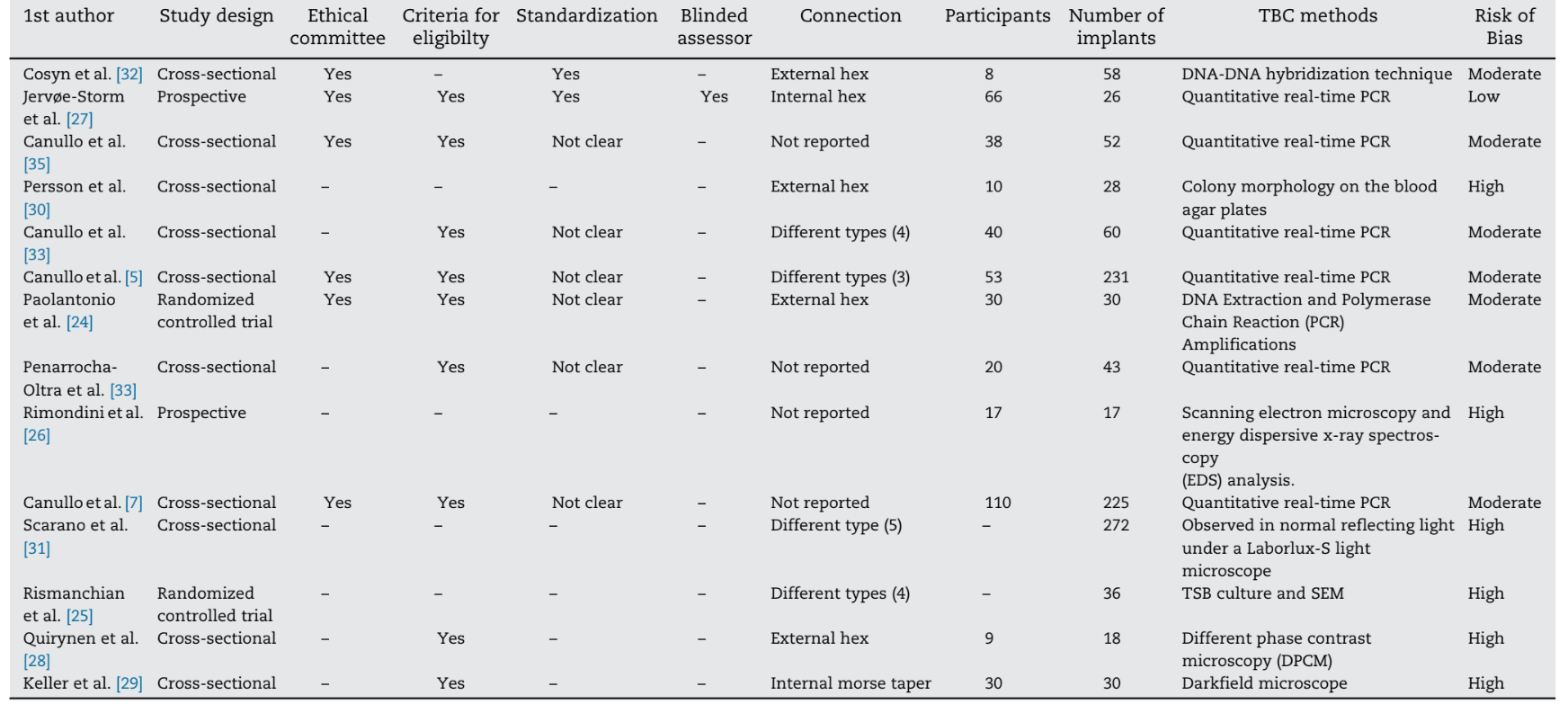
Considering the adequacy in the respective studies, the items were graded and the percentage of positively graded items was calculated. Quality assessment was performed in two different phases, namely phase I where quality assessment was based on the published full-text articles performed independently by both reviewers and in phase II where disagreements were resolved upon discussion. After collecting the scores at phase II of quality assessment, an overall estimation of plausible risk of bias (low, moderate or high) was completed for each selected study. While a low risk of bias was estimated when all the criteria were met, a moderate risk was considered when one or more criteria were partly met and a high risk of bias was estimated when one or more criteria were not met (Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. http://www.cochrane.org/resources/handbook).
2.6. Measures and analysis of results
Descriptive statistics, meta-regression and meta-analysis were performed, based on the comparable studies reporting the same outcome measures. The microbiota present at the IAI of implants in function for at least 1 year was considered for data analysis. BCs of gram-negative bacteria associated with chronic periodontitis (P. gingivalis, T. forsythia, T. denticola, P. intermedia, and Fusobacterium nucleatum) were extracted and defined as primary outcome variable. The included microbiota for the analysis are regularly detected at periimplantitis sites and are found to increase the risk for peri-implant bone loss and disease progression. Mean differences were combined using random-effects models. Heterogeneity between studies, subgroup analyses, meta-analysis, and forest plots were calculated using a software program (Comprehensive Meta-Analysis V3; Biostat, Englewood, NJ, USA).
Results
3.1. Study selection
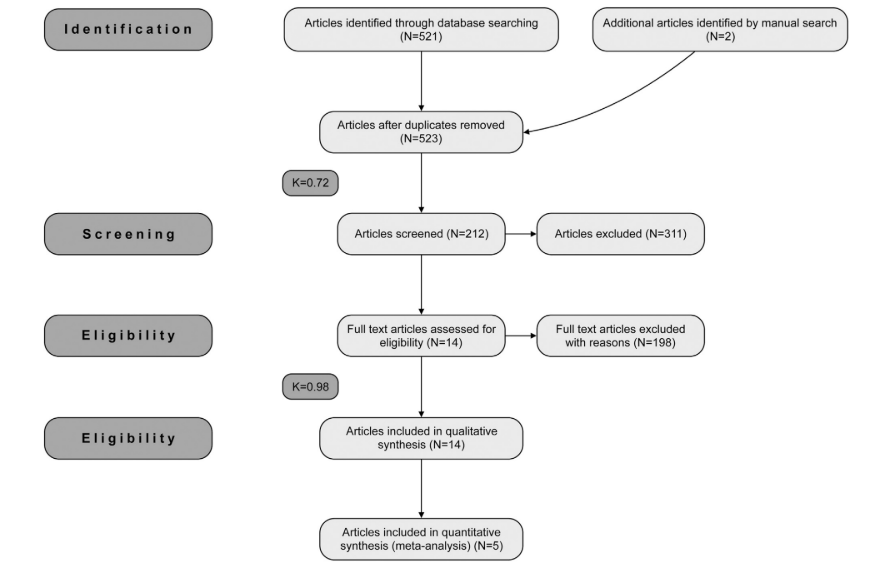
A total of 523 potentially relevant titles and abstracts were found after the electronic and manual search. During the first stage of selection, 309 articles were excluded based on the titles and abstracts (k=0.72). During the second phase, complete full-text articles of the remaining 212 publications were evaluated and 198 articles were excluded since they did not fulfill the inclusion criteria (k=0.98). Finally, a total of 14 articles, reporting data from 1126 implants, were selected that fulfilled inclusion criteria and quality assessment required for this systematic review (Fig. 1).
3.2. Study characteristics
The 14 selected articles were published between 1993 and March of 2015, two of which were RCTs, two prospective cohort studies, and ten cross-sectional studies. Only one prospective clinical study was written following the STROBE statement for observational studies (http://www.strobe-statement.org). Hence, a direct comparison between the selected articles was not possible.
3.3. Risk of bias within studies
None of the retrospective studies were able to fulfill all the requisites. One publication was associated with a low risk of bias, seven with moderate risk of bias and six with high risk of bias. The included articles received minimum grading when evaluating submission to ethical committees (6/14), presence of blinded evaluators (2/ 14), standardization of the procedures (1/14) and presence of eligible criteria (9/14) (Table 1).
3.4. Measures and meta-regression analysis
3.4.1. Bacterial leakage at the IAI
All selected studies reported contamination of the IAI and the abutment surface in patients receiving the assembly of a two-stage implant system. Quantitative real-time polymerase chain reaction (PCR) was carried out for BC in 7 of the 14 studies where the following pathogens were analyzed: Aggregatibacter actinomycetemcomitans, P. gingivalis, T. forsythia, T. denticola, P. intermedia, Parvimonas micra, F. nucleatum, C. rectus, Eikenella corrodens, Candida albicans, Enterococcus faecalis, and Porphyromonas aeruginosa. While in one study the checkerboard DNA-DNA hybridization technique was used, in other six studies different techniques including a scanning electron microscopy was used in order to screen the colony morphology. In one study, progressive colonization by periodontal pathogenic bacteria was described in the internal portions of two-piece implants. In another study, intra-coronal components of screw-retained fixed restorations were heavily contaminated in all the specimens. Contamination of abutment screws most likely occurred from the peri-implant sulcus through the IAI and abutment-prosthesis interface. Likewise, significant differences in antibiotic-resistant nosocomial bacteria (E. faecalis and P. aeruginosa) were observed at the internal and external implant components between healthy peri-implant sulci and implants compromised with periimplantitis. Regarding the absence/presence of the bacteria analyzed, no relevant differences were found between the analysis at the peri-implant sulcus and the connections inside the abutments surfaces. The microbial composition at the neighbouring teeth resembled those found in the the peri-implant sulcus with a high frequency for P. gingivalis, T. forsythia, P. intermedia, P. micra and E. corrodens.
Two comparative studies between healthy peri-implant conditions versus implants affected by periimplantitis, reported bacterial contamination in both groups. Orange complex species (P. intermedia, P. micra, F. nucleatum) were the most prevalent in all sites analyzed for both groups. Inside of the implant connection, the prevalence of the analyzed species was more predominant in the periimplantitis group and varied from 1.1% A. actinomycetemcomitans to 98.9% F. nucleatum. Species with ≥50% of prevalence were: P. gingivalis, T. denticola, P. intermedia, F. nucleatum, C. rectus, E. corrodens, T. forsythia and P. micra.
3.4.2. Bacterial leakage at the IAI in relation to abutment connection design
The selected sample showed greater heterogeneity regarding the type of the IAI. Four studies reported on external hexagon connections and two studies either on internal hexagons or morse taper connections. Four studies used different IAI designs, while the type of IAI was not reported in the other 4 manuscripts.
The evaluation of four different IAIs implied that all the analyzed connections presented contamination after 5 years of functional loading. It also appeared that the connection design might have influenced the BC levels qualitatively and quantitatively, especially inside the implant connections, showing better results for the conical connection. Similarly, different types of abutments showed significant variation on the mean microgap size within the first 5h of loading. However, no significant influence of micro-leakage was found at 24 h, 48 h, and 14 days on BC levels. Yet, the use of standard abutments significantly decreased the microgap size compared to customized ones. The study concluded that the microleakage in the connection area was comparable for all of the analyzed abutments.
3.4.3. Meta-regression and analysis of subgroups
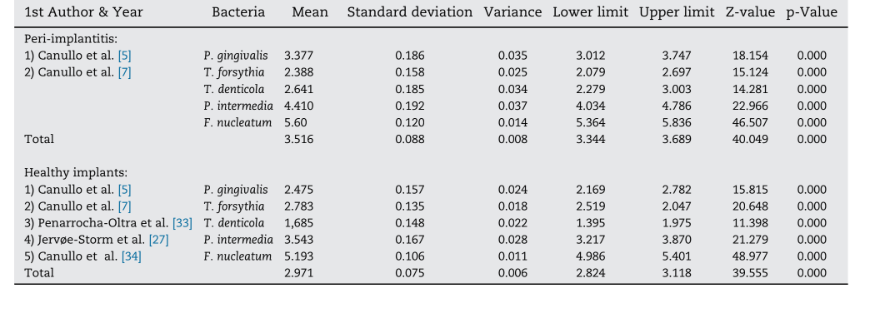
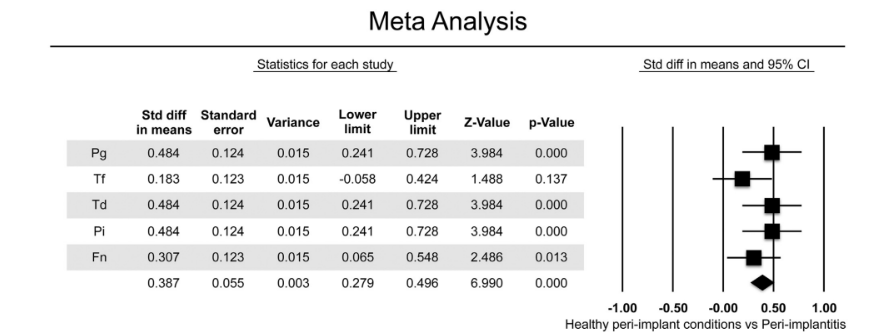
Five studies, including a total of 622 implants (n=223 with periimplantitis; n=399 with healthy peri-implant conditions) in function for at least 1 year, were included in the meta-analysis. BC of gram negative anaerobic showed relevance to chronic periodontitis and founded to increase the risk for peri-implant bone loss and disease progression due to the presence of periodontal patogens (P. gingivalis, T. forsythia, T. denticola, P. intermedia, F. nucleatum) (Table 2)]. Meta-analysis considered the bacteria that were evaluated in all selected studies. Two out of five studies compared the BC in healthy peri-implant conditions versus implants affected by periimplantitis. Meta analysis revealed higher mean values for the BC of all the gram-negative bacteria analyzed, except for T. forsythia in implants with periimplantitis (Fig. 2). Overall, the mean differences in BC were statistically significant between the two analyzed groups, with higher values in implants with periimplantitis (difference: 0.387 ± 0.055; 95% CI 0.279–0.496, p=0.000).
Discussion
This systematic review evaluated the microbial colonization at the IAI on bone level implants and related it to the possible onset of periimplantitis. One-piece implants were excluded from the analysis because of the supracrestal position of their IAJ: in fact, this location, due to the aerobiotic environment, leads to a completely different microbiologic composition.
In the present review, except for T. forsythia, significantly higher BC was identified at implants affected by periimplantitis compared to healthy peri-implant sulci for all gram-negative plausible periodontal pathogens. For the T. forsythia, only a trend toward higher BC was detected.
The included studies assessed the microbiota at the level of IAI in patients who received two-stage bone level implant systems with various implant-abutment connection designs. Nevertheless two studies reported the contamination of the IAI independent from the connection design. No distinction could be made between screw- and cemented- retained restorations assuming that the crown-abutment junction is located more coronal, and that the gap is filled with cement. Furthermore, the included manuscripts did not found differences between submerged and non-submerged implants in microbial colonization.
Presence of bacterial contamination at the IAI placed at the alveolar bone level was demonstrated to be associated with significant inflammatory cell infiltration and bone loss. Increased accumulation of acute inflammatory cells adjacent the IAI suggests the persistence of chemotactic stimuli from this region sustaining continuous recruitment of neutrophilic granulocytes. Additionally, the presence of an inflammatory infiltration of peri-implant tissue at the fixture-abutment interfaces was also led by microbial internal contamination.
A number of studies reported that microbial contamination could occur at the level of IAI both in implants with healthy and diseased tissue conditions. Despite the fact that there were no clinical signs of periimplantitis, the presence of the bacterial species associated with this condition were clearly elevated. When clinical and microbiological characteristics in subjects and implants with healthy tissue conditions or periimplantitis were evaluated and data from healthy and diseased implant sites were compared within the same subject (534 patients; 1507 dental implants), clear trends were observed. Microbial analysis obtained from three locations (peri-implant sulcus (PIS), inner parts of the implant connections (PI), gingival sulcus of the neighboring teeth) along with clinical parameters (bleeding on probing, probing pocket depth, plaque index), presence of periimplantitis was evident in 10.3% of the patients and in 7.3% of the implants. The microbial analysis within the 53 patients affected by peri-implantitis revealed no relevant differences between the analysis at the PIS and PI.
Microgap at the IAI may also yield to mechanical and biological complications including abutment screw fractures and peri-implant diseases. Microgap size and microbial leakage at different times at the IAI of 4 different abutments to Straumann implants denoted significant effect on the mean microgap size (p<0.001) and on the mean number of bacterial colonies (CFU/mL) leaking from the IAI within the first 5h of the experiment (p=0.012). However, the micro-leakage at 24 h, 48 h, and 14 days was no longer influenced significantly (p=0.145).
Clinical and microbial differences between healthy peri-implant conditions and periimplantitis revealed that the microbial prevalence was higher in the periimplantitis group at three locations and the differences in prevalence between different types of bacteria were more marked inside the connection than in the PIS (57 patients; 122 implants).
When opportunistic pathogens (E. faecalis, P. aeruginosa) were identified in the presence of peri-implant disease at the level of PIS of each implant, gingival sulcus of the adjacent teeth and the connection and abutment at the inner portion of each implant, significant differences on the presence and amount of nosocomial bacteria were detected around diseased implants. This might suggest the importance of connection decontamination in case of treatment of periimplantitis.
Different attempts were proposed to reduce the bacterial colonization at the IAI in case of healthy implants. However, the application of 0.2% chlorhexidine solution at two stage surgeries is considered a more common practice. Yet, controversial opinions exist on the effectiveness of chlorhexidine solution in preventing microbial colonization at the IAI. Bacterial endotoxins typically penetrate the IAJ, but 0.2% chlorhexidine solution could not significantly eliminate the penetration, at least in the long run. On the other hand, no indications were provided in the Literature in case of implants affected by periimplantitis.
Not only microbial leakage through the gap between the supra-structure and the abutment but also implant designs and materials may affect the potential risk of harboring oral microorganisms. Typically, morse taper connections developed less bacteria as opposed to four-groove conical internal connection. Similarly, morse taper connections presented favorable results in this respect compared to trilobed cemented connection. On the other hand, bacterial microbiota present inside the implant connection and in the PIS fluid of implants with healthy peri-implant conditions with four different implant systems after at least 5 years of functional loading, demonstrated microbiological contamination in all types of connections regardless of the site (peri-implant sulcus, inner portion of the connection, abutment surface and gingival sulcus of neighboring teeth).
From a clinical standpoint, current evidence may suggest removal of the crown/abutment complex and the disinfection/ sterilization of the connection units both at the implant and abutment aspects, in case of peri-implant disease, as an adjunct to maintenance regimens of dental implants.
Conclusions
This meta-analysis indicated that bacteria could easily be colonized at the implant-abutment interface. It is evident from a clinical point of view that inner portions of IAI should always be considered contaminated, even in clinically healthy conditions.
Marco Tallarico, Luigi Canullo, Martina Caneva, Mutlu Özcan
References
- Hermann JS, Buser D, Schenk RK, Schoolfield JD, Cochran DL. Biologic width around one- and two-piece titanium implants. Clin Oral Implants Res 2001;12:559–71.
- Tsuge T, Hagiwara Y, Matsumura H. Marginal fit and microgaps of implant-abutment interface with internal anti-rotation configuration. Dent Mater J 2008;27:29–34.
- Schwarz F, Hegewald A, Becker J. Impact of implant-abutment connection and positioning of the machined collar/microgap on crestal bone level changes: a systematic review. Clin Oral Implants Res 2014;25:417–25.
- Weng D, Nagata MJH, Bell M, Bosco AF, de Melo LGN, Richter EJ. Influence of microgap location and configuration on the periimplant bone morphology in submerged implants. An experimental study in dogs. Clin Oral Implants Res 2008;19:1141–7.
- Canullo L, Penarrocha-Oltra D, Covani U, Botticelli D, Serino G, Peñarrocha M. Clinical and microbiological findings in patients with periimplantitis: a cross-sectional study. Clin Oral Implants Res 2015;27:376–82.
- Canullo L, Peñarrocha D, Clementini M, Iannello G, Micarelli C. Impact of plasma of argon cleaning treatment on implant abutments in patients with a history of periodontal disease and thin biotype: radiographic results at 24-month follow-up of a RCT. Clin Oral Implants Res 2015;26:8–14.
- Canullo L, Peñarrocha D, Covani U, Rossetti PHO. Microbiological and clinical findings of implants in healthy condition and with periimplantitis. J Oral Maxillofac Implants 2015;30:834–42.
- Quirynen M, Bollen CM, Eyssen H, van Steenberghe D. Microbial penetration along the implant components of the Brånemark system. An in vitro study. Clin Oral Implants Res 1994;5:239–44.
- Passos SP, Gressler May L, Faria R, Özcan M, Bottino MA. Implant-abutment gap versus microbial colonization: Clinical significance based on a literature review. J Biomed Mater Res B Appl Biomater 2013;101:1321–8.
- Callan DP, Cobb CM, Williams KB. DNA probe identification of bacteria colonizing internal surfaces of the implant-abutment interface: a preliminary study. J Periodontol 2005;76:115–20.
- Hermann JS, Schoolfield JD, Schenk RK, Buser D, Cochran DL. Influence of the size of the microgap on crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged implants in the canine mandible. J Periodontol 2001;72:1372–83.
- Piattelli A, Scarano A, Paolantonio M, Assenza B, Leghissa GC, Di Bonaventura G. Fluids and microbial penetration in the internal part of cement-retained versus screw-retained implant-abutment connections. J Periodontol 2001;72:1146–50.
- Pérez-Chaparro PJ, Duarte PM, Shibli JA, Montenegro S, Lacerda Heluy S, Figueiredo LC, et al. The current weight of evidence of the microbiologic profile associated with peri-implantitis: a systematic review. J Periodontol 2016;87:1295– 304.
- Monje A, Suarez F, Galindo-Moreno P, García-Nogales A, Fu JH, Wang HL. A systematic review on marginal bone loss around short dental implants (<10mm) for implant-supported fixed prostheses. Clin Oral Implants Res 2014;25:1119–24.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Methods of systematic reviews and meta-analysis preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12.
- Lindhe J, Meyle J, Group D of European Workshop of Periodontology. Peri-implant diseases: consensus report of the sixth european workshop on periodontology. J Clin Periodontol 2008;35:282–5.
- Lang NP, Berglundh T, Working Group 4 of the Seventh European Workshop on Periodontology. Periimplant diseases: where are we now? Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 2001;38: 178–81.
- Miller SA, Forrest JL. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid Based Dent Pract 2001;1:136–41.
- Graziani F, Figuero E, Herrera D. Systematic review of quality of reporting, outcome measurements and methods to study efficacy of preventive and therapeutic approaches to peri- implant diseases. J Clin Periodontol 2012;39:224–44.
- Pjetursson BE, Zwahlen M, Lang NP. Quality of reporting of clinical studies to assess and compare performance of implant-supported restorations. J Clin Periodontol 2012;39:139–59.
- Mombelli A, Décaillet F. The characteristics of biofilms in peri-implant disease. J Clin Periodontol 2011;38:203–13.
- Mombelli A. Microbiology and antimicrobial therapy of periimplantitis. Periodontol 2000 2002;28:177–89.
- Leonhardt Å, Dahlén G, Renvert S. Five-year clinical, microbiological, and radiological outcome following treatment of periimplantitis in man. J Periodontol 2003;74:1415–22.
- Paolantonio M, Perinetti G, D’Ercole S, Graziani F, Catamo G, Sammartino G. Internal decontamination of dental implants: an in vivo randomized microbiologic 6-month trial on the effects of a chlorhexidine gel. J Periodontol 2008;79:1419–25.
- Rismanchian M, Hatami M, Badrian H, Khalighinejad N, Goroohi H. Evaluation of microgap size and microbial leakage in the connection area of 4 abutments with Straumann (ITI) implant. J Oral Implantol 2012;38:677–85.
- Rimondini L, Marin C, Brunella F, Fini M. Internal contamination of a 2-component implant system after occlusal loading and provisionally luted reconstruction with or without a washer device. J Periodontol 2001;72:1652–7.
- Jervøe-Storm PM, Jepsen S, Jöhren P, Mericske-Stern R, Enkling N. Internal bacterial colonization of implants: association with peri-implant bone loss. Clin Oral Implants Res 2015;26:957–63.
- Quirynen M, van Steenberghe D. Bacterial colonization of the internal part of two-stage implants: An in vivo study. Clin Oral Implants Res 1993;4:158–61.
- Keller W, Brägger U, Mombelli A. Peri-implant microflora of implants with cemented and screw retained suprastructures. Clin Oral Implants Res 1998;9:209–17.
- Persson LG, Lekholm U, Leonhardt A, Dahlén G, Lindhe J. Bacterial colonization on internal surfaces of Brånemark system implant components. Clin Oral Implants 1996;7:90–5.
- Scarano A, Assenza B, Piattelli M, Iezzi G, Leghissa GC, Quaranta A. A 16-year study of the microgap between 272 human titanium implants and their abutments. J Oral Implantol 2005;31:269–75.
- Cosyn J, Van Aelst L, Collaert B, Persson GR, De Bruyn H. The peri-implant sulcus compared with internal implant and suprastructure components: a microbiological analysis. Clin Implant Dent Relat Res 2009;13:286–95.
- Penarrocha-Oltra D, Rossetti PHO, Covani U, Galluccio F, Canullo L. Implant/abutment connection microleakage due to implant insertion maneuvers: cross-sectional microbiological analysis in implants after 5 years of loading. J Oral Implantol 2014;41:e292–6.
- Canullo L, Penarrocha-Oltra D, Soldini C, Mazzocco F, Penarrocha M, Covani U. Microbiological assessment of the implant-abutment interface in different connections: cross-sectional study after 5 years of functional loading. Clin Oral Implants res 2014;26:426–34.
- Canullo L, Rossetti PHO, Peñarrocha D. Identification of Enterococcus aaecalis and Pseudomonas aeruginosa on and in implants in individuals with peri-implant disease: a cross-sectional study. Int J Oral Maxillofac Implants 2015;30:583–7.
- Socransky SS, Haffajee AD, Ximenez-Fyvie LA, Feres M, Mager D. Ecological considerations in the treatment of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis periodontal infections. Periodontol 2000 1999;20:341–62.
- Socransky SS, Haffajee AD, Smith C, Duff GW. Microbiological parameters associated with IL-1 gene polymorphisms in periodontitis patients. J Clin Periodontol 2000;27:810–8.
- Broggini N, McManus LM, Hermann JS, Medina R, Schenk RK, Buser D. Peri-implant inflammation defined by the implant-abutment interface. J Dent Res 2006;85:473–8.
- Broggini N, McManus LM, Hermann JS, Medina RU, Oates TW, Schenk RK. Persistent acute inflammation at the implant-abutment interface. J Dent Res 2003;82:232–7.
- Cosyn J, Eghbali A, De Bruyn H, Collys K, Cleymaet R, De Rouck T. Immediate single-tooth implants in the anterior maxilla: 3-year results of a case series on hard and soft tissue response and aesthetics. J Clin Periodontol 2011;38:746–53.
- Tesmer M, Wallet S, Koutouzis T, Lundgren T. Bacterial colonization of the dental implant fixture-abutment interface: an in vitro study. J Periodontol 2009;80:1991–7.
- Nascimento CD, Pita MS, Fernandes FHNC, Pedrazzi V, de Albuquerque Junior RF, Ribeiro RF. Bacterial adhesion on the titanium and zirconia abutment surfaces. Clin Oral Implants Res 2014;25:337–43.
- Koutouzis T, Wallet S, Calderon N, Lundgren T. Bacterial colonization of the implant-abutment interface using an in vitro dynamic loading model. J Periodontol 2011;82:613–8.
- Assenza B, Tripodi D, Scarano A, Perrotti V, Piattelli A, Lezzi G. Bacterial leakage in implants with different implant-abutment connections: an in vitro study. J Periodontol 2012;83:491–7.
- Paolantonio M, Perinetti G, D’Ercole S, Graziani F, Catamo G, Sammartino G, et al. Internal decontamination of dental implants: an in vivo randomized microbiologic 6-month trial on the effects of a chlorhexidine gel. J Periodontol 2008;79:1419–25.
- Romanos GE, Biltucci MT, Kokaras A, Paster BJ. Bacterial composition at the implant-abutment connection under loading in vivo. Clin Implant Dent Relat Res 2016;18:138–45.

/public-service/media/default/446/xUzvr_671a187c9aa46.png)
/public-service/media/default/455/zGYoq_671a1ed13fef9.jpg)
/public-service/media/default/454/7jN0r_671a1b5b648ff.jpg)
/public-service/media/default/456/3eXLd_671a1f18319d2.jpg)