Dental implants treatment outcomes in patient under active therapy with alendronate: 3-year follow-up results of a multicenter prospective observational study
Abstract
Objective: To evaluate the 3-year clinical and radiographic data of fixed implant-supported dental prosthesis delivered to patients having taken alendronate 35–70 mg weekly for at least 3 years before implant placement.
Materials and Methods: Forty consecutive patients treated with oral bisphosphonates and requiring an implant-supported restoration were recruited in two private centers between January 2008 and December 2011. Implants were inserted through minimally invasive approach under antibacterial and antibiotic treatment, 6 months after alendronate administration stopping. After 4 months of submerged healing, implants underwent prosthetic loading. Hygiene maintenance and clinical assessments were scheduled every 4 months for 3 years. Outcome measures were the following: implant and prosthetic success, survival rates, any observed clinical complications, marginal bone remodeling, probing pocket depth and bleeding-on-probing.
Results: At the end of the study, eight patients dropped out. The final sample size resulted in 32 consecutive partially or fully edentulous patients (32 females; mean age 64.6 years) with 98 submerged implants. In only one patient, maxillary implant failed during healing period. No prosthesis failed during the entire follow-up, and no major complications were recorded. Implant and prostheses success resulted in an overall survival rate of 98, 98% and 100%, respectively. Three-year mean marginal bone loss was 1.35 ± 0.21 (CI 95% 1.24–1.38). Successful soft tissue parameters were found around all implants.
Conclusions: Oral bisphosphonate therapy did not appear to significantly affect implant survival and success in case of accurate treatment time selection, minimally invasive surgical approach and constant follow-up. Further prospective studies involving larger sample sizes and longer durations of follow-up are required to confirm these results.
Introduction
Osteoporosis is a progressive systemic skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture (Kanis et al. 2008). The most common medical treatment for osteoporosis involves the use of bisphosphonates (BPs) (Bernabei et al. 2014). In 2009, the Food and Drug Administration has approved the annual injection of zoledronic acid for the prevention of osteoporosis in menopausal populations (Khosla 2009). Bisphosphonates are stable analogs of naturally occurring inorganic pyrophosphates and are classified as antiresorptive medications (Fleisch et al. 1966), used to decrease osteoclast activity, prevent the resorption of bone and reduce its turnover (Reszka & Rodan 2004). They are commonly used to treat diseases that affect bone metabolism including multiple myeloma, secondary hypercalcemia caused by malignant tumors, bone metastasis in metastatic prostate or breast cancers, Paget’s disease, as well as osteoporosis (Sarzi Amadè et al. 2008).
The action of a BP depends on the drug’s chemical structure. Traditionally, BPs are divided into non-nitrogen-containing (non-N-BPs) drugs and nitrogen-containing (N-BPs). The non-nitrogenous bisphosphonates are metabolized in the cell into compounds that replace the terminal pyrophosphate moiety of ATP, forming a non-functional molecule that competes with adenosine triphosphate (ATP) in the cellular energy metabolism. The osteoclast initiates apoptosis and dies, leading to an overall decrease in the breakdown of bone. The addition of an amine group to the end of a side chain increases the drug’s potency; however, the mechanism of nitrogen-containing BPs is less well known. According to Reszka & Rodan (2004), N-BPs inhibit the farnesyl diphosphate synthase enzyme of the cholesterol biosynthesis pathway and disrupt the isoprenylation branch pathway, which inhibits proteins and other factors that play a rate-limiting role in osteoclast resorption of bone. N-BPs accumulate in maximum concentration in the osseous matrix and in osteoclasts, mainly during the first 24–48 h of medication. The end result is osteoclast cellular dysfunction and death (Jones et al. 2001). The resulting inhibition of normal bone resorption leads to reduced bone turnover, increased bone density, improved mineralization and reduced risk of fracture (Jones et al. 2001).
The alendronate is a nitrogen-containing bisphosphonate that has been extensively used for the treatment of osteoporosis (Tsetsenekou et al. 2012). The effects of locally and/or systemically administered alendronate on osseointegration have been extensively evaluated with experiments being performed in animals (Abtahi et al. 2013). However, according to a task force set up by the American Society for Bone and Mineral Research, there is little information available about the side effect of the oral N-BPs (Kos et al. 2010).
Two recent systematic reviews of the literature suggest that the intake of oral BPs did not influence short-term (1–4 years) implant survival rates, even when complications occurred (Madrid & Sanz 2009; Ata-Ali et al. 2014). Nevertheless, prospective study reporting implant survival and success rates are still lacking. As universally accepted guidelines have not been released yet, the clinical management of bisphosphonate-related ONJ remains controversial (Rupel et al. 2014). Since 2003, reports have been published on necrosis of the jaw bones possibly being associated with the administration of bisphosphonates (Hasegawa et al. 2012). There has been discussion regarding the risks associated with the performance of oral surgical procedures, particularly bone grafting and implant placement, on patients who are taking oral BPs (Sarzi Amadè et al. 2008; Memon et al. 2012; Kwon et al. 2014). The same authors concluded that prevention of BP-related ONJ is not completely possible, but non-invasive preventative procedures could help to decrease its incidence (Sarzi Amadè et al. 2008; Memon et al. 2012; Kwon et al. 2014). The American Association of Oral and Maxillofacial Surgeons recommends that patients suspended BPs 3 months prior to and 3 months after oral surgery if possible, especially if a patient had been using BPs for over 3 years (Ruggiero et al. 2009; Allen & Ruggiero 2014). For patients having a history of oral bisphosphonate treatment exceeding 3 years and those having concomitant treatment with prednisone, additional testing and alternate treatment options should be considered (Grant et al. 2008).
The purpose of this multicenter prospective observational study was to present the 3-year data on implants and prosthetic survival and success rates and peri-implant bone loss after placement of a fixed implant-supported dental prosthesis delivered to patients taking oral bisphosphonates (alendronate 70 mg tablet once a week or 5–10 mg once daily) for at least 3 years before implant placement. This study followed the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) guidelines.
Material and methods
Study design
This multicenter prospective observational study was designed to evaluate the clinical and radiological outcome of fixed implant-supported dental prosthesis delivered to patients taking alendronate 70 mg tablet once weekly or 5–10 mg once daily, for at least 3 years before implant placement. Forty patients were selected and treated in two private centers (20 in Rome, 20 in Sassari, Italy), between January 2008 and December 2011. Two experienced clinicians performed all implants and prosthetic procedures. The investigation was conducted according to the principles embodied in the Helsinki Declaration of 1964 for biomedical research involving human subjects, as amended in 2008. Patients were informed about clinical procedures, materials to be used, benefits, potential risks and complications, as well as follow-up evaluations required for the clinical trial, and gave their written consent to take part in this study.
Any healthy patient <90 years old, above the age of 18 years at the time of implant placement, needing an implant-supported prosthesis was asked to participate in the investigation in a consecutive order. Patients were included in the study after fulfilling the inclusion and not meeting any of the exclusion criteria.
The exclusion criteria were as follows:
- General medical (American Society of Anesthesiologist, ASA, class III or IV) and/or psychiatric contraindications;
- Pregnancy or nursing;
- Alcohol or drug abuse;
- Heavy smoking (>10 cigarettes/day);
- Radiation therapy to head or neck region within 5 years;
- High and moderate parafunctional activity;
- Absence of teeth/denture in the opposite jaw;
- Untreated periodontitis;
- Post-extractive implants;
- Full mouth bleeding and full mouth plaque index higher than or equal to 25%;
- <10 mm height and 5 mm width of bone to allow implants;
- Unavailability for regular follow-ups.
All the patients were treated according to a two-steps diagnostic and therapeutic protocol (Fig. 1). Diagnostic steps as follows:
- Anamnesis;
- Clinical examination and photographs;
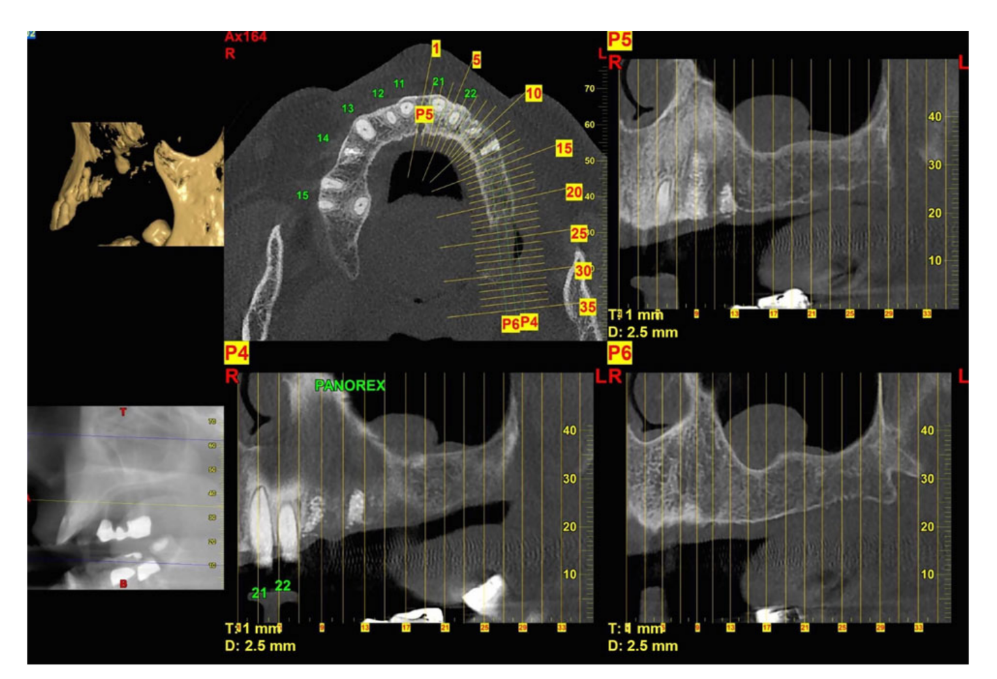
- Radiographic examination including peri-apical and panoramic X-rays, computed tomography (CT) scan, or cone beam CT (CBCT);
- Periodontal examination (probing and measurement of the indexes of oral health);
- Photographic documentation;
- Patient information (medicines to take and possible consequences).
Clinical procedures
Therapeutic protocol (Table 1):
- Professional hygiene;
- Elimination of all infection focuses (residual roots, caries, periodontal therapy):
- non-restorative-surgical treatment of all compromised teeth or with a poor prognosis;
- restorative treatment of teeth affected by decay;
- periodontal treatment and support therapy;
- assessment of the adequacy of removable and fixed prosthetics. In particular, it was evaluated the seal of the prosthetic structures and the stability of removable prosthesis, which have to be atraumatic.
At the end of the prevention stage, patients were motivated to a scrupulous domestic oral hygiene protocol and to follow professional recalls.
Two experienced surgeons (MT & SMM) performed all interventions. When planned, atraumatic tooth extraction was performed at least 8 weeks before implant placement. Crowns of multi-rooted teeth were sectioned. Roots were then individually removed and if needed, with the aid of a periotome. The residual extraction sockets were debrided thoroughly of granulation tissue and residual periodontal ligament fibers with curettes.
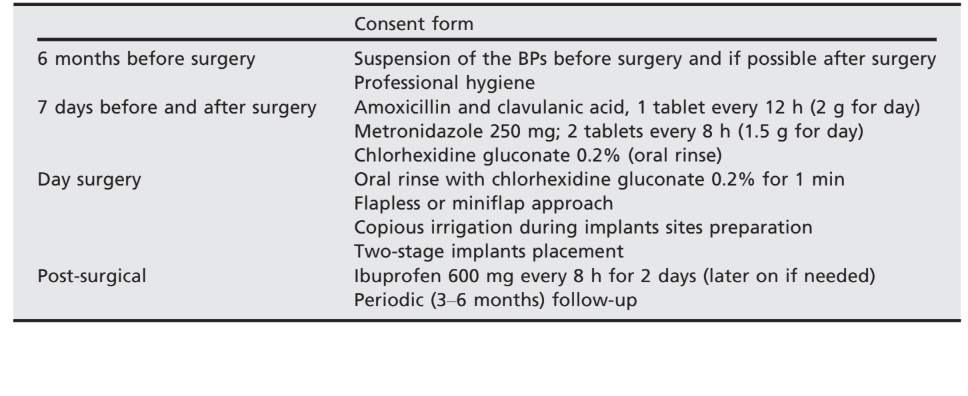
Pre-surgical protocol required the suspension of the BPs 6 months before surgery and until complete healing 4–6 months after implant installation. Antibiotics were administered prophylactically 7 days before surgery and continued for 7 days (Sarzi Amadè et al. 2008):
- Amoxicillin and clavulanic acid, one tablet every 12 h (2 g for day);
- Metronidazole 250 mg; two tablets every 8 h (1.5 g for day).
Prior to the start of surgery, patients rinsed with chlorhexidine 0.2% mouthwash for 1 min. Local anesthesia was induced using a 4% articaine solution with epinephrine 1 : 100.000 (Ubistein; 3M Italy SpA, Milan, Italy). Implants were placed in the planned anatomic sites using a flapless or a miniflap approach, (Fig. 2). Bone density was assessed during the drilling phase by clinician’s experience and sensation, and it was based on Lekholm and Zarb classification (Brånemark et al. 1985). Each drill was used under copious irrigation according to the protocol recommended by the manufacturer. The implant platform was positioned at the alveolar crest level or slightly below in the esthetic area.
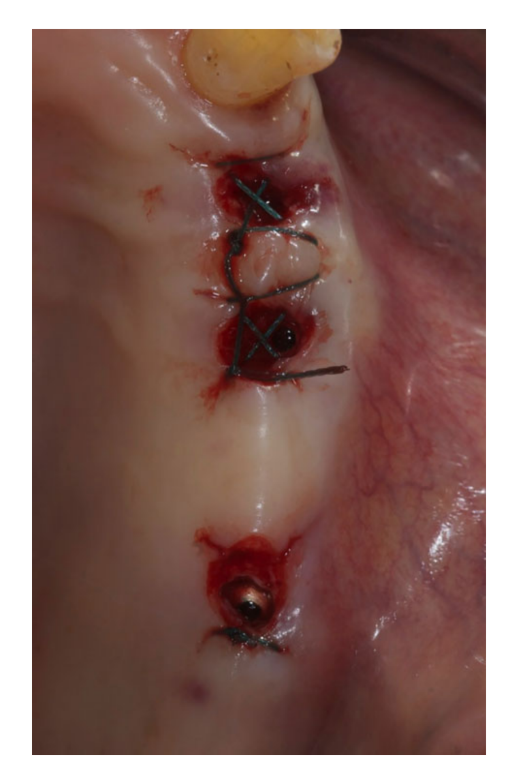
After implant placement, all patients received oral and written recommendations regarding medication, oral hygiene maintenance and diet. Post-surgical analgesic treatment was performed with ibuprofen 100 mg, administered every 8 h for 2 days after the surgery, and later on if needed. The patients were instructed to rinse with chlorhexidine 0.2% mouthwash twice a day without brushing the implant area until suture removal (10–14 days after).
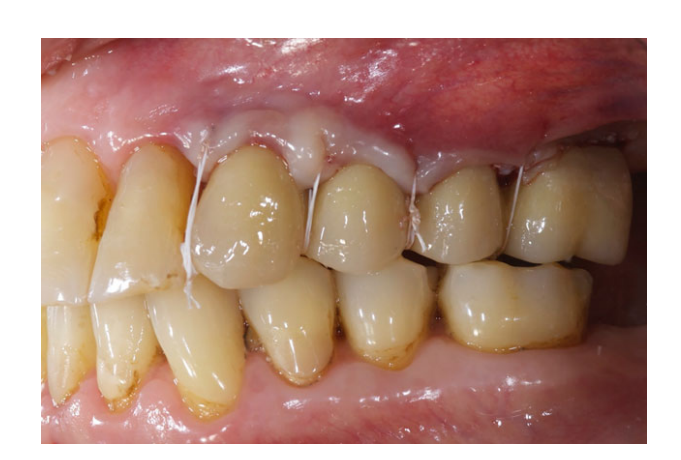
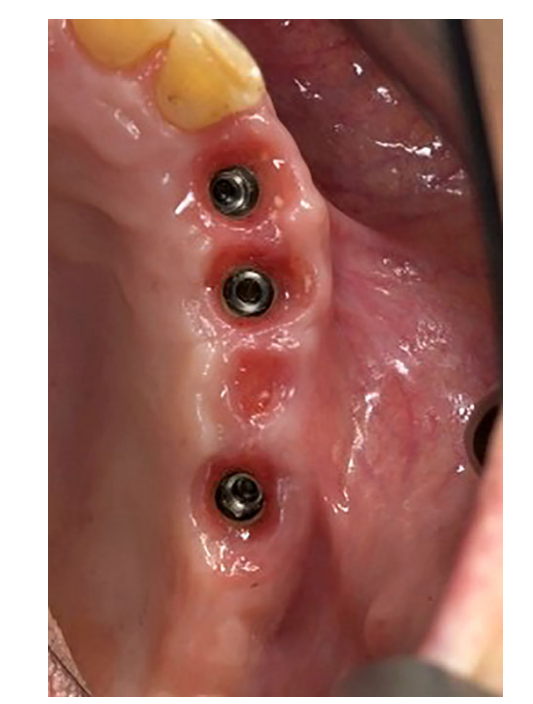
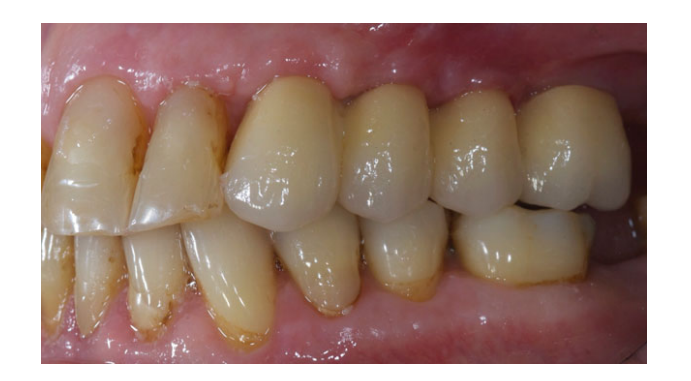
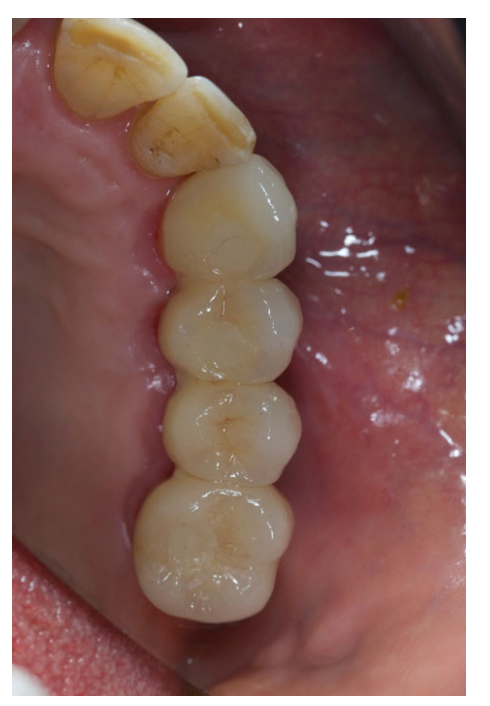
All the implants were placed submerged. The implants were exposed 3 months after implant placement (Figs 3 and 4). Open tray impressions were taken using a polyether material (ImpregumTM, 3M ESPE, Seefeld, Germany) with a custom open tray (Diatray Top, Dental Kontor, Stockelsdorf, Germany). At the time of prosthesis delivery (Figs 5 and 6), occlusion was adjusted and the restorations were either screwed or cemented using eugenol-free zinc oxide cement (Temp Bond NE, Kerr Corporation, Orange, CA, USA) 12 weeks after the first surgery, according to a conventional loading protocol (Gallucci et al. 2013). Follow-up visits were scheduled 1–6 months and then annually up to 3 years of function (Fig. 7). At every follow-up visit, occlusal adjustment of the dental prosthesis was performed if needed. The patients underwent a professional cleaning by a dental hygienist every 4–6 months. Periapical radiographs were obtained annually after definitive prosthesis delivery with the same customized Rinn holder (Rinn, Elgin, IL, USA).
Outcome measures
The primary outcome measures were the implant success and survival rates. At each follow-up examination, the implants were examined for tissue integration according to the strict parameters defined by Buser et al. (1990). Specifically, the integration was considered successful if the following parameters were met: (1) absence of recurring peri-implant infection with suppuration; (2) absence of persistent subjective complaints such as pain, foreign body sensation and/or dysesthesia, (3) absence of a continuous radiolucency around the implant and (4) absence of any detectable implant mobility. These criteria have proven to be effective in defining the success of an implant system and evaluating long-term results in clinical trials. Prosthesis success was evaluated following a modification of the evaluation criteria, suggested by the California Dental Association (CDA (Association CD 1976)).
The secondary outcomes were the following: any surgical and prosthetic complications occurred during the entire follow-up, marginal bone loss, probing pocket depth (PPD) and bleeding-on-probing (BOP).
- Complications: Any technical (fracture of the framework and/or the veneering material, screw loosening, etc.) and/or biologic (pain, swelling, suppuration, etc.) complications were considered.
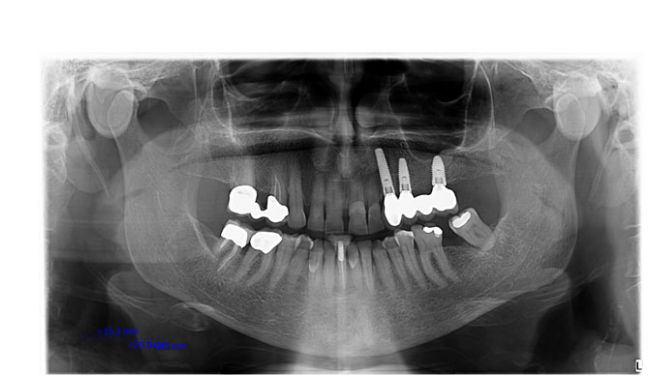
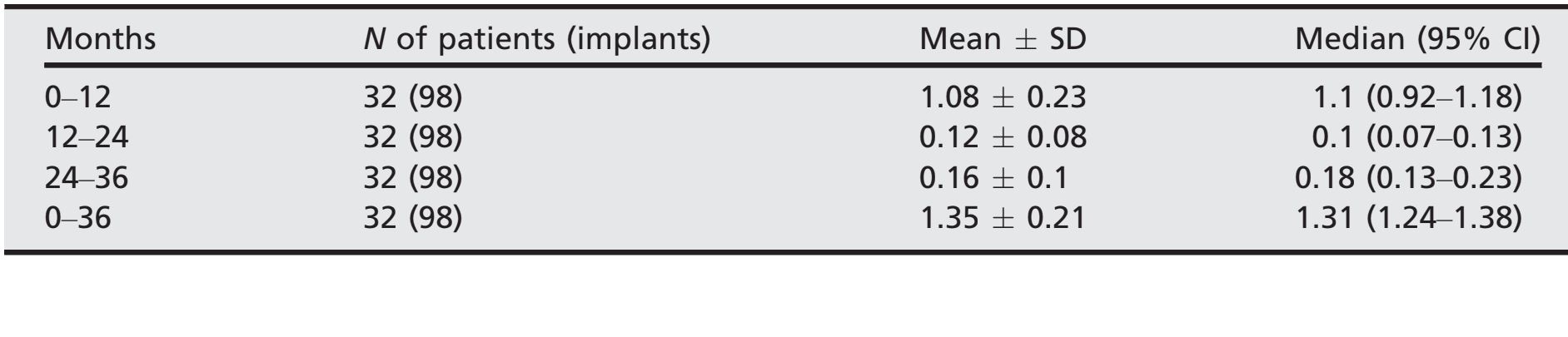
- Marginal bone loss: The distance from the most coronal margin of the implant collar and the most coronal point of bone-to-implant contact were taken as marginal bone level (MBL). MBL around the implants was evaluated on intraoral digital radiographs taken with the paralleling technique using a film holder (Rinn XCP, Dentsply, Elgin, IL, USA) at implant placement (baseline) and after 12, 24 and 36 months. The radiographs were accepted or rejected for evaluation based on the clarity of the implant threads. All readable radiographs were displayed in an image analysis program (DFW2.8 for windows, Soredex, Tuusula, Finland) on a 24-inch LCD screen (iMac, Apple, Cupertino, CA, USA) and evaluated under standardized conditions (SO 12646:2004). The software was calibrated for every single image using the known distance of the implant diameter or length. Measurements of the mesial and distal bone crest level adjacent to each implant were made to the nearest 0.1 mm and averaged at patient level.
- PPD and BoP were measured by a blindedoperator with a periodontal probe (PCP-UNC 15; Hu-Friedy Manufacturing, Chicago, IL, USA) at 6, 12, 24 and 36 months. Three vestibular and three lingual values were collected for each implant and averaged at patient level.
Two independents and fully blinded dentists (LC and EX) for each center evaluated the implant and prosthetic survival and success rate. Complications were assessed and treated by the treating clinicians (MT and SMM) who were non-blinded. The marginal bone loss (DMBL) was evaluated by an independent radiologist. An independent hygienist performed all the periodontal measurements.
Statistical analysis
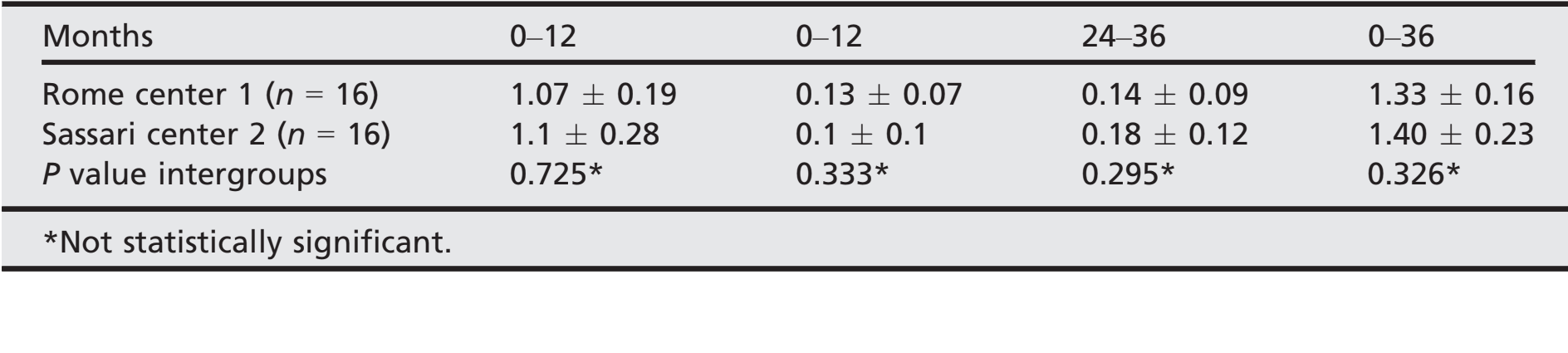
The statistical analysis was performed for numeric parameters such as marginal bone level and soft tissue parameters using SPSS for Mac OS X version 22.0 (SPSS, Chicago, IL, USA). A descriptive analysis was performed using mean standard deviation (SD), and median with 95% confidence interval (CI). Cumulative survival and success rates were calculated used the implants and prostheses as the statistical unit of the analyses. Differences of mean marginal bone levels between follow-ups, averaged at patient level, were compared by Wilcoxon signed-rank test for matched samples. The level of statistical significance was set at 0.05. Comparisons among centers were performed by one-way analysis of variance (ANOVA).
Results
At the end of the study, eight patients (20%) with 18 implants (15.5%) dropped out. One patient at the Rome center died of hepatic cancer. Five patients (two at the Rome center and three at the Sassari center) had serious health problems not related to the dental implant therapy, so that they could not come to the scheduled follow-up examinations. Two patients moved abroad. However, based on a phone interview, all of these patients had their implants in function without patient-related complications (pain, swelling). The final sample size resulted in a total of 98 implants with rough oxidized surface and tapered-body implant design (NobelReplace, Nobel Biocare AB) which were placed in 32 consecutive edentulous patients, (32 females) with a mean age of 64.6 (range 46–80). Each center treated the same number of 16 patients. Each patient was followed for ≥3 years of function (range 36–72 months; mean 47.6 months). There were no apparent baseline imbalances between the two groups apart from a higher number of longer implants used by the center of Rome and presence of more cases treated using computer-assisted template-based surgery in the Sardinia center. No deviation from the original protocol occurred. All the data collected were included in the statistical analysis.
- One of 98 implants (1.02%) failed during the healing period, before delivery of the final prostheses, resulting an overall implant cumulative survival rate of 98.98% at the 3-year follow-up. The only implant failure occurred in one patient of the Sardinia center (narrow platform implant 10 mm long). The implant was not replaced.
- At the 3-year follow-up examination, none of the definitive prostheses had failed resulting in a cumulative prosthetic survival rate of 100%.
- No prosthetic complication was observed. No major biological complications were recorded. Three patients had peri-implant mucosal inflammation with BoP after 6 months. Improved oral hygiene reduced the peri-implant inflammation, without any surgical interventions.
- After an initial mean marginal bone loss of 1.08 ± 0.23 (Median 1.1; CI 95% 0.92–1.18), all implants lost a mean of 0.12 ± 0.08 (median 0.1; CI 95% 0.07–0.13) between the 1- and 2-year follow- up, and 0.16 ± 0.1 (median 0.18; CI 95% 0.13–0.23) between the 2- and 3-year follow-up, with no statistically significant difference (P = 0.059). At the 3-year follow-up, the mean marginal bone loss was 1.35 0.21 (median 1.31; CI 95% 1.24–1.38). All the data are reported in Table 2. At each follow-up, bone level changes were similar for both centers (P ≥ 0.05). All the data are reported in Table 3.
- At the 3-year follow-up examination, the mean PPD values were 2.71 0.38 mm (95% CI = 2.54–2.86). The mean BoP val- ues were 1.29 ± 0.76 (95% CI = 0.93–1.63).
Discussions
The present prospective observational study was designed to evaluate the 3-year clinical and radiographic outcomes of a fixed implant-supported dental prosthesis delivered to patients taking alendronate 70 mg tablet once weekly or 5–10 mg once daily for at least 3 years before implant placement.
At the 3-year follow-up examination, the results of the present study showed an implant and prosthetic survival rate of 99% and 100%, respectively. Marginal bone changes, as well as BOP and PPD values seem to be stable after 3 years, confirming that implant therapy on patients taking oral BPs is a viable treatment according to the proposed therapeutic protocol. These results are consistent with other studies investigating the same topic. In a retrospective study, Memon et al. (2012) concluded that bis- phosphonates did not affect early implant success or crestal bone changes. Jeffcoat (2006) and Russo Dello et al. (2007), in two prospective studies, reported that oral BPs did not seem to increase the incidence of ONJ. Grant et al. (2008) in a retrospective study on 115 patients (468 implants) reported that oral bisphosphonate therapy did not appear to significantly affect implant success.
The main limitations of this study are the limited number of participants, followed-up for a short period. Nevertheless, even though eight patients dropped out, 98 implants were placed in 32 patients treated with the same protocol, and followed for at least 3 years, allowing the results of the present study to be generalized to a larger population with similar characteristics.
Animal study showed that administration of BPs interferes with normal bone remodeling after tooth extraction, jeopardizing the long- term healing around implants (Kim et al. 2013). One of the most serious though infrequent complications is bisphosphonate-related osteonecrosis of the jaws (BRONJ) (Kwon et al. 2014). The frequency of BRONJ may vary depending on the administration route and the strength of drugs used. The American Association of Oral and Maxillofacial Surgeons reported that the prevalence of BRONJ was about 0.8% to 12% when BPs are administered intravenously (Ruggiero et al. 2009; Allen & Ruggiero 2014). The prevalence is reportedly lower when BPs are administered orally. However, it was suggested that special attention should be paid when patients undergo BPs treatment for more than 3 years because the prevalence of BRONJ increases along the period of BPs treatment (Sarzi Amade` et al. 2008). After a poor enthusiastic preliminary results based on uncontrolled retrospective studies, some systematic review suggested more enthusiastic results. Madrid & Sanz (2009) concluded that a patient receiving oral bisphosphonates for a period of <5 years is “safe” to undergo dental procedures, specifically dental implants (Madrid & Sanz 2009). Furthermore, there is a general consensus on the non-contraindication of implant placement in non-cancer patients under oral BPs, mostly prescribed for osteoporosis (Madrid & Sanz 2009). Most recently, Kumar & Honne (2012) evaluating survival of dental implants in bisphosphonate users vs. non-users concluded that short-term BPs therapy does not increase or decrease the survival rate of dental implants compared to non-user.
Although the submerged technique is not a prerequisite for osseointegration (Tallarico et al. 2011), in the present study all the implants were placed submerged, without any surgical complications. This could represent one of the key factors together with significant antibiotic treatment and the temporary stopping of the alendronate administration, preventing bacterial contamination of the implant site.
In the present study, although the limited number of the patients, implant surgery did not result in bisphosphonate-related osteonecrosis of the jaw after 3 years after implant placement. Nevertheless, sufficient evidence exists to suggest that all patients undergoing implant placement should be questioned about bisphosphonate therapy including the drug taken, the dosage and length of treatment before surgery. Potential risks of BRONJ need to be explained to the patients before implant installation. For patients having a history of oral bisphosphonate treatment exceeding 3 years and those having concomitant treatment with prednisone, additional testing and alternate treatment options should be considered and prevention is still the most effective mean to avoid possible complications. In addition, the anatomic location of the implant and the duration of drug therapy at the time of placement were not significant factors in the success rate or bony changes.
Conclusion
Within the limitations of this study, according to the proposed therapeutic protocol and per- forming a minimally invasive submerged surgery, oral bisphosphonate therapy did not appear to significantly affect implant survival and success in the medium-term follow-up. These conclusions should be confirmed by further prospective studies involving larger sample sizes and longer durations of follow-up.
Marco Tallarico, Luigi Canullo
References
- Abtahi, J., Agholme, F., Sandberg, O. & Aspenberg, P. (2013) Effect of local vs. systemic bisphosphonate delivery on dental implant fixation in a model of osteonecrosis of the jaw. Journal of Dental Research 92: 279–283.
- Allen, M.R. & Ruggiero, S.L. (2014) A review of pharmaceutical agents and oral bone health: how osteonecrosis of the jaw has affected the field. International Journal of Oral and Maxillofacial Implants 29: e45–e57.
- Ata-Ali, J., Ata-Ali, F., Penarrocha-Oltra, D. & Galindo-Moreno, P. (2014) What is the impact of bisphosphonate therapy upon dental implant survival? A systematic review and meta-analy- sis. Clinical Oral Implants Research doi: 10.1111/clr.12526. [Epub ahead of print].
- Bernabei, R., Martone, A.M., Ortolani, E., Landi, F. & Marzetti, E. (2014) Screening, diagnosis and treatment of osteoporosis: a brief review. Clinical Cases in Mineral and Bone Metabolism 11: 201– 207.
- Brånemark, P.I., Zarb, G.A. & Albrektsson, T. (1985) Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry. Chicago: Quintessence ed.
- Buser, D., Weber, H.P. & Lang, N.P. (1990) Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow cylinder and hollow-screw implants. Clinical Oral Implants Research 1: 33–40.
- California Dental Association, Task Force on Quality Evaluation. (1976) Quality Evaluation for Dental Care: Guidelines for the Assessment of Clinical Quality and Professional Performance and the Standards for the Program Design to Assure the Quality of Care. Los Angeles: California Dental Association ed. Chicago.
- Fleisch, H., Russell, R. & Straumann, F. (1966) Effect of pyrophosphate on hydroxyapatite and its implications on calcium hemostasis. Nature 212: 901–903.
- Grant, B.T., Amenedo, C., Freeman, K. & Kraut, R.A. (2008) Outcomes of placing dental implants in patients taking oral bisphosphonates: a review of 115 cases. Journal of Oral and Maxillofacial Surgery 66: 223–230.
- Hasegawa, Y., Kawabe, M., Kimura, H., Kurita, K., Fukuta, J. & Urade, M. (2012) Influence of dentures in the initial occurrence site on the prognosis of bisphosphonate-related osteonecrosis of the jaws: a retrospective study. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 114: 318–824.
- Jeffcoat, M.K. (2006) Safety of oral bisphosphonates: controlled studies on alveolar bone. International Journal of Oral and Maxillofacial Implants 21: 349–353.
- Jones, D.H., Nakashima, T., Sanchez, O.H., Kozieradzki, I., Komarova, S.V., Sarosi, I., Morony, S., Rubin, E., Sarao, R., Hojilla, C.V., Komnenovic, V., Kong, Y.Y., Schreiber, M., Dixon, S.J., Sims, S.M., Khokha, R., Wada, T. & Penninger, J.M. (2006) Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440: 692–696.
- Kanis, J.A., McCloskey, E.V., Johansson, H., Oden, A., Melton, L.J. & Khaltaev, N. (2008) A reference standard for the description of osteoporosis. Bone 42: 467–475.
- Khosla, S. (2009) Increasing options for the treatment of osteoporosis. New England Journal of Medicine 361: 818–820.
- Kim, I., Ki, H., Lee, W., Kim, H. & Park, J.B. (2013) The effect of systemically administered bisphosphonates on bony healing after tooth extraction and osseointegration of dental implants in the rabbit maxilla. International Journal of Oral and Maxillofacial Implants 28: 1194–1200.
- Kos, M., Brusco, D., Kuebler, J. & Engelke, W. (2010b) Clinical comparison of patients with osteonecrosis of the jaws, with and without a history of bisphosphonates administration. International Journal of Oral and Maxillofacial Surgery 39: 1097–1102.
- Kos, M., Kuebler, J.F., Luczak, K. & Engelke, W. (2010a) Bisphosphonate-related osteonecrosis of the jaws: a review of 34 cases and evaluation of risk. Journal of Cranio-Maxillofacial Surgery 38: 255–259.
- Kumar, M.N. & Honne, T. (2012) Survival of dental implants in bisphosphonate users versus non- users: a systematic review. The European Journal of Prosthodontics and Restorative Dentistry 20: 159–162.
- Kwon, T.G., Lee, C.O., Park, J.W., Choi, S.Y., Rijal, G. & Shin, H.I. (2014) Osteonecrosis associated with dental implants in patients undergoing bisphosphonate treatment. Clinical Oral Implants Research 25: 632–640.
- Madrid, C. & Sanz, M. (2009) What impact do systemically administrated bisphosphonates have on oral implant therapy? A systematic review. Clinical Oral Implants Research 20: 87–95.
- Memon, S., Weltman, R.L. & Katancik, J.A. (2012) Oral bisphosphonates: early endosseous dental implant success and crestal bone changes. A retrospective study. International Journal of Oral and Maxillofacial Implants 27: 1216–1222.
- Reszka, A.A. & Rodan, G.A. (2004) Nitrogen-containing bisphosphonate mechanism of action. Mini Reviews in Medicinal Chemistry 4: 711– 719.
- Ruggiero, S.L., Dodson, T.B., Assael, L.A., Landesberg, R., Marx, R.E. & Mehrotra, B. (2009) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate- related osteonecrosis of the jaw – 2009 update. Australian Endodontic Journal 35: 119–130.
- Rupel, K., Ottaviani, G., Gobbo, M., Contardo, L., Tirelli, G., Vescovi, P., Di Lenarda, R. & Biasotto, M. (2014) A systematic review of therapeutical approaches in bisphosphonates-related osteonecrosisofthejaw(BRONJ). Oral Oncology 50: 10491057. Russo Dello, N.M., Jeffcoat, M.K., Marx, R.E. & Fugazzotto, P. (2007) Osteonecrosis in the jaws of patients who are using oral biphosphonates to treat osteoporosis. International Journal of Oral and Maxillofacial Implants 22: 146–153.
- Sarzi Amadè, D., Tallarico, M., Loreti, M.C., Montecchi, P.P. & Niccoli, A. (2008) Clinical guidelines for prevention of osteonecrosis of the jaws in patients in treatment with bisphospho- nates: literature review and report of three cases. Minerva Stomatologica 57: 429–446.
- Tallarico, M., Vaccarella, A. & Marzi, G.C. (2011) Clinical and radiological outcomes of 1- versus 2- stage implant placement: 1-year results of a randomised clinical trial. European Journal of Oral Implantology 4: 13–20.
- Tsetsenekou, E., Papadopoulos, T., Kalyvas, D., Papaioannou, N., Tangl, S. & Watzek, G. (2012) The influence of alendronate on osseointegration of nanotreated dental implants in New Zealand rabbits. Clinical Oral Implants Research 23: 659–666.
