A systematic review on the definition of periimplantitis: Limits related to the various diagnoses proposed
Abstract
Objective: The aim of this comprehensive systematic review was to present the different definitions of periimplantitis proposed in the literature.
Materials and methods: Electronic and manual literature searches were conducted by three independent reviewers to identify manuscripts reporting data on the definition of periimplantitis with clinical diagnosis, written in English and published up to October 2015. Several databases were referenced, including PubMed, Embase, the Cochrane Library and the Grey Literature Database.
Results: Forty-nine articles were considered suitable for the review. Current evidence suggests the use of unequivocal case definitions for periimplantitis, defined by changes in the level of crestal bone, presence of bleeding on probing and/or suppuration, with or without concomitant deepening of periimplant pockets. However, several reference points were used to measure these changes, including different levels of severity and years of follow-up.
Conclusion: The available scientific literature suggested an absence of a unanimous definition of periimplantitis. Future studies that apply consistent case definitions should be considered.
Introduction
The term “periimplantitis” was introduced in the early 1960s to describe infectious pathological conditions of the periimplant tissue. Today, periimplantitis is the most frequent complication of dental implants and occurs with a frequency ranging from 1% to 47% at implant level. Different from periimplant mucositis (defined as the presence of reversible inflammatory soft-tissue infiltrate, without additional bone loss beyond the initial physiological bone remodeling), periimplantitis has been described as being characterized by an inflammatory process around an implant, including both soft-tissue inflammation and progressive loss of supporting bone beyond the physiological crestal bone remodeling. However, as highlighted in recent literature reviews and consensus conferences, different definitions of periimplantitis have been reported. This may be due in part to the lack of consensus on terminology, etiology, diagnosis and prognosis systems.
Periimplantitis has been described as a disease with an infectious component that is similar to chronic periodontitis. The 8th European Workshop on Periodontology has agreed that the definitions published in 200810 and 20118 should be adopted. The suggested definition should include the following: changes in the level of crestal bone, positive bleeding on probing (BOP) and/or suppuration (SUP), with or without concomitant periimplant pockets (probing pocket depth, PPD). Nowadays, although plaque accumulation is still considered the main etiological factor, it has been shown that there are other potential related risk factors of the disease, including patient, surgical and prosthetic factors that may certainly contribute to its development.
In the MeSH (Medical Subject Headings) database, the term “periimplantitis” was introduced in 2011 and defined as an inflammatory process with loss of supporting bone in the tissue surrounding functioning dental implants. Despite this very clear and comprehensive disease definition, inconsistencies and confusion emerge in applying the terminology clinically. All of these factors together have led to different interpretations and definitions of this common emerging disease. Besides, recently, the noninfectious foreign-body reaction hypothesis has further complicated the understanding of this issue. The aim of the present systematic review was to present the different definitions of periimplantitis proposed in the literature.
Materials and methods
The present paper was prepared in partial fulfillment of a consensus statement held in Rome, Italy, in January 2016. This systematic review was written according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (http://www.prisma-statement.org/PRISMAStatement/PRISMA-Statement.aspx).
The focused question was: Is there an unanimous definition of periimplantitis, including clinical diagnosis. The research question was adapted to the PICO format:
P = population: human patients derived from clinical studies, systematic reviews, narrative reviews, consensuses statements, commentaries or editorials, who presented with at least one dental implant in function for a minimum of one year, affected by periimplantitis;
I = intervention: clinical data collected with the aim of establishing the severity of the periimplant disease and of defining novel criteria by which to classify periimplant diseases;
C = comparator/control: clinical outcomes of periimplantitis compared with clinical signs of periodontitis, as well as with healthy patients;
O = outcomes: clinical parameters and radio- graphic assessment of periimplantitis: BOP, PPD, bleeding index, presence of SUP and marginal bone loss (MBL).
Search strategy
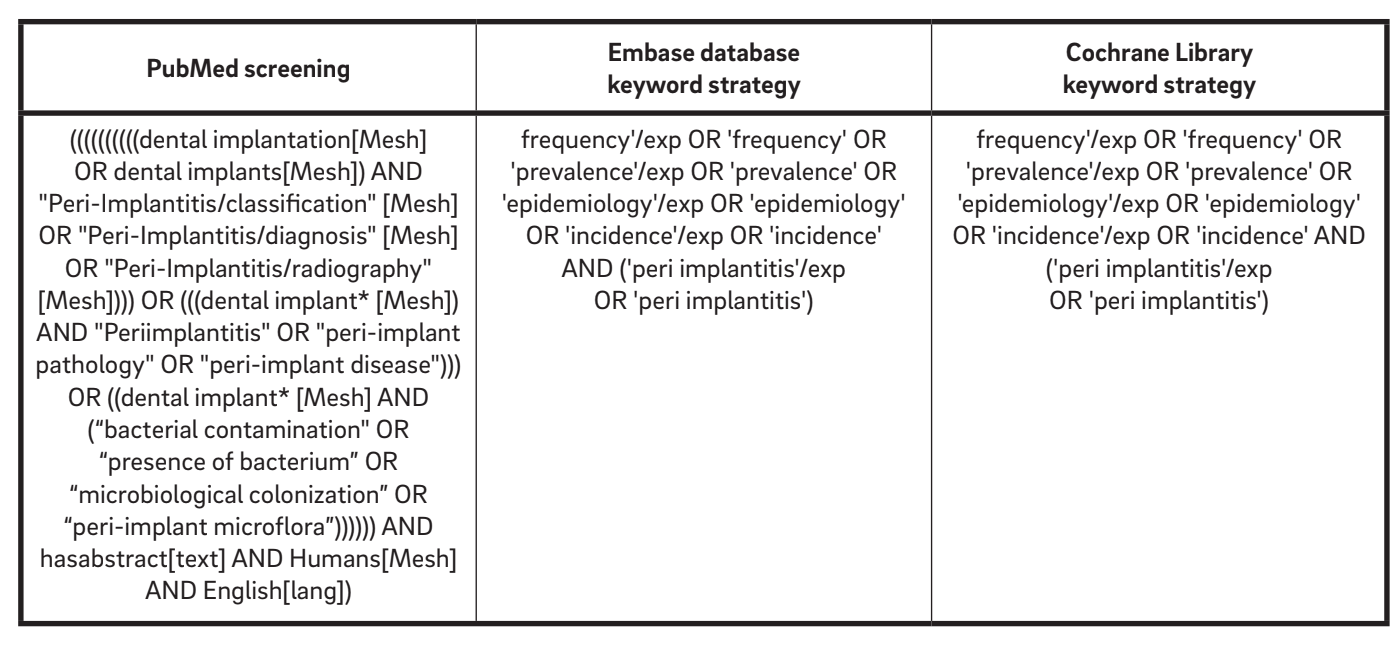
An initial search strategy encompassing the English literature from 1967 up to October 2015 was performed online to identify relevant studies that met the inclusion criteria. The following electronic databases were consulted: PubMed database of the U.S. National Library of Medicine, Embase (Excerpta Medica dataBASE) and the Cochrane Library. According to the AMSTAR (A Measurement Tool to Assess Systematic Reviews) check-list, the Grey Literature Database was screened in the New York Academy of Medicine Grey Literature Report in order to find possible unpublished works. Screening was performed independently and simultaneously by two examiners (MT and AM). A third reviewer (LC) reassessed the included and excluded studies. The electronic databases were searched using a combination of boolean keywords, including MeSH and several free-text terms (Table 1).
Eligibility criteria
The following inclusion criteria were defined for the selection of articles:
- written in English;
- entailing clinical examination of human patients;
- randomized controlled clinical trials of implants of ≥ 1 year in function;
- prospective and retrospective cohort studies of implants of ≥ 1 year in function;
- cross-sectional studies of ≥ 1 year in function; and
- systematic reviews, narrative reviews, consensus statements, commentaries or editorials.
Articles were excluded if they were
- animal studies;
- in vitro studies;
- reports of locally or systemically compromised sites and/or conditions;
- reports with < 15 cases;
- reports involving mini-implants, one-piece implants or blade implants; or
- reports on implants < 1 year in function.
Papers without abstracts, but with titles related to the objectives of this review were selected so that the full text could be screened for eligibility. Full-text papers were obtained for all abstracts and titles that appeared to meet the inclusion criteria and were assessed for inclusion by the same two examiners. The reference lists of the selected studies was screened for additional papers that may have met the eligibility criteria of the study. Additionally, manual searches of the reference lists of selected systematic reviews were conducted, limited to the following journals: Clinical Implant Dentistry and Related Research, Clinical Oral Implants Research, International Journal of Oral and Maxillofacial Implants, Journal of Clinical Periodontology and Journal of Periodontology. Any disagreement between the two reviewers was resolved after an additional discussion. Furthermore, inter-investigator agreement was calculated in the second stage. A final reviewer (LC) evaluated possible inconsistencies between the two reviewers. All of the full texts of the selected papers were stored in shared folders accessible to all of the reviewers.
Qualitative assessment of parameter s to define periimplantitis
A descriptive evaluation was performed to analyze qualitatively the range of parameters considered to define periimplantitis as an irreversible inflammatory condition that results in hard-tissue breakdown. Accordingly, the following common parameters were appraised: PPD, BOP, SUP and radiographic MBL. Such parameters from the various articles were pooled to analyze the variance or uniformity among the reported case definitions of periimplantitis. Graphs for presenting the variance were generated. While PPD was classified into three different groups (< 3 mm, 3–5 mm and > 5 mm), radiographic MBL was categorized into four main ranges, depending on the main reference taking prosthesis delivery as the baseline: ≤ 1 mm, > 1–2 mm, > 3–4 mm and ≥ 5 mm.
Results
Screening process
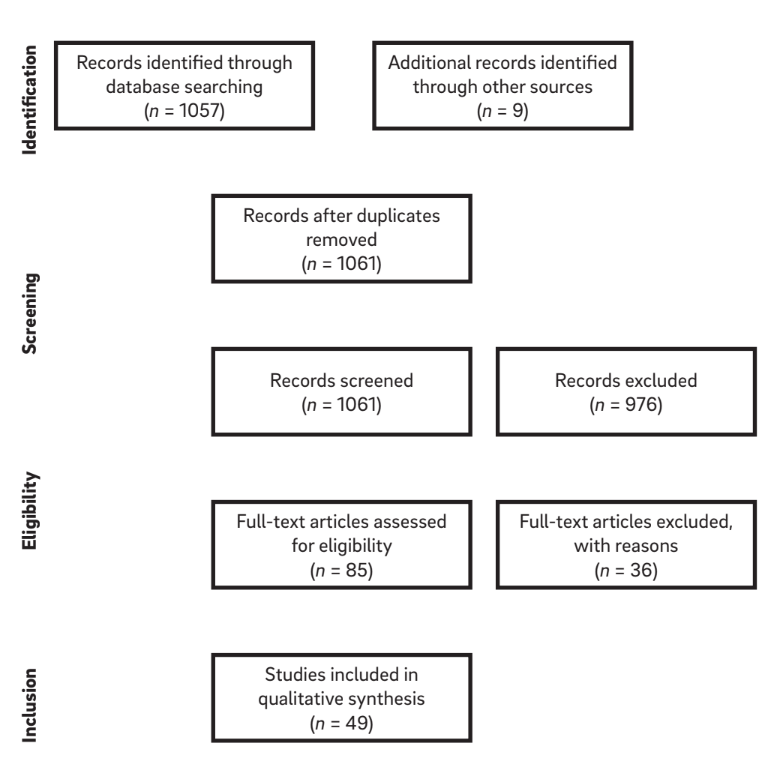
The combinations of search terms and a manual search of references in selected articles resulted in a list of 1,061 titles. Of these, 976 articles were excluded on the basis of the evaluation of the title and abstract, leaving 85 articles eligible for inclusion (k = 0.84). After application of the eligibility criteria, a total of 49 articles were considered for review. After full-text article selection and reading, the relevant information from each article was extracted. A diagram of the search strategy is shown in Figure 1.
Definitions of “periimplantitis”
Eighteen manuscripts, including narrative and systematic reviews, consensus statements and original papers, were selected and data were extracted. In 1965, Levignac reported a periimplant soft-tissue inflammation with subsequent destruction of bone and labeled it “periimplantitis.” In the 1987, Mombelli et al. described periimplantitis as an infectious disease that shares features with chronic periodontitis.13 The same author emphasized the infectious nature of this pathological condition, focusing on the bacterial load of the implant surface and the subsequent appearance of a soft-tissue inflammatory reaction adjacent to dental implants that sometimes resulted in loss of supporting bone. Like periodontitis, the etiopathogenesis of periimplantitis was shown to be triggered by bacterial infection that activates a cytokine cascade, leading to inflammatory bone loss.
“Periimplantitis” became an accepted term in the consensus report from the 1st European Workshop on Periodontology in 1993. It has been described as an irreversible inflammatory destructive reaction around implants in function that results in loss of supporting bone. The 6th European Workshop on Periodontology presented a modified definition, not only to acknowledge that periimplantitis is a treatable condition, but also to include the collective term of “peri-implant disease” for both periimplant mucositis and periimplantitis.
In order to improve the quality of research on periimplant diseases, the 7th European Workshop on Periodontology recommended the use of unequivocal case definitions: changes in the level of crestal bone and presence of BOP and/or SUP, with or without concomitant deepening of peri-implant pockets.8 Finally, the American Academy of Periodontology in 2013 defined “periimplantitis” as an inflammatory reaction associated with the loss of supporting bone beyond the initial biological bone remodeling around an implant in function.
The extent and severity of periimplant diseases have been rarely reported. Froum and Rosen proposed a combination of BOP and/or SUP, PPD and extent of radiographic MBL around the implant to classify periimplantitis into early, moderate or advanced disease categories. Likewise, Decker et al. proposed a prognosis system based on the diagnosis for each category following the Kwok and Caton prognosis classification for natural dentition. In their study, the authors stated that PPD, extent of radiographic MBL, presence of SUP and implant mobility were found to be the most critical factors for categorizing cases as having a favorable, questionable, unfavorable or hopeless prognosis.
Recently, Albrektsson et al. modified the concept of periimplantitis as a loss of bone surrounding an implant as a clinically unfavorable, disbalanced foreign-body reaction, specifically stating that osseointegration is a process whereby bone reacts to the dental implant by forming a calcified structure adjacent to it. Indeed, at times, this foreign-body reaction may actually result in osteoclastic activity that may destroy the supporting bone. The authors believe that the term “periimplantitis” is quite appropriate, because it is not a primary disease, but a complication of a clinically unfavorable, disbalanced foreign-body reaction that is the starting point of the pathological process and consequent tissue sequelae.
Currently, as foreseen by the consensus of the 7th European Workshop on Periodontology, it is assumed that the infection itself is always caused by plaque and its products; However, numerous risk factors are recognized as being specifically associated with periimplantitis, such as surgical- or prosthetic-related factors, implant characteristics, smoking and host response.
Definition of periimplantitis with clinical and radiographic diagnosis
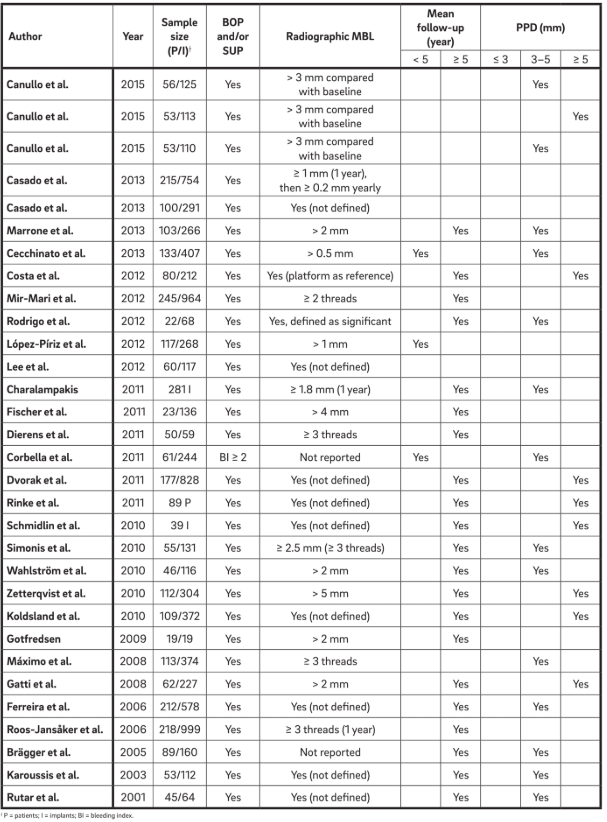
Thirty-one manuscripts (Table 2) were selected and data were extracted. Informations from 1,711 patients with 5,432 implants were analyzed. The term “periimplantitis” has generally been used to describe any implant with varying degrees of bone loss, and a clear definition was either not presented or was extracted directly from the terminology.
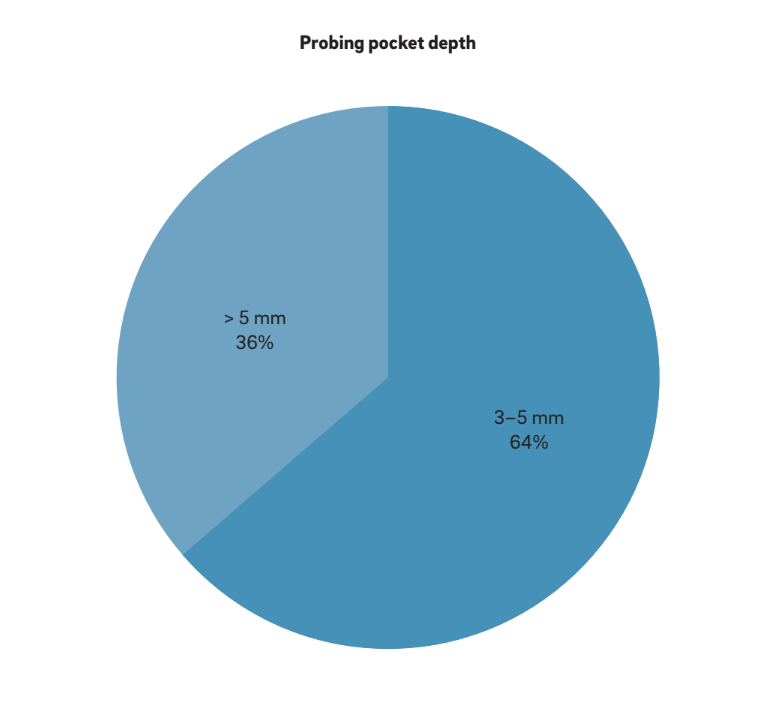
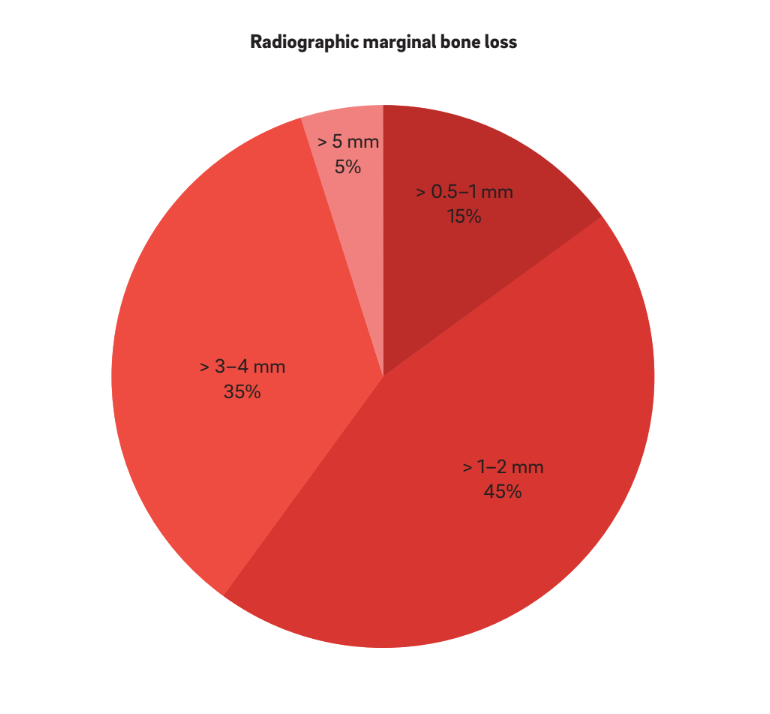
Four main characteristics have been used to define “periimplantitis”. Interestingly, all of the authors consider BOP and SUP as indicators of periimplantitis. This approach considers purely plaque- and foreign-body-induced peri-implantitis, where an inflammatory response is often triggered by the biofilm or its products and/ or foreign substances, such as residual cement. Moreover, 22 studies clearly reported PPD as a crucial parameter for determining periimplantitis. No study considered PPD < 3 mm as indicative of periimplantitis. While the vast majority (64%) of the studies defined PPD = 3–5 mm as indicative of periimplantitis, the remaining 36% considered PPD > 5 mm as the reference (Fig. 2). A radiographic MBL ≥ 0.5–1 mm, > 1–2 mm, > 3–4 mm and ≥ 5 mm, taking prosthesis delivery as baseline, was considered as defining periimplantitis in 15%, 45%, 35% and 5% of the studies, respectively (Fig. 3). As such, it was speculated that a radiographic MBL < 1 mm should be considered as physiological bone remodeling.
BOP and/or SUP were prerequisite in all of the analyzed studies. In most of the studies, the combination of clinical and radiographic measurements were used for case definition. In two prospective studies, the radiographic MBL were not reported, and clinical measurements alone were used to assess biological complications. In these cases, the presence of BOP and/or SUP on probing and PPD ≥ 4 mm were prerequisite for a diagnosis of periimplantitis. In nine studies, one randomized controlled trial, three prospective and five retrospective studies, BOP and radiographic assessments were performed alone, without reporting any PPD measurements. In these cases, a MBL ranging from 0.5 mm to > 4 mm34 was considered to be associated with periimplantitis.
Before 2012, changes in the level of crestal bone were either not defined or not clearly reported, making the diagnosis of periimplantitis difficult. However, even in studies that defined the entity of MBL, different diagnostic criteria were used. In one long-term study, periimplantitis was defined as the presence of BOP, PPD ≥ 4 mm and MBL > 0.5 mm. However, another study used MBL > 4 mm as a reference value.34 Most of the studies considered MBL > 2 mm for the diagnosis of periimplantitis. Previously, our group used a radiographic MBL > 3 mm, from the baseline radiograph taken at the time of prosthesis delivery, to diagnose periimplantitis. In three other studies, MBL was considered in relation to the time that the prosthesis was in function. All of the studies but five calculated MBL in millimeters. In the other studies, the implant threads were used as reference.
Eight studies applied PPD > 5 mm for the diagnosis of periimplantitis. Marrone et al. defined periimplantitis as the presence of BOP, PPD > 5 mm and MBL > 2 mm. Charalampakis et al. applied the criteria of the presence of BOP and/or SUP, PPD ≥ 5 mm and MBL ≥ 1.8 mm after one year in function. Zetterqvist et al. included cases of PPD > 5 mm and MBL ≥ 3 mm. Two other studies, one prospective and one retrospective, applied the presence of BOP and/or SUP, PPD > 5 mm and radiographic signs of MBL, without specifying the baseline bone level. Positive BOP and/or SUP, radiographic MBL ≥ 3 mm and PPD ≥ 6 mm were used by Koldsland et al.
At the 7th and 8th European Workshop on Periodontology, periimplant mucositis and periimplantitis were described as follows: “changes in the level of crestal bone, presence of bleeding on probing and/or suppuration; with or without concomitant deepening of peri-implant pockets.” Periimplant mucositis was defined with positive BOP and/or SUP and periimplantitis with positive BOP and/or SUP, in combination with radiographic MBL ≥ 2 mm. The same parameters were used by Zitzmann and Berglundh to define periimplantitis. However, Atieh et al. used the same criteria, plus PPD ≥ 5 mm, as the definition of periimplantitis in their systematic review paper.
Discussion
Periimplant diseases present in two forms: periimplant mucositis and periimplantitis. Both are characterized by an inflammatory reaction in the tissue surrounding an implant. Periimplant mucositis has been defined as a reversible inflammatory reaction in the soft-tissue surrounding an implant in function, whereas periimplantitis has been defined as a more profound inflammatory lesion characterized by a deepened periimplant pocket and loss of supporting bone around a functional implant.
Studies published in early 2010 suggested that mucositis and periimplantitis are equivalent to periodontitis, since both are described as an imbalance between bacterial load and the host response. Based upon this, both diseases are closely related to the formation of a biofilm containing microbiota rich in Gram-negative bacteria in the presence of a susceptible host. However, it has been shown that microorganisms may be present, but are not a necessity for periimplantitis. In addition, both periodontitis and periimplantitis share several common systemic risk factors or indicators (e.g., smoking, poor oral hygiene, diabetes or history of periodontitis, osteoporosis). Similarly, periimplantitis, as occurs with periodontitis, seems to be influenced by a particular genetic profile (i.e., interleukin-1 polymorphism). Others have rejected the description of a disease comparable to periodontitis, because of the anatomical differences that exist between periodontal and periimplant structures (e.g., different collagen fiber orientation [perpendicular vs. horizontal], vascularity or repair capacity, and the mechanical resilience provided by the periodontal ligament). In fact, periodontitis is characterized by inflammatory destruction of the supporting apparatus of the dentition (periodontium), including the periodontal ligament and alveolar bone. Owing to the different composition of the two supporting tissues, similar tissue reactions around an implant and a tooth seem most unlikely. The term “osseo-insufficiency” was proposed by Zarb and Koka to describe the difference between periimplantitis and periodontitis-induced bone loss. The anatomical image of bone resorption due to periodontitis or periimplantitis differs, in many situations with very wide bone craters being typical for the implant but not for the tooth. Hence, periimplantitis may be considered distinct from periodontitis in that it significantly differs regarding onset and progression and has poor treatment predictability; consequently, its treatment must be focused on early diagnosis and controlling the risk factors or indicators to prevent it from occurring.
To date, there have been no standardized parameters to clinically differentiate the various stages and severities of periimplantitis. The criteria used to diagnose periimplantitis remain inconclusive. Most existing studies used clinical parameters in combination with radiographic findings to define periimplantitis. However, clinical parameters such as BOP and PPD around implants are less predictable, since they are influenced by more confounding factors compared with natural dentition. Furthermore, any factor that facilitates plaque formation (e.g., poor oral hygiene) or host defense capability (e.g., smoking habit, excessive alcohol consumption, genetic traits, history of periodontitis or use of bisphosphonates) might contribute to the development of periimplantitis. The diagnosis and progression of periimplantitis may be characterized by increased measurements for clinical parameters (PPD, BOP, SUP or even mobility), MBL and microbiology. Regarding clinical parameters, PPD is a valid method of assessment, as correlation exists between bone levels recorded and radiographic probe penetration. Nevertheless, in a cross-sectional study, the intraoperatively measured periimplant bone levels were more apical than the radiographic bone levels. SUP occurs more frequently in implants with than without progressive bone loss, particularly in smokers, and may be associated with episodes of active tissue destruction. In a systematic review, Berglundh et al. defined periimplantitis as having a PPD ≥ 6 mm or MBL ≥ 2.5 mm. Lang and Berglundh, in the 2011 European Federation of Periodontology consensus, stated that clinical and radiographic data should routinely be obtained after prosthesis installation on implants in order to establish a baseline for the diagnosis of periimplantitis during maintenance of implant patients. A meta-analysis by Derks and Tomasi clearly showed a positive relationship between the prevalence of periimplantitis and function time. The presence of bone loss and PPD alone may not be enough to establish a diagnosis of periimplantitis. One important factor that potentially influences the wide range of periimplantitis prevalence is the lack of consensus regarding the clinical parameters. For example, one study reported that if PPD > 4 mm was used as criterion, then 74.8% individuals had periimplantitis, but if this measurement was changed to > 6 mm, then the prevalence dropped to 43.9%. When radiographic MBL was considered for defining periimplantitis, 25.3% individuals showed > 2 mm, while 13.1% had > 3 mm. Indeed, if PPD is considered, some further heterogeneity can be found. Probing around implants is influenced by many factors, such as the size of the probe, the probing force, the direction of the probe, the health and thickness of the periimplant soft-tissue, and the design of the implant neck and the superstructure.1 In fact, the platform-switched design, as well as defective restorations, can complicate probing and, thus, hide the true extent of periimplantitis. Furthermore, the presence of discrepancies in the buccolingual hard- and soft-tissue levels may result in different PPD readings.
Owing to the lack of standard parameters to determine the presence and severity of periimplant disease, it is difficult to develop a clinical strategy based upon PPD in managing this common problem in implant dentistry. However, Froum and Rosen proposed a classification system to determine periimplantitis severity based upon PPD, MBL and clinical signs of BOP and/or SUP,28 but this system remains to be validated. Furthermore, in a series of studies by Merli et al., the inter-rater agreement in the diagnosis of periimplant disease was judged as merely good, owing to the unclear definition of periimplantitis and mucositis, with complete agreement obtained only in half of the cases (52%).
The vast majority (45%) of the studies included in the present review found radiographic MBL > 1–2 mm after prosthetic loading. Hence, the following criteria for defining periimplantitis are proposed: a radiographic MBL > 1 mm after implant prosthesis delivery or 2 mm at least six months after implant prosthesis placement as a good indicator of periimplantitis. BOP does not possess a high predictive value owing to the weak soft-tissue connection around dental implants. Likewise, PPD largely relies on implant design (bone vs. tissue level), apicocoronal position and biotype. From the extracted data, it seems logical to consider radiographic MBL as the most uniform and accurate indicator of periimplantitis. Although, the cut-off value depends on the patient’s inflammatory pattern, the type of surgery, the apicocoronal implant position, the implant’s macrodesign and the crestal module, considering the rapid disease progression over time, strict radiographic control must be followed if any clinical symptom is detected. Furthermore, the clinician must use a combination of the many available clinical parameters, such as PPD, inflammatory status of the mucosa, BOP on light probing, radiographic MBL, and possibly bacterial and/or periimplant crevicular fluid biomarkers to establish an accurate diagnosis of periimplantitis. Unlike in the case of periodontitis, bacterial testing may not reliable in diagnosing peri-implantitis. This suggests that periodontal and periimplant ecosystems differ significantly and, hence, periimplant disease might not always be approached as an infectious disease. Similarly, such difference has been shown to apply to the pathogenesis. Furthermore, no evidence was found that primary infection caused marginal bone resorption.
Conclusion
The available scientific literature suggested an absence of a unanimous definition of periimplantitis. Actual definitions of periimplantitis were based solely on clinical parameters without consideration of other potential related risk factors of the disease. Future studies that apply consistent case definitions should be considered.
Marco Tallarico, Alberto Monje, Hom-Lay Wang, Pablo Galindo Moreno, Erta Xhanarid & Luigi Canulloe
References
- Levignac J. [Periimplantation osteolysis— periimplantosis—periimplantitis]. Rev Fr Odontostomatol. 1965 Oct;12(8):1251–60. French.
- Hämmerle CH, Glauser R. Clinical evaluation of dental implant treatment. Periodontol 2000. 2004 Feb;34:230–9.
- Schwarz F, Becker K, Sager M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J Clin Periodontol. 2015 Apr;42 Suppl 16:S202–13.
- Atieh MA, Alsabeeha NH, Faggion CM, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013 Nov;84(11):1586–98.
- Zitzmann NU, Margolin MD, Filippi A, Weiger R, Krastl G. Patient assessment and diagnosis in implant treatment. Aust Dent J. 2008 Jun;53 Suppl 1:S3–10.
- Sanz M, Chapple IL; Working Group 4 of the VIII European Workshop on Periodontology. Clinical research on peri-implant diseases: consensus report of Working Group 4. J Clin Periodontol. 2012 Feb;39 Suppl 12:202–6.
- American Academy of Periodontology. Academy report: peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013 Apr;84(4):436–43.
- Lang NP, Berglundh T; Working Group 4 of the Seventh European Workshop on Periodontology. Periimplant diseases: where are we now?—Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011 Mar;38 Suppl 11:178–81.
- Chan HL, Lin GH, Suarez F, MacEachern M, Wang HL. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes.J Periodontol. 2014 Aug;85(8):1027–41.
- Lindhe J, Meyle J; Group D of the European Workshop on Periodontology. Peri-implant diseases: consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008 Sep;35(8 Suppl):282–5.
- Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012 Oct;23 Suppl 6:67–76.
