Crestal sinus lift using an implant with an internal L-shaped channel: 1-year after loading results from a prospective cohort study
Purpose: To evaluate the clinical and radiographic outcomes of a one-stage crestal sinus elevation procedure using a self-tapping endosseous implant system (iRaise, Maxillent, Herzliya, Israel) developed for sinus augmentation, 1 year after loading.
Materials and methods: Patients needing restoration in the posterior maxilla with a residual alveolar crest of 3 to 8 mm in height and 5 mm in width distal to the canine as measured on CBCT scan were treated using the iRaise sinus lift system. Outcome measures were: implant and prosthetic failures, any complications, increased bone height (iBH), marginal bone loss (MBL), implant stability quotient (ISQ), radiographic tissue remodelling patterns using the sinus grafting remodelling index (SGRI), volumetric measurements of sinus graft, patient self-reported post-surgical swelling, consumption of pain medication and histological analysis.
Results: A total of 30 consecutive participants with a mean age of 54.2 ± 9.4 years underwent a transcrestal elevation of the sinus membrane, insertion of bone graft, and implant placement. A total of 50 implants were placed (30 iRaise system implants and 20 adjunctive iSure implants, Maxillent). The mean follow-up was 15.8 ± 2.1 months after implant loading. One patient dropped out at the 1-year after loading follow-up examination. No implants and no prostheses failed during the entire follow-up. One patient experienced a small membrane tear. Before implant insertion, the mean residual alveolar ridge height was 4.64 ± 0.86 mm (range: 3.4–6.4 mm; 95% CI: 4.39–5.01 mm). One year after loading, the bone height was 16.86 ± 3.13 mm (95% CI 15.83–18.07 mm). At the 1-year after loading follow-up, the mean MBL was 0.19 ± 1.05 mm (95% CI 0.02–0.78 mm). The mean ISQ at implant placement was 65.2 ± 5.4 (95% CI 63.6–67.4) and increased during the healing period reaching the mean value of 73.6 ± 3.7 (95% CI 73.1–75.9; range 62–79). The difference was statistically significant (8.4 ± 5.3; 95% CI 5.9–39.7; P = 0.0000). One year after loading, SGRI score was evaluated in 23 implants. Overall, the mean SGRI value was 2.29 ± 2.41 mm (95% CI 1.22–2.98 mm). Bone volume at implant placement was 2.41 ± 0.25 CC (95% CI 2.22–2.48 CC). During the 6-month, submerged healing period, a slight bone contraction of 11.3% were observed. (2.13 ± 0.24 CC;95% CI 2.02–2.26; difference = 0.27 ± 0.25 CC; 95% CI 0.10–0.36; P = 0.0011). At the first year post-loading period, the bone graft remained stable (2.11 ± 0.22 CC; 95% CI 2.02–2.24). The difference was not statistically significant (0.02 ± 0.07 CC; 95% CI 0.01–0.04; P = 0.2166). From the patient’s point of view, the mean pain value was 0.52 ± 0.74 (range 0–3); mean swelling value was 0.27 ± 0.52 (range 0–2); and the mean consumption of analgesic was 0.87 ± 4.94 tablets (range 0–4) 3 days after surgery. Morphological and histomorphometric analyses showed that all the samples had a normal structure without inflammatory infiltrate, six months after healing. The following fractions (%) were found: bone (immature bone + mature bone): 44.07 ± 4.91; residual biomaterial: 23.98 ± 2.64; medullary spaces: 31.95 ± 3.16.
Conclusions: Sinus floor augmentation can be successfully accomplished with a transcrestal approach using a dedicated implant system. A physiologic contraction of 11.3% of the original volume of the bone graft was experienced during the first 6 months of healing; afterwards, no additional graft volume reduction was observed. Long-term clinical studies are needed to confirm these preliminary results.
Introduction
Staged sinus floor elevation is the common solution for implant placement in severe atrophic maxilla. However, maxillary sinus floor elevation using a lateral approach implies execution of a large mucosal periosteal flap that inevitably affects post-operative recovery and the additional cost of the augmentation procedure. Sinus membrane perforations, nose bleeding, post-operative pain, swelling, haematoma and sinus infection are possible complications.
The elevation of the maxillary sinus floor through the alveolar crest (transalveolar) was first described by Tatum in the late 1970s and was first published by Boyne in 1980. The technique has been repeatedly modified. The main concerns of this technique are the limited amount of bone augmentation, no visual access to the site, risk of perforation of the Schneiderian membrane and post-operative symptoms.
A closed transcrestal hydraulic Schneiderian membrane elevation and a simultaneous bone graft augmentation can be accomplished using a dedicated dental implant. This approach mainly differs from previously described hydraulic techniques because the Schneiderian membrane elevation and the bone graft are both performed through the implant.
In general, sinus lift procedures adequately increase the vertical dimension of the resorbed alveolar process in the posterior maxilla, thus allowing the placement of implants of sufficient length. The graft’s volume contraction rate reported in literature ranges from 20% to 50% for both autogenous bone, as well as bone substitutes, such as demineralised freeze-dried bone allograft (DFDBA), mineralised freeze-dried bone allograft (FDBA), and xenografts. In more recent years, resorbable bioceramics, made of a mixture of hydroxyapatite and beta tricalcium phosphate, have gained popularity, demonstrating bioactivity and osteoconductivity in different histological studies. Nevertheless, the use of bone substitutes is questionable when more than 3 mm of bone height is present.
An interim 6-month report from this study on the first 18 patients showed a physiologic contraction of 13.9% of the original bone graft volume using the iRaise Sinus Lift System. The present study evaluated the clinical and radiographic outcomes of a one-stage crestal sinus elevation procedure using a self-tapping endosseous implant system (iRaise, Maxillent, Herzliya, Israel) developed for sinus augmentation, using cone-beam computed tomography (CBCT), 1 year after loading. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for improving the quality of observational studies (http://www.strobe-statement.org).
Materials and methods
This study was designed as a prospective observational study. Patients were treated at a private practice in Rome, Italy, between September and December 2014. All patients were informed about the nature of the study and gave their written consent for surgical and prosthetic procedures and for the use of clinical and radiologic data. Surgical procedures were performed by two clinicians (MT and SMM) who have extensive experience in implant placement and sinus augmentation procedures.
Consecutive patients aged 18 years or over, able to sign an informed consent, requiring implant supported restorations to rehabilitate their atrophic posterior maxilla, were considered eligible for inclusion. The selected site had to have a residual alveolar crest of at least 3 to 8 mm in height and 5 mm in width distal to the canine as measured on a CBCT scan.
Exclusion criteria were:
- General contraindications to implant surgery;
- Subjected to irradiation in the head and neck area < 1 year before implantation;
- Uncontrolled diabetes;
- Pregnant or nursing;
- Substance abuse;
- Heavy smoker (≥ 11 cigarettes/day);
- Psychiatric therapy or unrealistic expectations;
- Immunosuppressed or immunocompromised;
- Treated or under treatment with oral or intravenous aminobisphosphonates;
- Lack of opposite occluding dentition/prosthesis in the area intended for implant placement;
- Severe bruxism or clenching;
- Healed sites (at least 3 months after teeth extracted);
- Untreated periodontitis;
- Poor oral hygiene and motivation (full mouth bleeding on probing and full mouth plaque index > 25%);
- Patients participating in other studies, if this prevents the present protocol from being properly followed.
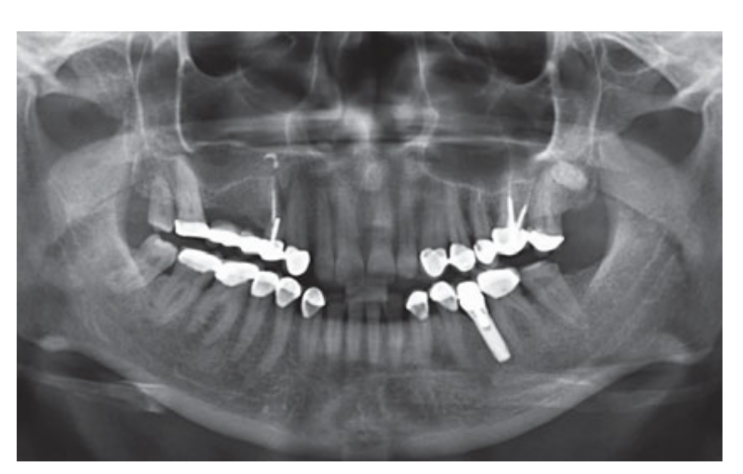
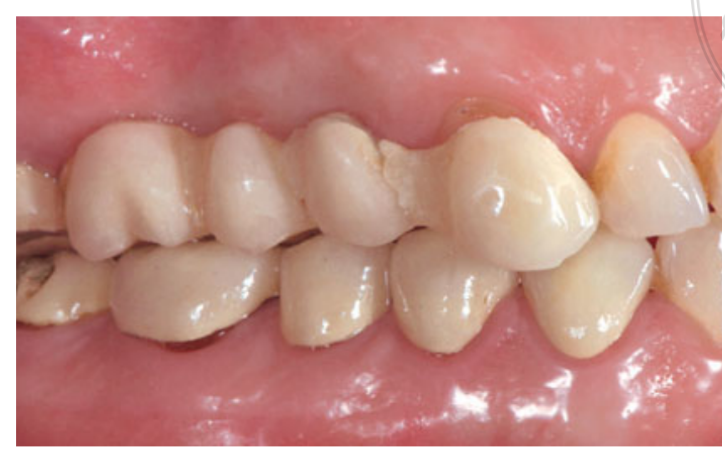
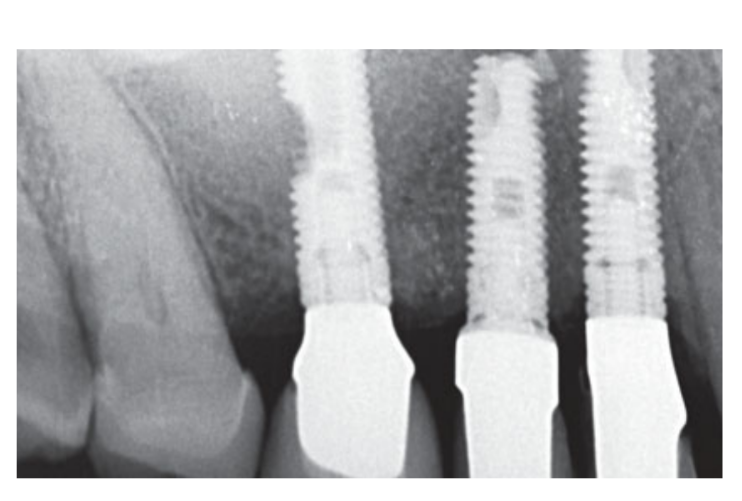
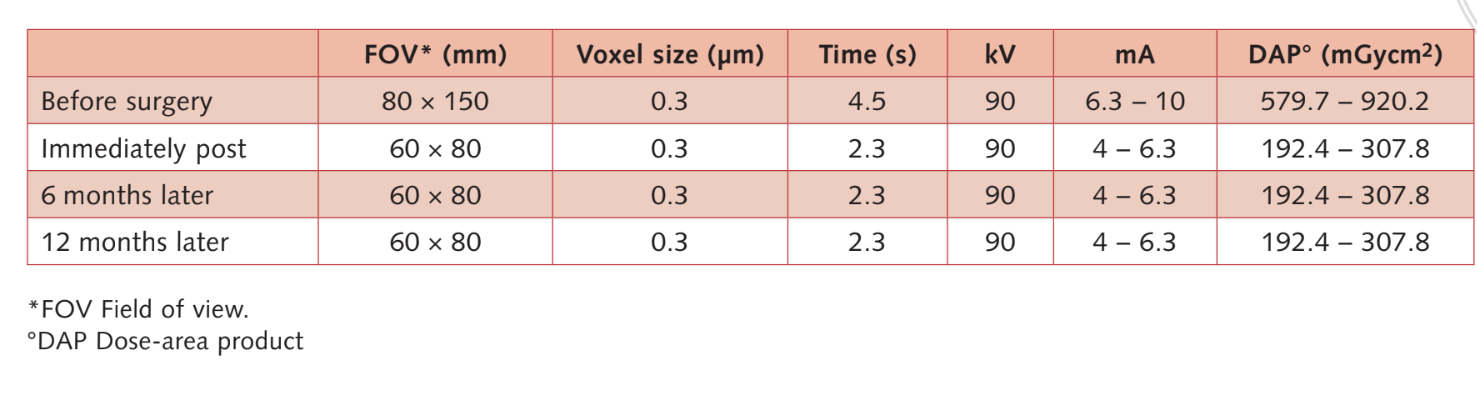
Moderate smokers were included and patients were categorised as non-smokers or moderate smokers (≤ 10 cigarettes/day). Preoperative panoramic or periapical radiographs and pictures were used for initial screening (Figs 1 and 2). Cone-beam computed tomography scans were performed for each patient within 2 weeks before surgery (field of view 80 A~ 150 mm; voxel size 0.3 μm; 4.5 s; 90 kV; 6.3–10 mA; 579.7–920.9 mGy cm2), immediately after the procedure, 6 months after implant placement, and 1 year after loading (field of view 60 Å~ 80 mm; voxel size 0.3μm; 2.3 s; 90 kV; 5–8 mA; 192.4–307.8 mGy cm2) as part of the regular treatment protocol. Exposure parameters were set as low as reasonably possible.
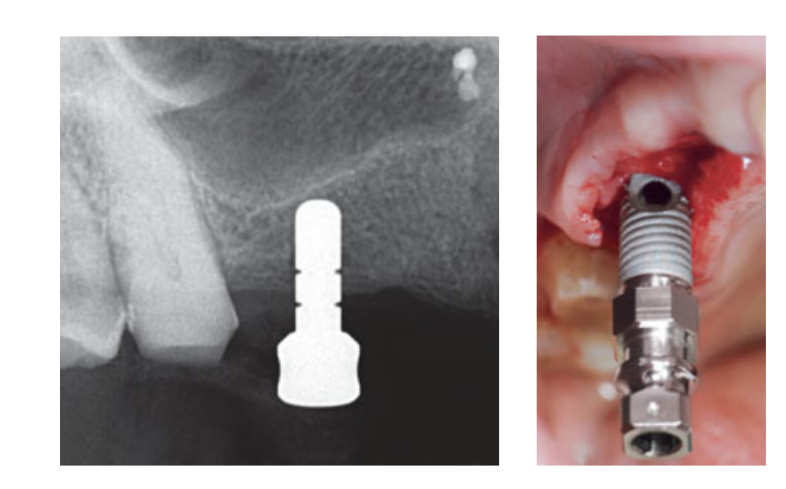
Intranasal spray therapy (thiamphenicol glycinate acetylcysteinate 810 mg/4 mL) and cortisone (betamethasone 1 mg) were administered twice a day starting the day before surgery. On the day of surgery, a single dose of antibiotic (2 g amoxicillin and clavulanic acid, or 600 mg clindamycin, if allergic to penicillin) was administered prophylactically 1 h prior to surgery. Chlorhexidine 0.2% mouth rinse was administered for 1 min prior to surgery. Local anaesthesia using articaine with adrenalin 1:100,000 was administered. A midline incision was made and a full-thickness mucoperiosteal flap was elevated. Implant recipient sites were prepared according to the drilling protocol suggested by the manufacturer (Fig 3a). Drill stops and depth guides were used to achieve a precise and safe control of osteotomy depth. The length of the implant (ranging from 13 to 16 mm) was selected in advance based on the residual bone height, measured using the preoperative CBCT scans, from the bone crest to the floor of the sinus along the implant planned axis, and confirmed by using dedicated radiographic tools (Maxillent). The iRaise Sinus Lift implants (Maxillent) were inserted into the osteotomy sites aligning the opening in the implant mid-buccally and leaving one visible screw thread above the opening (Fig 3b). The single-use tube connector was screwed to the im- plant tubing port. The connector does not touch the implant except for the silicone ring (medical silicone intended for implantation). Then 2 to 3 mL of saline solution was gently injected into the sinus through the tube connector and the internal L-shaped channel of the implant (diameter 1.5 mm), isolated from the prosthetic connection, and thus from the oral cavity. The saline solution was retracted back into the syringe and slight physiologic bleeding was noted in the retracted saline solution. Afterward, a syringe filled with 1 mL of flowable bone graft material (MBCP Gel, Biomatlante, Vigneux-de-Bretagne, France) was mixed with 0.1 mL of 0.9% sterile saline, which was slowly injected through the same port. The MBCP Gel is a 100% synthetic injectable bone substitute composed of 60% biphasic calcium phosphate and 40% hydroxyapatite suspended in a soluble polymer, with a granulometry ranging from 80 to 200 μm. After the grafting procedure was complete, the hydraulic system was disconnected and the entire length of the implant was inserted into the osteotomy site and the grafted sinus cavity and left to heal for 6 months, according to a submerged protocol. Additional implants (iSure, Maxillent) were placed in the treated area after the iRaise (Maxillent) surgical sequence was completed, or 6 months later in cases where patients had residual bone height ≤ 3 mm at the planned site.
Intranasal spray therapy (thiamphenicol glycinate acetylcysteinate 810 mg/4 mL) and cortisone (betamethasone 1 mg) were continued for 10 days after surgery. Antibiotics were continued for 7 days (1 g amoxicillin and clavulanic acid or 300 mg clindamycin twice a day) after surgery. Chlorhexidine 0.2% mouth rinse was used for 1 min twice a day for 2 weeks, and a soft diet was recommended for 1 month. Ibuprofen 400 mg or paracetamol 1 g was administered in case of pain. Sutures were removed after 1 week and oral hygiene instructions were reinforced.
Six months after implant placement and sinus augmentation, the healing abutments were connected. In patients requiring staged implants, a calibrated trephine bur with a 3.0 mm external diameter was used to prepare the site and a core sample was taken for histological analysis.
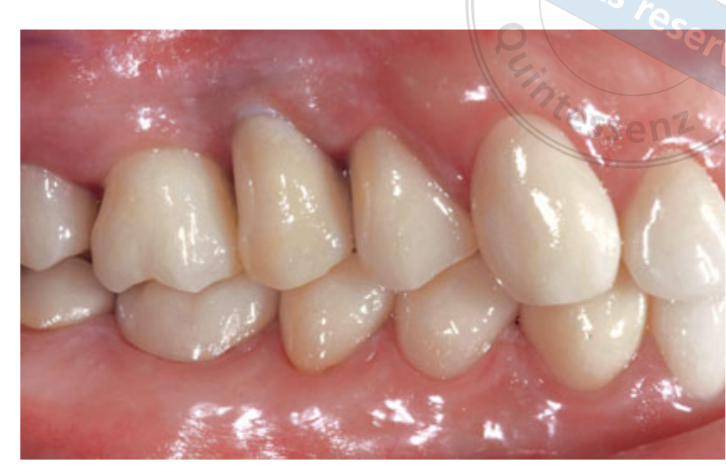
Two weeks after second surgery an open tray impression was taken with a polyether material (Impregum, 3M ESPE, Seefeld, Germany). No temporary restorations were used. Final restorations were fabricated by computer-aided design (CAD)/ computer-aided manufacturing (CAM) technology and delivered 4 (single crowns) to 6 weeks (fixed prostheses) later. The occlusion was then carefully checked. Recall appointments for oral hygiene maintenance were made every 4 months after loading. The occlusion was also evaluated at each visit (Figs 4 and 5).
Outcome measures
The outcome measures of this study were:
- An implant was considered a failure if it presented any mobility, assessed by tapping or rocking the implant head with the metallic handles of two instruments, and/or infection dictating implant removal or any mechanical complications (e.g. implant fracture) rendering the implant useless, though still mechanically stable in the bone. Radiographic evaluation utilised an intraoral radiograph taken with a paralleling technique strictly perpendicular to the implant-bone interface.
- A prosthesis was considered a failure if it needed to be replaced by an alternative prosthesis.
- Any biologic (pain, swelling, or suppuration, etc) and/or mechanical (screw loosening or fracture of the framework and/or the veneering material, etc) complications.
- Marginal bone level changes were assessed by intraoral digital periapical radiographs (Digora Optime device, Soredex, Tuusula, Finland; Imaging plate, PSP [Photo Stimulable Phosphor plate] size 2, pixel size 30 μm, resolution 17 lp/mm.) made with the paralleling technique using commercially available film holders (Rinn XCP; Dentsply Rinn, Elgin, IL, USA) at implant placement (baseline), implant loading, and 1 year after loading. In case of an unreadable radiograph, the radiograph was taken again. All radiographs were displayed in an image analysis software package (DfW 2.8 for Windows, Soredex) that was calibrated using the known length or diameter of the dental implants. The distance from the most coronal margin of the implant collar to the top of the bone crest was measured to the nearest 0.01 mm, and taken as the marginal bone level. Mesial and distal values were aver- aged for each implant. The negative difference between time points was taken as marginal bone loss (MBL).
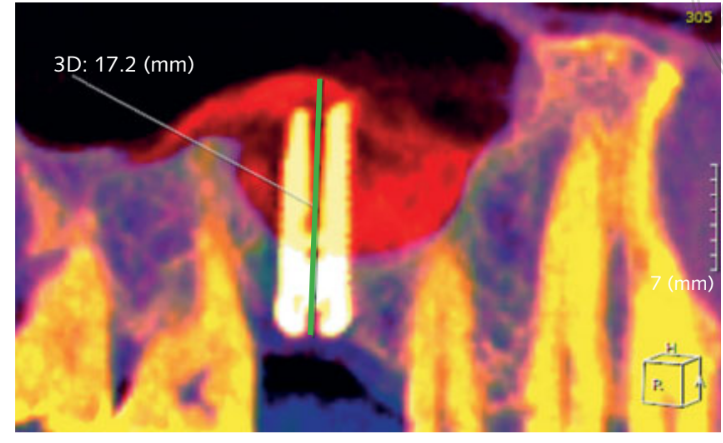
- Increased bone height (iBH) was calculated on the CBCT scan as the distance between the bone crest and the most superior radiopaque sign of the graft material, measured along the implant’s long axis. Bone gain was determined by the difference between the iBH and the preoperative residual alveolar bone height (aBH), calculated as the distance between the bone crest and the floor of the sinus, measured along the long axis on the ideal implant position (Fig 6).
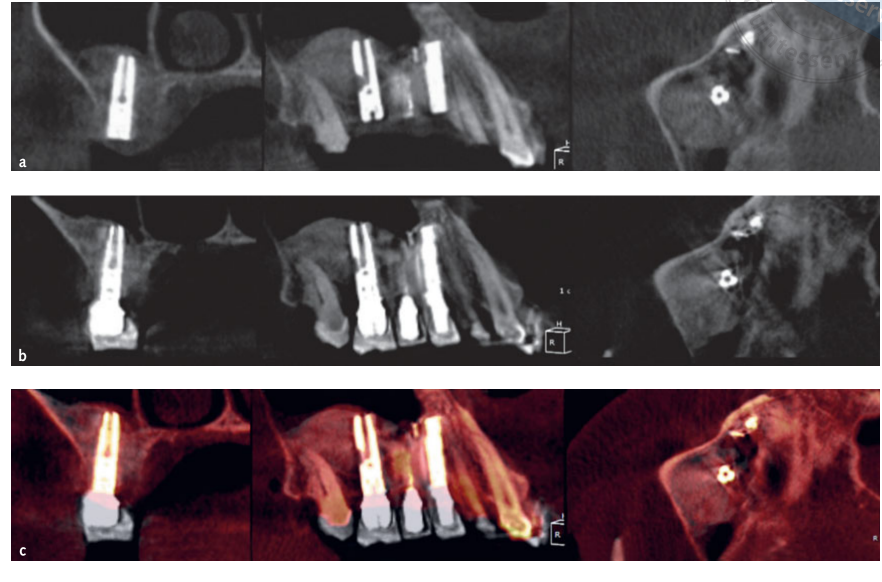
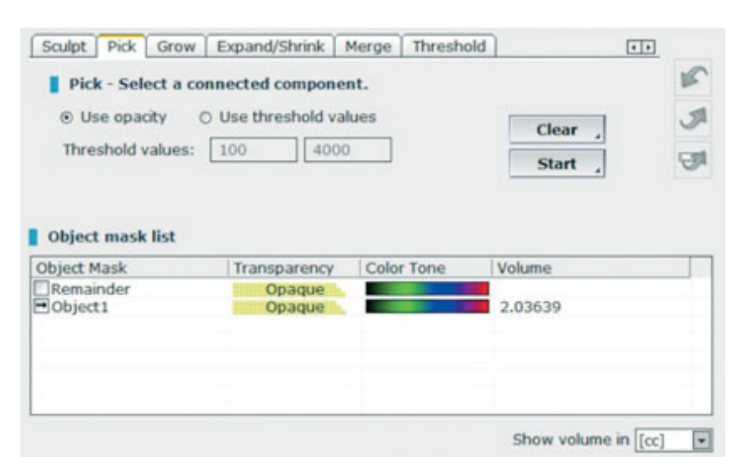
- Volumetric measurements of sinus grafts were performed on the CBCT scan using OnDemand 3D software, Fusion adjunctive module (Cybermed, Yuseong-gu, Daejeon, Korea) (Fig 7). Fusion is a visualisation tool that utilises an advanced registration technique to super-impose volume data using voxel information. The technology behind Fusion, known as MI or Mutual Information, calculates the statistical dependence between two volumes, the intensity and correlation values of entropy and compares the difference in the entropy of the sum of individual images and the joint entropy of combined images to fuse data together. Superimpositions of the post-operation and pre-operation patient DICOM data were made automatically by drawing a volume of interest (VOI) overlay over an area involving unchanged anatomical landmarks (e.g. teeth, basal skull, implants) and manually checked for a complete match ensuring the highest accuracy for the superimposition. Next the volumes of grafted material were calculated by segment in the sinus cavity using the segmentation tool (OnDemand 3D, Cybermed). This tool provides volumetric information based on the opacity of the grafted material (Figs 8 and 9). The segmented area included implants and graft. However, the implants could be distinguished clearly from the grafted materials by their density and structure and were excluded from the measurements. The CBCT scans were taken before implant placement, immediately after, and at the 6- and 12-month follow-ups according to the ALARA (as low as reasonably achievable) principle (Table 1). SMAR (Soredex Metal Artifact Reduction) technology were used to minimise scatter from metal artefacts. The same clinician, who was not previously involved in the study, assessed all the 2- and 3-dimensional radiographic measurements.
- Insertion torque was recorded at implant placement using the surgical unit (iChiro Pro, BienAir, Bienne, Suisse).
- Implant stability quotient (ISQ) was recorded by the surgeon using resonance frequency analysis (Osstell Mentor device, Osstell, Göteborg, Sweden). Two measurements were taken for each implant: one buccopalatal from the buccal side, and one mesiodistal from the mesial side. Both measurements were averaged, with the result being displayed by the device in ISQ units, ranging from 1 to 100. The values were recorded at implant placement (baseline) and at the 6-month follow-up.
- Patient self-reported post-surgical pain, on an ordinal scale (0 = no pain; 1 = mild pain; 2 = moderate pain; 3 = severe pain): assessed 3 days after surgery at the post-operative check-up by a blinded assessor.
- Patient self-reported post-surgical swelling, on an ordinal scale (0 = no swelling; 1 = mild swelling; 2 = moderate swelling; 3 = severe swelling): assessed 3 days after surgery at post-operative check-up by the blinded assessor.
- Consumption of painkillers: number of tablets used (out of 12 tablets provided [Ibuprofen 400 mg, or paracetamol 1 g for those allergic to NSAIDs]): recorded 3 days after surgery at the post-operative check-up by the blinded assessor.
- Histomorphometric analysis was utilised in order to compute the volume tissue fractions (expressed as a percentage of the total volume) that composed the sample (bone, biomaterial, soft tissue). Histological samples were harvested and fixed in 10% formalin and then dehydrated in increasing amounts of alcohol scale (70%, 80%, 90%, 96%, 100%), infiltrated in alcohol resin in decreasing ratio (3 alcohol:1 resin, 1:1, 1:3, pure resin), and finally embedded in pure methyl-methacrylate resin (Technovit 7200 VLC, Exact Kulzer, Bio-Optica, Milano, Italy). After that, samples were cut in the middle with a diamond blade to obtain two sections for each sample. These sections were glued on plastic slides, ground by sanding down to a thickness of 100 μm and finally polished with alumina 0.1%. The samples were stained with a dichromic staining composed of toluidine blue and pyronine yellow, which highlights all different tissue fractions. Slides were observed and photographed with a light and polarised microscope (Nikon Eclipse, Nikon Instruments S.p.A, Florence, Italy).
Statistical analysis
All data analysis was carried out according to a pre-established analysis plan. Descriptive analysis was performed using the mean ± standard deviation (SD), median, and 95% confidence interval (CI) (SPSS for Mac OS X version 22.0, IBM Corporation, Armonk, New York, USA). Comparisons between the various follow-up end-points and the baseline measurements were made by a paired student t-test to detect any changes. All statistical comparisons were conducted at the 0.05 level of significance. The patients were used as the statistical unit.
Results
In total, 33 patients were screened for eligibility, but three then refused to participate in the research protocol. Therefore, 30 consecutive participants (17 women, 13 men) with a mean age of 54.2 ± 9.4 years (range: 28–75) and severe atrophy of the posterior maxilla underwent transcrestal elevation of the sinus membrane, insertion of bone graft, and implant placement at the planned site, with no deviation from the original protocol. The mean follow-up was 15.8 ± 2.1 months after implant loading (range: 12–18). The main patient and implant characteristics are reported in Table 2.
A total of 50 implants were placed (30 iRaise implant systems and 20 adjunctive iSure implants. One patient dropped out at the 1-year after loading follow-up examination (patient did not want to come back for the evaluations). No implants and no prostheses failed during the follow-up period. One patient experienced a small membrane tear that was sealed with a collagen sponge (Parasorb Cone, RESORBA Medical GmbH, Nürmberg, Germany). The procedure was aborted and successful repeated 2 months later. No other intra-operative or post-operative adverse events were observed.
Before implant insertion, the mean residual alveolar ridge height was 4.64 ± 0.86 mm (range: 3.4–6.4 mm; 95% CI: 4.39 to 5.01 mm). Immediately after implant placement, bone height was 17.92 ± 2.63 mm (95% CI 17.02 to 18.90). One year after loading, the bone height remained stable, with a mean value of 16.86 ± 3.13 mm (95% CI 15.83 to 18.07). The difference was 1.09 ± 1.60 mm (95% CI 0.00 to 1.16; P = 0.0010). The mean bone gain (iBH - aBH) compared to the baseline was 12.15 ± 2.96 mm (95% CI 11.22 to 13.38; P = 0.0000).
The mean marginal bone level at implant placement was -0.16 ± 0.86 mm (95% CI -0.41 to -0.21).
At implant loading, the mean marginal bone level was 0.11 ± 1.06 mm (95% CI -0.15 to 0.60). At the 1-year after loading follow-up, the mean MBL was 0.19 ± 1.05 mm (95% CI 0.02 to 0.78). The difference from baseline was 0.36 ± 0.53 mm; 95% CI 0.08 to 0.47; P = 0.00095). The data are reported in Table 3.
The mean insertion torque at implant placement was 31.6 ± 8.1 Ncm (95% CI 29.9 to 35.7) (iChiro Pro, BienAir). The mean ISQ at implant placement was 65.2 ± 5.4 (95% CI 63.6 to 67.4) and increased during the healing period reaching a mean value of 73.6 ± 3.7 (95% CI 73.1 to 75.9; range 62–79) at the healing abutment connection (6 months after implant placement). The difference was statistically significant (8.4 ± 5.3; 95% CI 5.9 to 39.7; P = 0.0000).
At implant placement, 24 out of 30 implants presented bone graft over the implant apex. Overall (n = 30), the mean value was 2.95 ± 2.56 mm (95% CI 2.18 to 4.0). One year after loading mean bone graft over the top of the apex was observed in 23 implants. Overall (n = 29), the mean value was 2.29 ± 2.41 mm (95% CI 1.22 to 2.98).
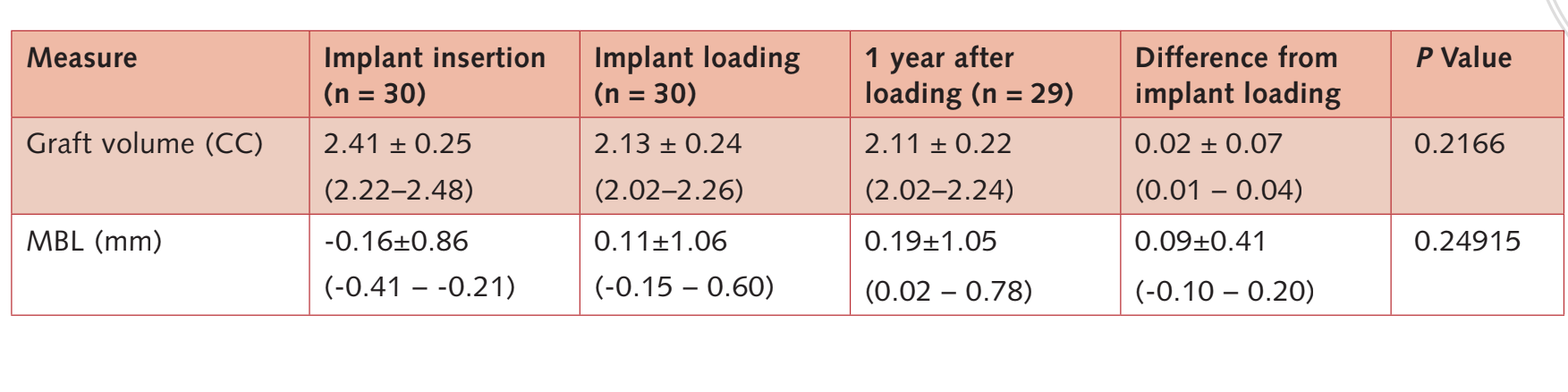
Bone volume at implant placement was 2.41 ± 0.25 CC (95% CI 2.22 to 2.48). During the 6-month submerged healing period, a slight bone contraction of 11.3% was observed. (2.13 ± 0.24 CC; 95% CI 2.02 to 2.26; difference = 0.27 ± 0.25 CC; 95% CI 0.10 to 0.36; P = 0.0011). During the first year post-loading period, the bone graft remained stable (2. 11 ± 0.22 CC; 95% CI 2.02 to 2.24).
The difference was not statistically significant (0.02 ± 0.07 CC; 95% CI 0.01 to 0.04; P = 0.2166).
The data are reported in Table 3.
The patients reported on their experiences after surgery. The mean pain value was 0.52 ± 0.74 (range 0–3); mean swelling value was 0.27 ± 0.52 (range 0–2); and the mean consumption of analgesic was 0.87 ± 4.94 tables (range 0–4).
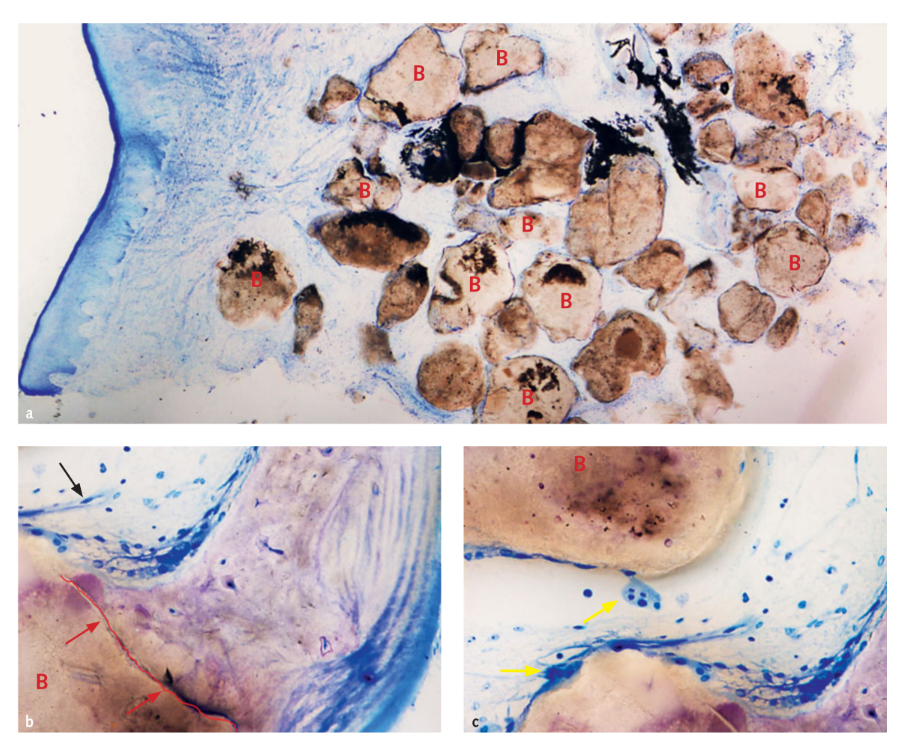
In three cases where a staged, adjunctive implant was placed, a sample of graft was taken for histological analysis. Morphological and histomorphometric analyses showed that after 6 months of healing, all samples had a normal structure without the presence of an inflammatory infiltrate (Fig 10). The biomaterial appeared surrounded by mineralised matrix indicative of large areas of mineralisation both at low (Fig 10a) and at high magnification. In figure 10b, the microphotograph reveals the close contact between graft and new bone matrix. Blood vessels were evident in the marrow spaces presumably in order to sustain the tissue during the phases of formation and organisation (Figs 10b and 10c). Many bone cells were detected, in particular osteoblast-like cells on the borders of new trabecular bone in close contact with osteoclast-cells during the active phase of new bone formation and remodelling (Fig 10c). Histomorphometric analysis showed the following tissue fractions (%): bone (immature bone + mature bone): 44.07% ± 4.91; residual biomaterial: 23.98% ± 2.64; medullary spaces: 31.95% ± 3.16.
Discussion
The aim of the present prospective study was to report the 1-year after loading data on a novel implant system that allows for simultaneous sinus graft and implant placement using a transcrestal approach. Because it was designed as a prospective cohort study, the primary limitation is the lack of a control group. Nevertheless, the present study is one of the first to evaluate clinical and radiographic data from a crestal sinus lift procedure using a dedicated implant with an internal L-shaped channel 1 year after loading. Another limitation of the present study could be the technology used to measure bone volume contraction. Although this technology allows for superimposition and segmentation of different bone volumes, no scientific evidence is present. Therefore, the data should be interpreted with caution.
During the entire follow-up period, no implant failed, and only one membrane tear was experienced, with no other adverse events. Therefore, the major clinical consideration of this prospective study was that elevation of the sinus membrane can be safely performed using hydraulic pressure directly through the implant channel, allowing for bone grafting (also through the channel) and implant placement at the same time. The presented approach can be performed with a minimal residual bone of 3 mm between the bone crest and the sinus floor.
According to the recent Cochrane review by Esposito et al on sinus lift procedures, the use of bone substitute is questionable when more than 3 mm of bone height is present. Nevertheless, a variety of grafting materials, either alone or in combination, have been validated for effective use in sinus augmentation procedures, including autogenous bone, allogeneic bone, xenogeneic bone, and alloplastic materials (e.g. hydroxyapatite [HA], beta tricalcium phosphate [β-TCP]). In the present study, a mixture of 60% of HA and 40% of β-TCP was used. An interim manuscript reporting the 6-month after implant placement data on 18 patients concluded that a physiologic contraction of 13.9% of the original volume of the graft was experienced during the healing period. The present study analysed the overall sample size of 30 patients reporting a slight bone contraction of 11.3% during the 6-month period of submerged healing. Afterwards, the bone graft remained stable, with a contraction of 0.95%. A recent systematic review by Shanbhag et al on sinus augmentation procedures performed with a conventional lateral approach reported reductions in augmentation volumes during early healing ranging from 18% to 22% when using bone substitutes, with no significant differences between grafting materials. Specifically, in a randomised controlled trial by Kühl et al, within the first 6 months of maxillary sinus augmentation, the volume decreased by 15% for biphasic calcium phosphate, (BCP, 60% of HA and 40% of β-TCP) and 18% when the investigators added particulated autologous bone at the test site, with no statistically significant differences between the two groups. Several other studies also confirmed a reduction in graft material volume. Hatano et al suggested that autogenous bone or bone substitute has a dimensional contraction in height of 20% up to 2 years. Although autologous bone is considered the gold standard in accordance with Wiltfang, many concerns are related to an observed unpredictable resorption. This statement was confirmed by Shanbhag et al in a systematic review when using autologous bone blocks that reduced in volume 45% over time after 6 to 24 months. The same authors confirmed that anorganic bovine bone used either alone or in combination with autologous bone showed a volume change of 15% to 21%, suggesting a better volume stability compared with autologous bone alone. The results of these studies support the findings of this study.
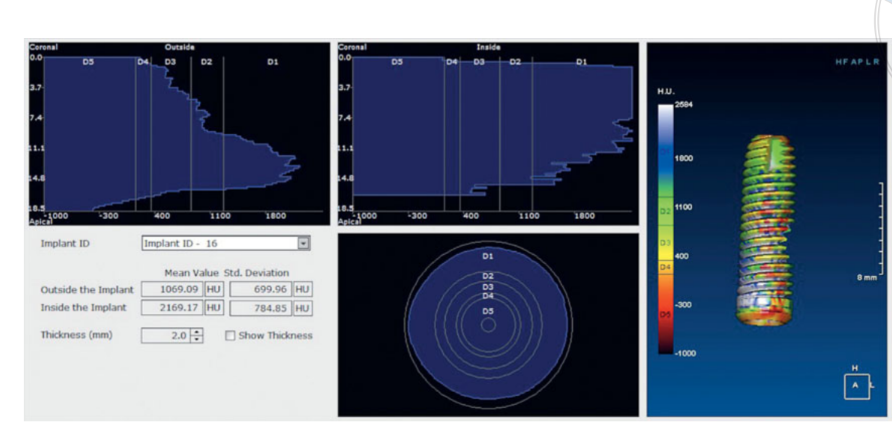
Histology showed that after 6 months the samples months, were characterised by 44.07% ± 4.91 of bone at different stages of mineralisation, 23.98% ± 2.64 of residual graft particles and 31.95% ± 3.16 of medullary spaces. Although about half of the original grafted material was still present 6 months after submerged healing, radiologic data from a previous preliminary study23 indicated that graft density significantly improved from 322.0 ± 100.42 Hounsfield Units (HU) (range: 177–449 HU; 95% CI: 246.4 to 377.6) to 1,062.0 ± 293.7 HU (range: 573–1,489 HU; 95% CI: 876.12 to 1,259.88). This difference was statistically significant (740.0 ± 295.35 HU; range: 324–1,231; 95% CI: 426.04 to 811.96; P = 0.0001).
Hounsfield Units refer to a D1-D5 scale, which are based on medical computer tomography values and are not completely reliable when it comes to CBCT scans. However, according to Cassetta et al, the HU was equal to gray density values multiplied by a conversion ratio of 0.7. Hence, previous preliminary research of the present study indicated a mean graft density (Figure 11) comparable to a D2 value according to the Lekholm and Zarb classification. In addition, clinical data showed that the mean ISQ at implant placement was 65.2 ± 5.4 (95% CI 63.6 to 67.4) and increased during the healing period reaching the mean value of 73.6 ± 3.7 (95% CI 73.1 to 75.9; range 62–79). The difference was statistically significant (8.4 ± 5.3; 95% CI 5.9 to 39.7; P = 0.0000). All of these data are in agreement with the high implant survival rates, with no failure experienced during the entire follow-up period.
Short implants have been suggested as an alternative to sinus lift procedures. Advantages of short implants include a less technically demanding operation, therefore potentially fewer complications, and lower expense (no bone graft). Data from several randomised controlled trials showed that short 4 to 8 mm implants could be a suitable, simpler, cheaper and faster alternative to longer implants placed in augmented bone. It has also been demonstrated that sinus lift procedures using hydraulic pressure provide high predictability in clinical outcomes, together with extremely low morbidity and shortened surgical tissues. Therefore, both short and longer implants placed in conjunction with hydraulic sinus elevation represent viable treatment options. Nevertheless, randomised controlled trials are needed, and follow-up periods are still too short to draw definitive conclusions as to the best treatment for specific indications.
Conclusions
Sinus floor augmentation can be successfully accomplished with a transcrestal approach using a dedicated implant system that allows for hydraulic elevation of the Schneiderian membrane, injection of a flowable bone graft material and simultaneous dental implant placement, with minimal patient discomfort. A physiologic contraction of 11.3% of the original volume of the bone graft was experienced during healing. Afterwards, no additional graft volume reduction was observed. Long-term clinical studies are needed to confirm these results.
Marco Tallarico, David L Cochran, Erta Xhanari, Claudia Dellavia, Elena Canciani, Eitan Mijiritsky, Silvio Mario Meloni
References
- Nickenig HJ, Wichmann M, Zoller JE, Eitner S. 3-D based minimally invasive one-stage lateral sinus elevation – a prospective randomized clinical pilot study with blinded assessment of postoperative visible facial soft tissue volume changes. J Craniomaxillofac Surg 2014;42:890–895.
- Bechara S, Kubilius R, Veronesi G, Pires JT, Shibli JA, Mangano FG. Short (6-mm) dental implants versus sinus floor elevation and placement of longer (≥10-mm) dental implants: a randomized controlled trial with a 3-year follow-up. Clin Oral Implants Res 2016 Jul 12. doi: 10.1111/ clr.12923. [Epub ahead of print].
- Katranji A, Fotek P, Wang HL. Sinus augmentation complications: Etiology and treatment. Implant Dent 2008;17: 339–349.
- Tatum H Jr. Maxillary and sinus implant reconstructions. Dent Clin North Am 1986;30:207–229.
- Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg 1980;38:613–616.
- Misch CE. Maxillary sinus augmentation for endosteal implants. Organized alternative treatments plans. Int J Oral Implantol 1987;4:49–58.
- Smiler DG, Holmes RE. Sinus lift procedure using porous hydroxyapatite: a preliminary report. J Oral Implantol 1987;13:239–253.
- Wood R, Moor P. Grafting of the maxillary sinus with intraorally harvested autogenous bone prior to implant placement. Int J Oral Maxillofac Implants 1988;3:209–214.
- Kent JN, Block MS. Simultaneous maxillary sinus floor bone grafting and placement of hydroxylapatite-coated implants. J Oral Maxillofac Surg 1989;47:238–242.
- Misch CE, Dietsh F. Bone-grafting materials in implant dentistry. Implant Dent 1993;2:158–167.
- Better H, Slavescu D, Barbu H, Cochran DL, Chaushu G. Minimally invasive sinus lift implant device: a multicenter safety and efficacy trial preliminary results. Clin Implant Dent Relat Res 2014;16:520–526.
- Johansson B, Grepe A, Wannfors K, Hirsch JM. A clinical study of changes in the volume of bone grafts in the atrophic maxilla. Dentomaxillofac Radiol 2001;30:157–161.
- Hallman M, Sennerby L, Zetterqvist L, Lundgren S. A 3-year prospective follow-up study of implant-supported fixed prostheses in patients subjected to maxillary sinus floor augmentation with a 80:20 mixture of deproteinized bovine bone and autogenous bone Clinical, radiographic and resonance frequency analysis. Int J Oral Maxillofac Surg 2005;34:273–280.
- Wanschitz F, Figl M, Wagner A, Rolf E. Measurement of volume changes after sinus floor augmentation with a psychogenic hydroxyapatite. Int J Oral Maxillofac Implants 2006;21:433–438.
- Kirmeier R, Payer M, Wehrschuetz M, Jakse N, Platzer S, Lorenzoni M. Evaluation of three-dimensional changes after sinus floor augmentation with different grafting materials. Clin Oral Implants Res 2008;19:366–372.
- Klijn RJ, van den Beucken JJ, Bronkhorst EM, Berge SJ, Meijer GJ, Jansen JA. Predictive value of ridge dimensions on autologous bone graft resorption in staged maxillary sinus augmentation surgery using Cone-Beam CT. Clin Oral Implants Res 2012;23:409–415.
- Umanjec-Korac S, Wu G, Hassan B, Liu Y, Wismeijer D. A retrospective analysis of the resorption rate of deproteinized bovine bone as maxillary sinus graft material on cone beam computed tomography. Clin Oral Implants Res 2014;25:781–785.
- Meloni SM, Jovanovic SA, Lolli FM, Cassisa C, De Riu G, Pisano M, Lumbau A, Luglie PF, Tullio A. Grafting after sinus lift with anorganic bovine bone alone compared with 50:50 anorganic bovine bone and autologous bone: results of a pilot randomised trial at one year. Br J Oral Maxillofac Surg 2015;53:436–441.
- Frayssinet P, Trouillet JL, Rouquet N, Azimus E, Autefage A. Osseointegration of macroporous calcium phosphate ceramics having a different chemical composition. Biomaterials 1993;14:423–429.
- Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med 2003;14:195–200.
- Le Nihouannen D, Saffarzadeh A, Aguado E, Goyenvalle E, Gauthier O, Moreau F, Pilet P, Spaethe R, Daculsi G, Layrolle P. Osteogenic properties of calcium phosphate ceramics and fibrin glue based composites. J Mater Sci Mater Med 2007;18:225–235.
- Esposito M, Felice P, Worthington HV. Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus. Cochrane Database Syst Rev 2014;13:CD008397.
- Tallarico M, Meloni SM, Xhanari E, Pisano M, Cochran DL. Minimally invasive sinus augmentation procedure using a dedicated hydraulic sinus lift implant device: A prospective case series study on clinical, radiologic, and patient-centered outcomes. Int J Periodontics Restorative Dent 2017;37: 125–135.
- Tallarico M, Better H, De Riu G, Meloni SM. A novel implant system dedicate to hydraulic Schneiderian membrane elevation and simultaneously bone graft augmentation: An up-to 45 months retrospective clinical study. J Craniomaxillofac Surg. 2016;44:1089–1094.
- Shanbhag S, Shanbhag V, Stavropoulos A. Volume changes of maxillary sinus augmentations over time: a systematic review. Int J Oral Maxillofac Implants 2014;29:881–892.
- Kühl S, Payer M, Kirmeier R, Wildburger A, Acham S, Jakse N. The influence of particulated autogenous bone on the early volume stability of maxillary sinus grafts with biphasic calcium phosphate: a randomized clinical trial. Clin Implant Dent Relat Res 2015;17:173–178.
- Hatano N, Shimizu Y, Ooya K. A clinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/ xenograft mixture and simultaneous placement of dental implants. Clin Oral Implants Res 2004;15:339–345.
- Wiltfang J, Schultze-Mosgau S, Nkenke E, Thorwarth M, Neukam FW, Schlegel KA. Onlay augmentation versus sinus lift procedure in the treatment of the severely resorbed maxilla: a 5-year comparative longitudinal study. Int J Oral Maxillofac Surg 2005;34:885–889.
- Lekholm U, Zarb GA. Patient selection and preparation. In: Brånemark PI, Zarb GA, Albrektsson T, editors. Tissue-integrated prostheses: osseointegration in clinical dentistry. Chicago: Quintessence Publishing Co. 1985;1–356.
- Cassetta M, Stefanelli LV, Pacifici A, Pacifici L, Barbato E. How accurate is CBCT in measuring bone density? A comparative CBCT-CT in vitro study. Clin Implant Dent Relat Res 2014;16:471–478.
- Esposito M, Pellegrino G, Pistilli R, Felice P. Rehabilitation of posterior atrophic edentulous jaws: prostheses supported by 5 mm short implants or by longer implants in augmented bone? One-year results from a pilot randomised clinical trial. Eur J Oral Implantol 2011;4:21–30.
- Pistilli R, Felice P, Cannizzaro G, Piatelli M, Corvino V, Barausse C, Buti J, Soardi E, Esposito M. Posterior atrophic jaws rehabilitated with prostheses supported by 6 mm long 4 mm wide implants or by longer implants in augmented bone. One-year post-loading results from a pilot randomised controlled trial. Eur J Oral Implantol 2013;6:359–372.
- Pistilli R, Felice P, Piattelli M, Gessaroli M, Soardi E, Barausse C, Buti J, Corvino V. Posterior atrophic jaws rehabilitated with prostheses supported by 5 x 5 mm implants with a novel nano- structured calcium-incorporated titanium surface or by longer implants in augmented bone. One-year results from a randomised controlled trial. Eur J Oral Implantol 2013;6:343–357.
- Gulje FL, Raghoebar GM, Vissink A, Meijer HJ. Single crowns in the resorbed posterior maxilla supported by either 6-mm implants or by 11-mm implants combined with sinus floor elevation surgery: A 1-year randomised controlled trial. Eur J Oral Implantol 2014;7:247–255.
- Esposito M, Barausse C, Pistilli R, Sammartino G, Grandi G, Felice P. Short implants versus bone augmentation for placing longer implants in atrophic maxillae: One-year post-loading results of a pilot randomised controlled trial. Eur J Oral Implantol 2015;8:257–268.
- Esposito M, Pistilli R, Barausse C, Felice P. Three-year results from a randomised controlled trial comparing prostheses supported by 5-mm long implants or by longer implants in augmented bone in posterior atrophic edentulous jaws Eur J Oral Implantol 2014;7:383–395.
