Applications of Laser in Non-Surgical Periodontal Therapy: A Review
Machine translation
Original article is written in PT language (link to read it) .
Summary
Despite the high number of publications, there is controversy among clinicians regarding the application of lasers in periodontal treatment. The objective of this work is to review the existing literature and determine the state of the art concerning the application of lasers in non-surgical periodontal therapy.
Chronic periodontitis is characterized by the formation of pockets and progressive loss of alveolar bone. This tissue destruction is a consequence of specific bacterial aggression and the patient's immune response. The main goal of periodontal treatment is the removal of calculus and subgingival plaque in order to suppress ecological niches and achieve attachment gain, preventing disease progression, eliminating pathogenic bacteria and their toxins. The use of lasers has been progressively suggested for periodontal therapy as a more selective, more efficient, and less traumatic technique to promote periodontal repair. Each type of laser is named according to its active medium and emits monochromatic light energy. The word LASER is the acronym for “light amplification by stimulated emission of radiation.” This concept dates back to 1917 with Einstein's Theory of Stimulated Emission, but it was only in 1960 that the first laser, the Ruby laser, was created by Maiman. Lasers began to be widely used in Medicine and Surgery since the development of the Ruby laser by Maiman. Unfortunately, these early attempts resulted in a “crack” of the dental surface and thermal damage to dentin and enamel.
For the laser to have biological effects, the energy must be absorbed, and the degree of tissue absorption varies depending on the wavelength and optical characteristics of the target tissue. Since tissues have more than one component, the resulting effect is the combination of the effects of each of its components. CO2, Nd:YAG, and Er:YAG lasers are the most used in surgical procedures performed on the soft tissues of the oral cavity, and they were the first to have pieces that could be used intraorally. Their first application in oral tissues was reported by Goldman et al and Stern and Sognnaes. It was suggested that the Nd:YAG laser be used in oral soft tissue surgery, which quickly led to the development of lasers for periodontal therapy. Characteristics of lasers such as ablation or vaporization, hemostasis, and sterilization may make it an excellent adjunct or alternative instrument in the treatment of periodontal diseases. These types of lasers provide treatments with results comparable to or sometimes superior to those provided by conventional techniques and instrumentation. There are dozens of indications for the use of lasers that range from a simple excision of gingival tissue to the removal of carious tissue. Compared to conventional treatment, lasers can be used to reduce the number of pathogenic elements, provide superior hemostasis, decrease the healing period, and also the postoperative pain period and postoperative sequelae, ensuring greater comfort for the patient. Currently, there is a large amount of literature on the application of lasers in the treatment of periodontitis, although there is enormous controversy among clinicians regarding the subject. The objective of this work is to review the existing literature and determine the state of the art regarding the application of lasers in non-surgical periodontal therapy.
Methods
A search was conducted in the databases Medline, Cochrane, and ScienceDirect from 2002 to 2009 using the keywords: Laser, Laser therapy, and Non-surgical Periodontal Therapy, selecting all articles that linked the use of laser in non-surgical periodontal therapy. Additionally, the following journals were manually examined: Journal of Clinical Periodontology; Periodontology 2000; Journal of Periodontology.
CO2 Laser
With the CO2 laser, the rapid increase in temperature and intracellular pressure leads to cell lysis. Thus, during its use, no contact with the tissue is possible. Its wavelength is almost entirely absorbed by water. Since soft tissues are composed of approximately 75% to 90% water, about 98% of the energy is converted into heat absorbed by the surface of the tissue with the slightest approach or penetration of the laser. Thus, a brown area, caused by coagulation necrosis, is visible at the laser incision, which is why in current systems the laser focus should be applied about 3 to 5 mm from the target tissue.
The depth of the laser incision is proportional to the applied power and exposure time. This laser, when applied to soft tissue surgery, is used with a variable power of 5 to 15 watts, in pulsed mode. Higher energy levels are used for tissue removal, while lower levels are for hemostasis and coagulation.
Neodymium: YAG Laser
The Nd:YAG laser at 1,064nm penetrates water to a depth of 60mm. The heating effect of this laser is ideal for ablating the abnormal bleeding potential of tissue and for hemostasis of small capillaries. The penetration depth in soft tissue has been estimated to be 2+- 1mm. Recent studies concluded that thermal damage can occur in the underlying bone. Harmful intra-pulpal heating has also been reported in in vitro studies. Since it transmits through an optical fiber, it allows for easy application in hard-to-reach oral spaces such as periodontal pockets (Figure 1).
Erbium: YAG Laser
Only from 1997 was there an emphasis on the use of this laser on soft tissues, and the FDA approved its application on hard tissues such as enamel, cementum, and bone. Its wavelength of 2,940 nm is ideal for absorption by hydroxyapatite and water, making it efficient for the ablation of enamel and dentin. The heating generated by it produces micro-explosions in hard tissues, with minimal heating even concerning the pulp. The use of a water spray during radiation use is essential to achieve maximum effectiveness in residual removal, with minimal heat generation (Figure 2).

Diode laser
This laser was recently introduced in Dentistry, having demonstrated excellent capacity as a hemostatic agent. It is used for the removal of soft tissue by direct contact. Comparative studies have shown tissue effects similar to those of the Nd:YAG laser, but without the secondary effects on deeper tissues.
The first periodontal surgeries with Laser were performed with a CO2 laser in 1985, but these lasers initially could not access all areas of the oral cavity and could cause thermal damage to teeth and bone. Nowadays, lasers have more sophisticated systems, and the advancement in the understanding of the pathogenesis of periodontal disease has made lasers a very important tool as an adjunct to the treatment of periodontal disease. At the 6th European Consensus on Periodontology, a systematic review of the applications of laser in non-surgical periodontal therapy was conducted, concluding that they provide similar results in the short and long term between the use of the Er:YAG laser versus mechanical debridement in patients with chronic periodontitis. It also states that it was not possible to reach conclusions related to the power to be used, due to the multiple variability of studies, without a standardization of usage parameters. This Consensus concludes that the scientific evidence supporting the application of CO2, Nd:YAG; Nd:YAP lasers or diode lasers and their corresponding clinical benefit is insufficient due to the lack of available studies, and also because the studies use these lasers as adjuncts and not as alternatives to traditional treatment. The energy of the Nd:YAG laser is well absorbed by pigmented tissues (affinity for melanin), which allows its clinical application for cutting and coagulation. The main advantage is its bactericidal effect. The simultaneous use of the Nd:YAG laser during scaling and root planing disinfects the pockets, eliminates the superficial layer of bacterial plaque over the calculus, and allows for a simpler and more effective smoothing. Furthermore, it allows working in an almost blood-free field and reduces the amount of anesthesia requests from patients. Its hemostatic effect represents an advantage in the treatment of patients with coagulation disorders. Patients usually report less pain and postoperative edema.
The Nd:YAG laser reports the formation of a new periodontal attachment. When used carefully and at low power, it does not cause damage to dental surfaces and periodontium. The Er family lasers have indications for the removal of calculus, and can be used for bone ablation with minimal thermal damage to adjacent tissues. CO2 lasers are used for the remodeling of soft tissues. Diode lasers are used to reduce bleeding, providing a bactericidal effect, inflammatory reduction, and superior healing in periodontal pockets. In the studies by Neil and Melloning, patients treated with the Nd:YAG laser along with scaling and root planing showed a decrease in the gingival index compared to those who did not undergo laser therapy, and also a gain in periodontal attachment at the end of 6 months. Badder et al. described that the laser removes bacteria from soft tissues and their exotoxins (hyaluronidases and collagenases) responsible for the progression of periodontal disease (Figure 3).
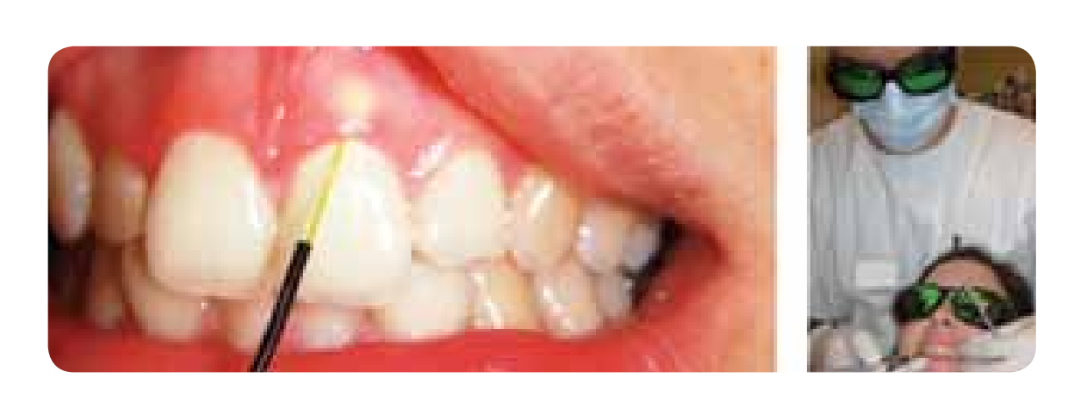
Many studies describe laser therapy as an adjunctive treatment and not a replacement for standard treatments. The curettage of periodontal pockets with laser is not effective if root scaling and planing are not performed to eliminate bacteria from the root surfaces.
The use of laser in the treatment of chronic periodontitis is based on the benefits of subgingival curettage, the induction of the laser in improving the level of attachment through regeneration of cementum, periodontal ligament, and support from the alveolar bone, and by significantly reducing pathogenic subgingival bacteria, and preparing a superior environment for the reepithelialization of the connective tissue on the root surfaces, outlining and sculpting both hard and soft tissues for a more predictable healing.
Scaling and root planing is the traditional method of controlling subgingival microflora. The goals of subgingival debridement are not only to eliminate bacteria from non-adherent plaque but also calculus deposits.
Watnabe et al. demonstrated the effectiveness of calculus removal with the Er:YAG laser without side effects, and a consequent reduction in the periodontal pocket. Schwarz et al. reported similar or superior results after 6 months with laser, compared to conventional mechanical debridement, where only bleeding upon probing and clinical attachment level showed significant differences after laser treatment. Aoki and colleagues studied the removal of tartar through the Er:YAG laser using scanning electron microscopy and histological examinations, observing results similar to those achieved by ultrasonic instrumentation. Yamaguchi et al. state that the Er:YAG laser radiation effectively eliminates most lipopolysaccharides from Gram (-) bacteria, as they have a high absorption peak for the wavelength of this laser.
Folwaczny et al. detected the antimicrobial effect of the Er:YAG laser on Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythensis, Treponema denticola, and Aggregatibacter actinomycetemcomitans with inhibition of the growth of these bacteria.
Lasers drastically reduce bacteria in surgical sites, which could potentially benefit both the patient and the Dentist in managing periodontal issues.
Protocols combining scaling and root planing with laser therapy have documented reductions in periodontal pockets and gains in clinical attachment level.
Advantages and disadvantages
The advantages of laser treatment in non-surgical periodontal treatment are the efficacy and effectiveness in the ablation of soft and hard tissues, with superior hemostasis, bactericidal effect, and minimal collateral damage with reduced use of local anesthesia. The decrease in the sound of lasers compared to traditional techniques reduces stress in patients.
As disadvantages include the initial cost of acquiring lasers and the long learning curve they require. Careless use of lasers can cause damage to adjacent structures, including eyes, if the necessary protective measures are not used. The dimensions of the devices can also be an obstacle to clinical application.
Discussion
The Er:YAG laser is used to eliminate calculus, condition root surfaces, and disinfect periodontal pockets. It allows for treatments without the need for local-regional anesthesia when used at low power. It has the potential to remove bacterial endotoxins from cementum, as lipopolysaccharides show a high absorption coefficient for this wavelength.
The Nd:YAG laser and diode lasers have as their main characteristics their bactericidal effect, but they can also cut soft tissues and debride the epithelium of the pocket. They allow for work in a nearly blood-free field and reduce the anesthesia used (Figure 4).
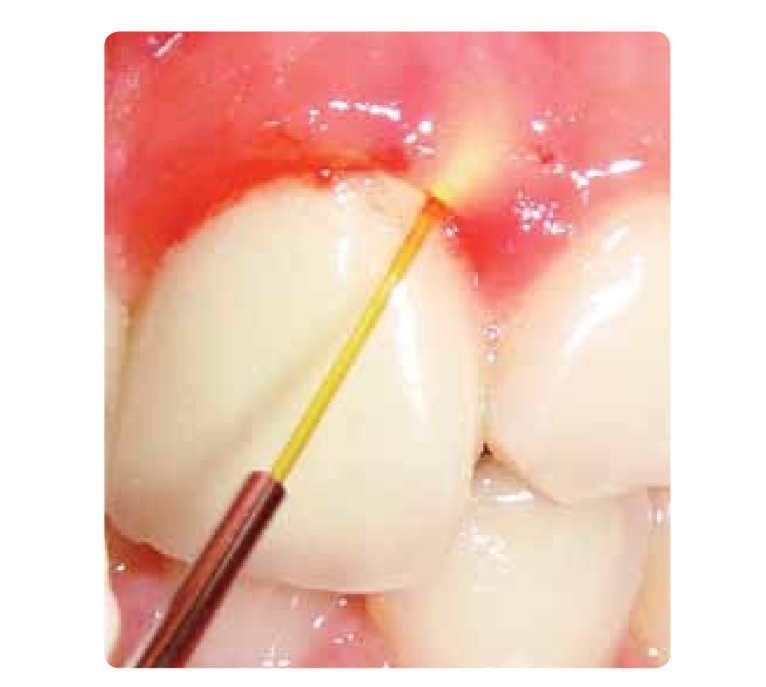
The Co2 laser used at low power in defocused mode can improve periodontal debridement and redefine the anatomy of soft tissues.
More studies with a high degree of evidence and standardization of equipment parameters are needed to allow for more solid statistical conclusions that support clinical decisions.
Conclusions
In summary, the laser can be used as an adjunct to non-surgical periodontal therapy, although there are not enough studies to consider lasers as an alternative to conventional treatments. The bactericidal effects, the elimination of calculus, the ability to remove bacterial plaque, the possibility of sculpting soft and hard tissues, and a faster and more predictable healing of soft and hard tissues make lasers a promising tool in non-surgical periodontal treatment.
João Filipe Mouzinho; João Fontes Pereira; Cristina Trigo Cabral
Bibliography
- Folwaczny M, Benner K, Flasskamp B, Mehl A, Hickel R. Effects of 2.94 µm Er:YAG laser radiation on root surfaces treated in situ: a histological study J Periodontol 2003; 74: 360-365
- Folwaczny M, Mehl A, Aggstallet H, Hickel R. Antimicrobial effects of 2.94 µm Er:YAG on root surfaces treated in situ: an in vitro study. J Clin Periodontol 2002; 29: 73-78
- Schwarz F, Berakdar M, Georg T, Reich E, Sculean A. Clinical evaluation of an Er:YAG laser combined with scaling and root planning for non-surgical periodontal treatment. A controlled, prospective clinical study. J Clin Periodontol 2003; 30: 26-34
- Maiman TH. Stimulated optical radiation in ruby lasers. Nature 1960; 187:493-94
- Wetcher SJ. Treatment of cervical intraepithelial neoplasia with CO2 laser; Laser vs cryotherapy. A review of effectiveness and cost. Obstet Gynocol Surg 1984; 39:469-73.
- Carruth JAS. Resection of the tongue with the carbon dioxide laser. J Laryngol Otolaryngol; 1982: 96:529-43
- Shapshay SM. ND:YAG laser surgery: Overview of applications. In: Joffe SN and Oguro Y. Advances in Nd:YAG laser surgery. New York; Springer-Verlag: 1988:150-55
- White JM, Goodies HE, Rose CL. Use of pulsed Nd:YAG laser for intraoral soft tissue surgery. Lasers Surg Med 1991; 11:455-61
- Goldman L, Hornby P, Meyer R, Goldman B. Impact of the laser on dental caries. Nature 1964 203:417
- Stern RH, Sognnaes RF. Laser inhibition of dental caries suggested by first test in vivo. J Am Dent Assoc 1972; 85:1087-90
- Myers TD. What lasers can do for dentistry And you. Dent Manage 1989; 29:26-8.
- Aoki A, Sasaki K, Watanabe H, Ishikawa I. Lasers in nonsurgical periodontal therapy. Periodontology 2000 2004; 36: 59-97.
- Schwarz F, Aoki A, Becker J; Sculean A. Laser application in non-surgical periodontal therapy: a systematic review. J Clin Periodontol 2008; 35 (suppl. 8 ): 29-44.
- Neill ME; Melloning JT. Clinical efficacy of the Nd: YAG laser for combination periodontitis therapy. Pract Periodontics Aesthetic Dent 1997; 9 (6 suppl): 1-5
- Hall RR, Hill DW, Beach AD. A carbon dioxide surgical laser. Ann Royall Coll Surg Eng 1971; 48:222-25
- Fuller TA. Fundamentals of laser in surgery and medicine. In: Dixon JA, ed. Surgical Application of Lasers, 2 nd ed. Chicago: Year Book Medical Publishers, Inc; 1988:16-33
- Halldorsson T, Langerholc J. Thermodynamic analysis of laser irradiation of biological tissue. Appl opt 1978; 17:3948
- Spencer P, Cobb CM, Wieliczka Dm, Glaros AG, Morris PJ. Change in temperature of subjacent bone during soft tissue laser ablation. J Periodontal 1998; 69:1278-82
- Fujii T, Baehni PC, Kawai O, Kywawkami T, Matsuda K, Kowashi Y. Scanning electron microscopic study of the effects of Er:YAG laser on root cementum. J Peridontol 1998; 69: 1283.90.
- Wyman A, Duffy S, Sweetland HM, Sharp F, Rogers K. Preliminary evaluation of a new high power diode laser. Lasers Surg Med 1992; 12:506-9.
- Sanz M, Teughels W. Innovations in Non-Surgical Periodontal Therapy: Consensus report of the sixth European Workshop on Periodontology; J Clin Periodontol 2008; 35 (Supl8) 3-7
- Cobb Charles. Laser in Periodontics: A review of literature. J Periodontol 2006. 4; 77: 545-564.
- Garcia-Ortiz de zárate F, España-Tost AJ, Berini-Aytés L, Gay-Escoda C. Applications of the Nd:YAG laser in dentistry. RCOE 2004; 9; 5: 539-545
- Yukna RA, Evans GH, Vastardis S, Carr RF. Laser Assisted periodontal regeneration in humans. Presented at the 81st General Session of the International Association for Dental Research, Gothenburg; Sweden; June 2003
- Bader H. Use of lasers in periodontics. Dent Clin North Am 2000; 44: 779-791
- Mortiz A, Schoop U; Goharkhay, et al. Treatment of periodontal pockets with a diode. Lasers Surg Med 1998; 22: 302-311
- Pfitzner A, Sigusch BW, Albrecht V, Glockmann E. Killing of periodontopathogenic bacteria by photodynamic therapy. J Periodontol 2004; 75:1343-1349.
- Watnabe H, Ishikawa I, Suzuki M, Hasegawa K. Clinical Assessments of the Er: YAG laser for soft tissue surgery and scaling. Journal of clinical Laser Medicine & Surgery 1996;14: 67-75
- Schwarz F, Sculean A, Georg T, Reich E. Periodontal treatment with Er:YAG laser compared to scaling and root planning. A controlled clinical study. J Periodontal 2001; 72: 361-67.
- Yamaguchi H, Kobayashi K, Osada R, Sakuraba E, Nomura T, Arai T, Nakamura J. Effects of irradiation of an erbium:YAG laser on root surfaces. Journal of Periodontology 1997; 68: 1151-115
- Ben Hatit Y, Blum R, Severin C, Maquin M, Jabro MH. The effects of a pulsed Nd: YAG laser on subgingival bacterial flora and on cementum: an in vivo study. J Clin Med Surg 1996; 14:137-143.
- Gutknecht N, Radufi P, Frazen R, Lampert F. Reduction of specific microorganisms in periodontal pockets with the aid of an Nd:YAG laser-an in vivo study. J Oral Laser Appl 2002; 2: 175-80.
- Coffelt DW, Cobb CM, Rapley JW, Killoy WJ. Determination of energy density threshold for laser ablation of bacteria: an in vitro study. J Clin Periodontal 1997; 24:1-7.
- Aoki A, Miuta M, Akiyama F, et al. In vitro evaluation of Er:YAG laser scaling of subgingival calculus in comparison with ultrasonic scaling. J Periodontal Res 2000; 35:266-77.
- Eberhard J, Ehlers H, Falk W, Acil Y, Albers HK, Jepsen S. Efficacy of subgingival calculus removal with the Er:YAG laser compared to mechanical debridement: an in situ study. J Clin Periodontal 2003; 30:511-18.
- Borrajo JL, Varela LG, Castro GL, Rodrigues-Nunez I, Torreira MG. Diode Laser (980nm) as an adjunct to scaling and root planning. Photomed Laser Surg 2004; 22:509-12.
- Harris DM, Gregg RH II, McCarthy DK, Colby LE, Tilt LV. Laser-assisted new attachment procedure in private practice. Gen Dent 2004; 52:396-403.
- Sculean A, Schwarz F, Georg T, Becker J. Clinical evaluation of the Er:YAG laser in combination with an enamel matrix protein derivative for the treatment of intrabony periodontal defects: a pilot study. J Clin Periodontal 2003; 30:97
