Clinical and radiological outcomes of 1- versus 2-stage implant placement: 1-year results of a randomised clinical trial
Aim: To compare the clinical and radiological outcomes of 1- versus 2-stage implant placement.
Materials and methods: Forty-seven patients were randomly allocated to 1- or 2-stage treatment groups immediately after implant placement. Twenty-nine patients received 38 1-stage early loaded implants and 18 patients received 51 2-stage early loaded implants. Outcome measures were failures of implants and/or prosthesis, complications, pain score, amount of analgesic consumption and peri-implant bone level changes at implant loading (12 weeks) and at the 1-year follow-up.
Results: After 1 year, no dropout occurred. In the 1-stage group, 2 implants (1 patient) failed to osseointegrate and the implant-supported prosthesis could not be placed, versus none in the 2-stage group. Two complications were reported in the 1-stage group versus only one in the 2-stage group. Pain score measurements, analgesic consumption and peri-implant bone level did not show any significant difference between the two groups. All patients would undergo the same procedures again.
Conclusions: The submerged technique is not a prerequisite for osseointegration, though 1-stage implant placement might be at a slightly higher risk for early failures.
Introduction
The integration and healing processes of dental implants were first described by Brånemark and Schroeder. Osseointegration was originally defined as the direct structural and functional connection between ordered, living bone and the surface of a load-carrying implant. Among the initially identified prerequisites for osseointegration and successful implant treatment were primary implant stability at time of placement and absence of micro-motion during the healing phase for preventing soft-tissue encapsulation of the implant. The original Brånemark concept prescribed a 2-stage surgery with a submerged healing period of at least 3 months in the mandible and 6 months in the maxilla. This approach is considered the gold standard procedure. Nowadays, clinical research is focusing on shorter and less invasive procedures. Different placement and loading protocols are currently used to shorten treatment times and decrease the amount of surgical interventions, enabling clinicians to choose between a 1- (non-submerged) and a 2-stage (submerged) approach. In the classic 2-stage surgical approach, the implant is covered during the healing phase by soft tissue. After bone healing, a second surgery is performed to connect a healing abutment. In the 1-stage surgical approach, transmucosal healing abutments are placed. Studies have shown that osseointegration can be predictably achieved also with 1-stage implants in both jaws, but divergent results have been presented. According to a Cochrane systematic review, the 1-stage approach seems to be preferable in partially edentulous patients since it avoids one surgical intervention and shortens treatment times. In addition, the use of 1-stage placement reduces patient discomfort, and allows for a healed peri-implant mucosa at the time of prosthetic rehabilitation. However, more randomised clinical trials with a larger number of patients are required to confirm these preliminary results. The aim of the present randomised clinical trial is to compare a 1-stage versus a 2-stage implant placement protocol in partially edentulous patients. The hypothesis was that there would be no difference in results between the two procedures concerning success rate and peri-implant marginal bone level changes. This trial is reported according to the CONSORT statement for improving the quality of parallel-group randomised trials.
Materials and methods
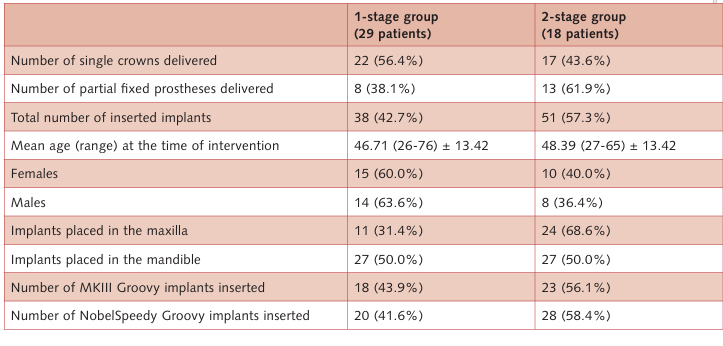
This trial was designed as a randomised, controlled, 2 x 2 factorial clinical trial. Patients requiring one or more dental implants who were 20 years or older and able to sign an informed consent form were recruited for placement of 1-stage or 2-stage, MKIII Groovy™ or NobelSpeedy™ Groovy (Nobel Biocare, AB, Göteborg, Sweden) implants. Randomisation of participants was made indirectly by independent randomisation within each intervention. Periapical radiographs were used for initial screening. Exclusion criteria were smoking more than 10 cigarettes a day within the past year, untreated periodontitis, poor oral hygiene and motivation, insufficient bone volume to accommodate dental implants, inadequate restorations of existing teeth at the moment of implant placement, absence of mutually protected occlusion, poor physical health, patients requiring bone augmentation and an implant insertion torque ≤ 35 Ncm. Moreover, the minimum distance of an implant to the adjacent teeth had to be at least 1.5 mm, and in cases of two or more adjacent implants at least 3 mm between them.
Eligible patients were asked to participate and were enrolled after detailed explanations of the study protocol. Patients were recruited and treated in a private practice in Rome between September 2008 and October 2009.
One experienced surgeon (MT) performed all interventions. When planned, tooth extractions were performed at least 6 weeks before implant placement. Patients were instructed to use chlorhexidine mouthwash 0.2% for 1 minute, twice a day, starting 3 days prior to implant placement and thereafter for 1 week. In both groups, a single 2 g dose of prophylactic antibiotic (amoxicillin and clavulanic acid) was administered 1 hour before surgery14. Local anaesthesia was induced using articaine with epinephrine 1:100,000. Small flaps were elevated. Patients received TiUnite® Brånemark System implants (41 MKIII Groovy™ and 48 NobelSpeedy™ Groovy, Nobel Biocare) placed according to the manufacturer’s instructions. The diameter of the final drill was chosen in relation to the bone quality, and a minimum insertion torque of 35 Ncm was to be obtained. In the 1-stage group the healing abutments were placed, while in the 2-stage group the cover screws were placed, both after the application of a 1% chlorhexidine gel. After implant placement, all patients received oral and written recommendations. Ibuprofen 600 mg was prescribed to be taken every 6 to 8 hours if needed. No implant-supported temporary restorations were used during the first 12 weeks after implant placement (unloaded period). Two-stage implants were exposed 8 weeks after implant placement. Open tray impressions were taken using a polyether material (Impregum™, 3M ESPE, Seefeld, Germany) with a custom open tray (Diatray Top, Dental Kontor, Stockelsdorf, Germany). Titanium abutments and titanium ceramic restorations were fabricated by computer-aided design (CAD)/computer-aided manufacturing (CAM) technology (Nobel Biocare Procera® System). At the time of prosthesis delivery, occlusion was adjusted and the restorations were either screwed or cemented using eugenol-free zinc oxide cement (Temp Bond NE, Kerr Corporation, Orange, CA, USA) 12 weeks after the first surgery, according to an early loading protocol15. Patients were recalled every 3 months for maintenance.
Primary outcome measures were failures of the prostheses and of the implants, and any complications that occurred up to the end of the follow-up16. A successful prosthesis is a prosthetic restoration that is stable and in good function. A failed implant is an implant that has been removed, while a surviving implant is an implant that remains in the jaw and is stable, even though not all of the individual success criteria are fulfilled. Secondary outcome measures were marginal peri-implant bone level changes evaluated on intraoral radiographs taken with the parallel technique by means of a custom radiograph holder, and patient’s perception of pain. The distance from the implant platform to the most coronal bone-to-implant contact measured on radiographs with a digital calliper was defined as bone crest level (BCL). Measurements were approximated to the nearest 0.01 mm. Two measurements (mesial and distal) were taken for each implant in both groups, at the time of implant placement, at the time of implant loading (12 weeks) and then at the 1-year examination. The average radiographic values for the mesial and distal surfaces were calculated and averaged for each patient. One blinded assessor (GCM), who was otherwise not involved in the study, performed all radiographic measurements. Patient‘s perception of pain was evaluated by a questionnaire. Each patient was asked to score the intensity of pain perception in the first week after implant placement and then in the first week after abutment connection (not applicable in the 2-stage group). Furthermore, the number of analgesic tablets taken at each intervention was recorded, and the patient was asked whether he or she would undergo the same procedures again. Pain was evaluated using a 0 to 10 numbered scale, 0 corresponding to no pain at all, and 10 as the maximum pain imaginable. The questionnaires were collected and analysed by an independent blinded outcome assessor (AV) for both groups after implant placement, and at the abutment surgery only for the 2-stage group.
The original goals of this study were to com- pare both 1- and 2-stage implant placement protocols and implant designs (Brånemark System® MKIII Groovy™ and NobelSpeedy™ Groovy) using resonance frequency analysis (RFA, Osstell® Mentor device, Osstell, Göteborg, Sweden). During the study the authors noted that repeated abutment dis/ reconnections (i.e. unscrewing the healing abutment and then screwing the transducer), could produce peri-implant soft tissue inflammation and patient discomfort, therefore RFA assessment was discontinued. The authors decided not to consider RFA in this study. A posteriori, it was also decided to consider the randomised different implant types as a single implant type.
To achieve a larger sample size, each eligible private patient that visited between September 2008 and October 2009 was enrolled in this randomised controlled trial (RCT), but no sample size calculation was performed.
Four opaque envelopes containing the two possible options for implant type and the two possible options for stages of the procedure were prepared for each patient by an assistant (AV). A surgical nurse chose one of the two envelopes for each randomised intervention. The surgical nurse dispensed either MKIII Groovy™ or NobelSpeedy™ Groovy dental implants according to the outcome of the randomisation performed just before the surgery. The allocation of the patients to a 1- or 2-stage procedure was concealed since the surgical nurse opened the corresponding envelope immediately after implant placement.
The statistical analysis was performed for numeric parameters such as marginal bone level and perception of pain. XL Stat Professional (Addinsoft, New York, NY, USA) and Microsoft Visual Basic® for Application (VBA) were used. In addition, G*power 3.117 was used for a post-hoc power analysis (Power = 1–b error probability). A descriptive analysis was performed using mean and standard deviation (SD). The patient was used as the statistical unit of the analysis. The mean bone crest resorption (BCR) was calculated for both groups in the unloaded period (12 weeks mean BCL minus baseline mean BCL), and at the 1-year follow-up (1-year mean BCL minus baseline mean BCL). Inferential statistics for all clinical and radiological parameters were performed to evaluate the results of the two interventions, using a parametric t test (Student t test) with 95% confidence intervals. The current study tested the null hypothesis that there was no difference between the two surgical approaches with regard to BCR and pain perception against the alternative hypothesis of a difference. All statistical comparisons were conducted at the 0.05 level of significance.
Results
A flow diagram of the progress through the phases of the trial is reported in Figure 1. Fifty-two patients were examined for eligibility, but five patients could not be enrolled in the trial for the following reasons: three were heavy smokers and two had poor oral hygiene. Forty-seven patients were considered eligible and were consecutively enrolled in the study. Eighteen patients were randomly allocated to the submerged group and 29 patients to the non-submerged group.
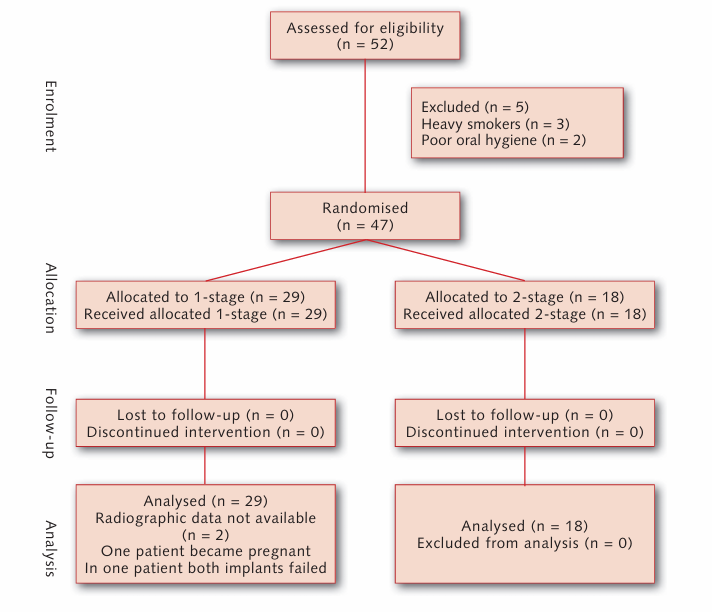
No dropout occurred in the 1-year follow-up, and all of the data collected were evaluated in the statistical analysis. The following deviations from the original protocol occurred: RFA assessments were not considered as well as the two different implant designs. All patients were treated according to the allocated interventions. Baseline demographic and intervention characteristics are shown in Table 1.
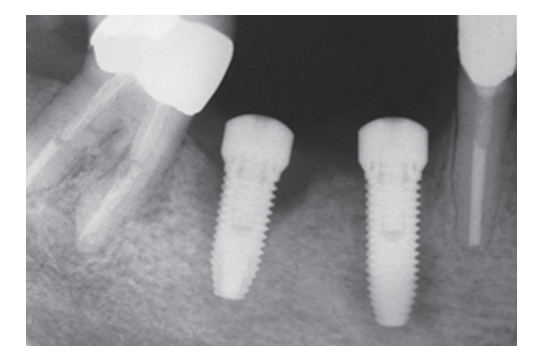
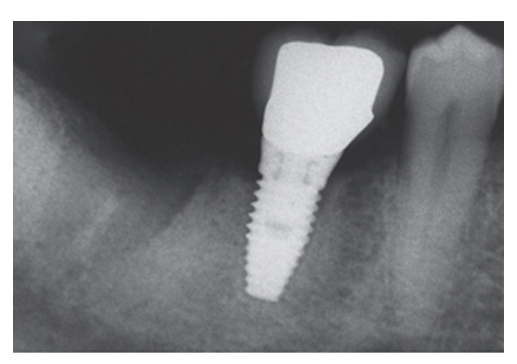
In the 1-stage group, 2 implants (one patient) out of 38 implants failed to osseointegrate (Fig 2) and were lost, while in the 2-stage group no implant was lost, resulting in a cumulative survival rate at 1 year of 94.7% in the 1-stage group, and 100% in the 2-stage group. All implant failures occurred before taking the final impression. Failed implants were not replaced, and a removable partial denture was used instead. Two asymptomatic complications were reported in the 1-stage group, 2 weeks post-operatively and 6 weeks after prosthesis connection. Both implants displayed poor stability and were horizontally mobile. Periapical radiographs showed a slightly radiolucent peri-implant line (Fig 3) without pain and swelling. ISQ values were ≤50 in both cases (ISQ 48 and 44, respectively). These patients were treated with the application of the cover screw according to the submerged protocol and both implants were clinically stable at the end of the 1-year follow-up period. One case of asymptomatic periapical peri-implantitis, possibly due to an endodontically treated adjacent tooth, occurred in the 2-stage group 3 months after prosthesis connection. This patient was subjected to surgery and systemic antibiotics to treat the apically infected implant.
Three out of 38 submerged implants in three patients showed a minimal exposure of the cover screw, limited to the occlusal portion of the mucosa. The implant necks were never exposed. In these three cases, the authors’ choice was to monitor the patients once a week, performing no intervention. One patient (one implant) in the 1-stage group became pregnant after prosthesis connection and couldn’t complete the 1-year radiographic follow-up. However, clinical examinations were performed during the entire follow-up, and no biomechanical or biological complication occurred. No failure of the definitive prostheses occurred 1 year after implant placement. The results of bone crest level changes are summarised in Tables 2 and 3. The mean BCL at the moment of implant placement was 0.14 ± 0.27 mm (median 0.07 mm) for the non-submerged group and 0.10 ± 0.31 mm (median 0.07 mm) for the submerged group. At loading, the mean BCL was 0.66 ± 0.37 (median 0.58 mm; 95% CI: 0.44–0.72 mm) in the non-submerged group and 0.59 ± 0.37 (median 0.54 mm; 95% CI: 0.38–0.70 mm) in the submerged group. At the 1-year follow-up, mean BCL was 1.00 ± 0.41 (median 0.92 mm; 95% CI: 0.77–1.07 mm) in the non-submerged group and 0.87 ± 0.45 (median 0.82 mm; 95% CI: 0.61–1.03 mm) in the submerged group. Any changes in mean BCL from baseline were statistically significantly different (P < 0.05, Table 2).
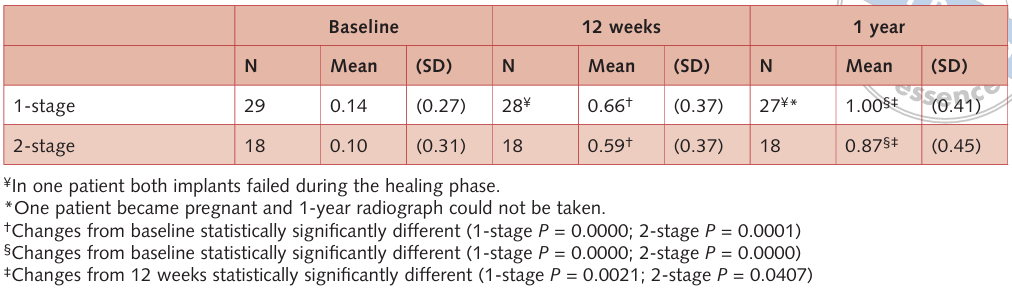
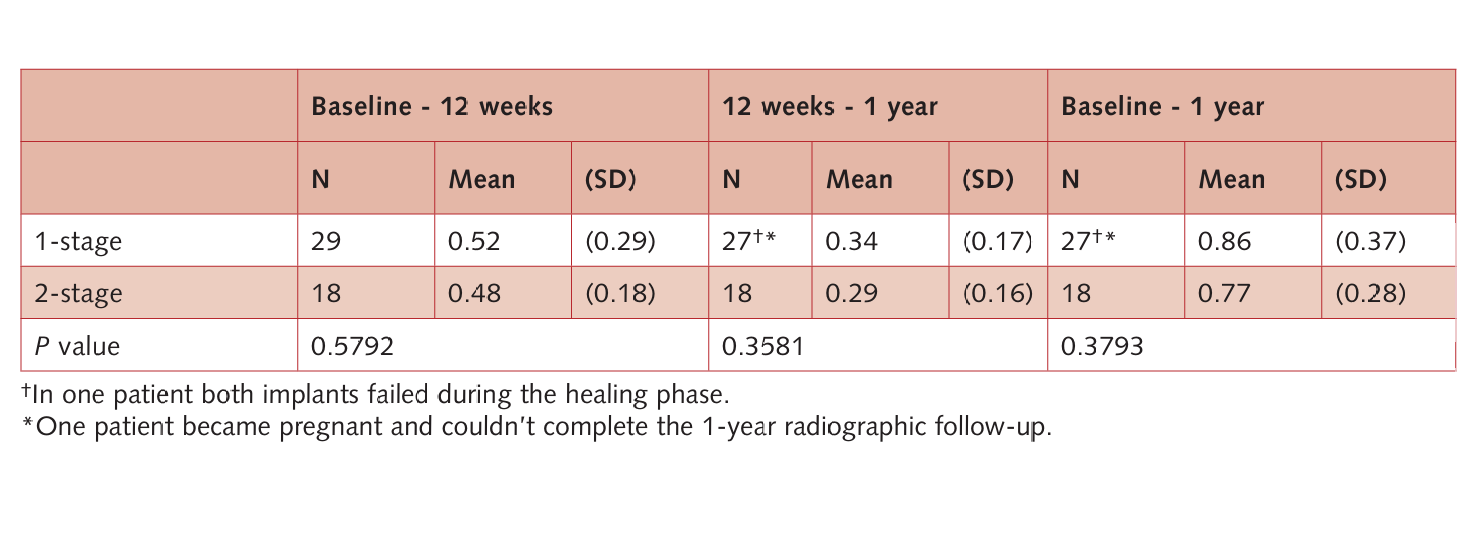
Both groups gradually lost a slight amount of marginal peri-implant bone. In the unloaded period, the mean BCR was 0.52 ± 0.29 (median 0.49 mm; 95% CI: 0.38–0.60 mm) in the non-submerged group and 0.48 ± 0.18 (median 0.48 mm; 95% CI: 0.39–0.57 mm) in the submerged group. After 1 year, the mean BCR was 0.86 ± 0.37 (median 0.77 mm; 95% CI: 0.63–0.91 mm) in the non-submerged group and 0.77 ± 0.28 (median 0.73 mm; 95% CI: 0.60–0.86 mm) in the submerged group. Therefore, in the loaded period, the mean BCR was 0.34 ± 0.17 (median 0.34 mm; 95% CI: 0.28–0.40 mm) in the non-submerged group and 0.29 ± 0.16 (median 0.24 mm; 95% CI: 0.17–0.31 mm) in the submerged group. There was no statistically significant difference in mean BCR between the two interventions (P > 0.05, Table 3) at any of the follow-ups. Instead, in both groups, the mean BCR in the unloaded period was statistically greater than in the loaded period (P = 0.041 in the 1-stage group and P = 0.002 in the 2-stage group).
Patients of both groups reported low levels of pain. The mean pain score in the first week after implant placement was 2.07 ± 1.90 (median 2.00; 95% CI: 1.31–2.69) in the 1-stage group and 2.44 ± 1.65 (median 2.00; 95% CI: 1.24–2.76) in the 2-stage group, while the mean amount of analgesic tablets taken was 1.93 ± 2.23 (median 1.00; 95% CI: 0.19–1.81) in the 1-stage group and 1.39 ± 0.92 (median 1.00; 95% CI: 0.58–1.42) in the 2-stage group. There was no statistically significant difference between the two interventions in both pain perception (P = 0.498; Power = 0.105) and analgesic tablets taken (P = 0.335; Power = 0.270). Three days after surgery, all patients except the one with failed implants had no postoperative pain. Only patients in the submerged group had a second intervention to connect the abutment, however, the mean pain score was only 0.67 ± 1.14 (median 0.00; 95% CI: -0.53–0.53) and just 4 analgesics in 3 out of 18 patients were taken. All patients declared they would undergo the same procedures again.
Discussion
The present trial was originally designed to evaluate two different implant types and if the classic submerged implant placement can be considered a prerequisite for osseointegration. It was then decided to analyse and report data comparing the different staging procedures only. The present data cover a follow-up period of 1 year after implant placement. This RCT revealed no clinically or statistically significant differences for implants and prosthesis failures, mean peri-implant marginal bone resorption, and pain perception between 1- and 2-stage groups.
The main limitations of the current trial were the use of two different implant designs due to the different original goal of this study. However, the implants presented the same parallel wall design, external-hex connection, oxidised surface and they were randomly distributed between the two groups in approximately equal number. In addition, other limitations of the current study were the small sample size and the different size of the two groups due to a non-optimal randomisation procedure.
The results of the present study seem to be in agreement with previous results reported in a recent Cochrane systematic review. However, in the literature there are only a few well-conducted RCTs comparing 1- versus 2-stage surgical approaches. Four trials, three analysed in the Cochrane systematic review, presented radiographic bone crest level measurements, but only Cordaro et al and the authors of the present study analysed the mean marginal bone level changes between implant placement and prosthesis delivery, as suggested in the implications for research by Esposito et al. In the other RCTs, the unloaded healing period after implant placement was 3 to 6 months for mandibular implants and 6 months for maxillary ones. The main limitation of these studies is that the healing period immediately after implant placement was not evaluated.
According to Cordaro et al, no statistically significant difference in the mean BCR was found between groups. However, in the 1-stage group, a minor difference was reported in the mean BCR between implant placement and provisional restoration (12-week unloaded period) compared to the current study (0.26 ± 0.34 mm and 0.52 ± 0.29 mm, respectively). In Cordaro’s study, all implants were placed immediately after tooth extraction apical to the mesiodistal crestal bone level of the socket. In the present study, all implants were placed crestally, in healed sites. The results of the current study showed that most of the 1-year BCR occurred in the unloaded period. The difference between the mean BCL from baseline to prosthesis connection was highly statistically significant, in both 1- and 2-stage implant placement protocols (P = 0.0000 and P = 0.0001, respectively). In the authors’ opinion, the marginal bone loss occurring early after implant placement should also be evaluated during the unloaded period.
On the basis of this study, the submerged technique may not be a prerequisite for osseointegration. According to all of the aforementioned RCTs, no statistically significant difference was found for peri-implant bone changes at any follow-up, irrespective of the two interventions. Furthermore, perception of pain seemed to be independent of the surgical approach. However, the number of patients that showed failures and complications was slightly higher in the 1-stage group than in the 2-stage group. The choice between 1-stage and 2-stage implant placement protocols essentially depends on primary implant stability at implant placement and on other risk factors. There might be situations where trends clearly favour the 2-stage approach, such as patients wearing removable dentures during implant healing.
Two implants showed mobility, probably due to overloading, without pain and swelling. However, these complications were successfully treated, possibly because they were noticed early and implants were immediately removed from occlusion to allow for osseointegration. Owing to the limitations of this study, the results should be interpreted with caution. More well-designed RCTs are needed to clarify which could be the most effective implant staging procedure.
Conclusions
No difference was observed when comparing 1- with 2-stage procedures, however 1-stage implant placement might be at a slightly higher risk of failures and complications, though it avoids one surgical intervention.
Authors: Marco Tallarico, Anna Vaccarella, Gian Carlo Marzi
References
- Brånemark PI, Hansson BO, Adell R, Breine U, Landström J, Hallén O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaws. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl 1977;16:1-132.
- Schroeder A, Pohler O, Sutter F. Tissue reaction to an implant of a titanium hollow cylinder with a titanium surface spray layer. SSO Schweizerische Monatsschrift für Zahnkeilkunde 1976;86:713-727.
- Schroeöder A, Van Der Zypen E, Stich H, Sutter F. The reactions of bone, connective tissue, and epithelium to endosteal implants with titanium-sprayed surfaces. J Maxillofac Surg 1981;9:15-25.
- Brånemark PI, Zarb GA, Albrektsson T. Tissue-integrated prostheses: osseointegration in clinical dentistry. Chicago: Quintessence Publishing, 1985.
- Brunski JB, Moccia AF Jr, Pollack SR, Korostoff E, Trachtenberg DI. The influence of functional use of endosseous dental implants on the tissue–implant interface. II. Clinical aspects. J Dent Res 1979;58:1970-1980.
- Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand 1981;52:155-170.
- Barber HD, Seckinger RJ, Silverstein K, Abughazaleh K. Comparison of soft tissue healing and osseointegration of IMZ implants placed in one-stage and two-stage techniques: a pilot study. Implant Dent 1996;5:11-14.
- Becktor JP, Isaksson S, Billstrom C. A prospective multi- center study using two different surgical approaches in the mandible with turned Branemark implants: conventional loading using fixed prostheses. Clin Implant Dent Relat Res 2007;9:179-185.
- Cecchinato D, Olsson C, Lindhe J. Submerged or non-submerged healing of endosseous implants to be used in the rehabilitation of partially dentate patients. J Clin Periodontol 2004;31:299-308.
- Cordaro L, Torsello F, Roccuzzo M. Clinical outcome of submerged versus non-submerged implants placed in fresh extraction sockets. Clin Oral Implants Res 2009;20: 1307-1313.
- Eliasson A, Narby B, Ekstrand K, Hirsch J, Johansson A, Wennerberg A. A 5-year prospective clinical study of submerged and nonsubmerged Paragon system implants in the edentulous mandible. Int J Prosthodont 2010;23:231-238.
- Esposito M, Grusovin MG, Chew YS, Coulthard P, Worthington HV. Interventions for replacing missing teeth: 1- versus 2-stage implant placement. Cochrane Database Syst Rev 2009;(3):CD006698.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332.
- Esposito M, Grusovin MG, Loli V, Coulthard P, Worthington HV. Does antibiotic prophylaxis at implant placement decrease early implant failures? A Cochrane systematic review. Eur J Oral Implantol 2010;3:101-110.
- Cochran DL, Morton D, Weber HP. Consensus statements and recommended clinical procedures regarding loading protocols for endosseous dental implants. Int J Oral Maxillofac Implants 2004;19:109-113.
- Van Steenberghe D. Outcomes and their measurement in clinical trials of endosseous oral implants. Ann Periodontol 1997;2:291-298.
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175-191.
- Heydenrijk K, Raghoebar GM, Meijer HJ, van Der Reijden WA, van Winkelhoff AJ, Stegenga B. Two-part implants inserted in a one-stage or a two-stage procedure. A pro- spective comparative study. J Clin Periodontol 2002;29: 901-909.