Novel digital workflow for full-arch rehabilitation
Introduction
Modern dentistry is undergoing a technological revolution, driven by the integration of advanced digital tools in diagnostics, planning and treatment. Among these, computer-guided implantology has become a corner-stone, particularly in complex procedures like aesthetics and full-arch rehabilitation. This approach not only increases surgical precision, but also reduces complications, enhances patient comfort, and streamlines the workflow. By combining CBCT imaging, intra-oral scanning and planning software, clinicians can carry out virtual simulations that transition seamlessly into clinical execution. Surgical guides allow implants to be placed almost exactly as pre-planned.
Full-arch rehabilitation remains one of implantology’s greatest challenges, requiring restoration of function, aesthetics and long-term stability in patients with limited anatomical reference points. The digital workflow enables accurate planning tailored to each patient, improving control and predictability throughout treatment.
In addition, intra-oral scanners allow for fast, precise and non-invasive data acquisition. Their high-quality output now rivals that of traditional impressions, offering an improved patient experience and immediate usability in prosthetic design.
The integration of intra-oral scan data with CBCT data forms a unified digital model – a digital twin – that supports prosthetic planning, surgical guide fabrication and identification of anatomical constraints. Artificial intelligence (AI) adds further potential by processing large datasets to assist in diagnosis, implant positioning and biomechanical analysis.
Clinically, these technologies lead to more accurate implant placement, optimised prostheses and shorter, less invasive procedures – often making immediate loading easier. However, success depends also on implant characteristics and proper training. Decades of research have shown that implants with superior hydrophilicity potentially improved clinical outcomes by accelerating healing and facilitating immediate loading protocols.
Clinicians must understand digital workflows, software logic and how to manage complex edentulous cases, where diagnosis, precision and accuracy are vital. Guided surgery translates virtual planning into highly accurate clinical procedures. 3D-printed guides help minimise deviations and reduce surgical trauma. Still, maintaining high standards throughout the workflow is essential— from impressions and guide design to delivery of the definitive prosthesis. Collaboration with dental laboratories is key. Digital communication allows for custom prostheses that are virtually validated and precisely manufactured using CAD/CAM. This enhances efficiency, reduces errors and elevates overall treatment quality.
Ultimately, the convergence of CBCT, AI, intra-oral scanning and 3D printing is reshaping dentistry into a predictive, preventive and personalised discipline. Full-arch rehabilitation exemplifies the benefits of this shift, making computer-guided implantology not just a technique but a patient-centred philosophy built on precision, safety and clinical excellence.
Case report
An 85-year-old partially edentulous patient was referred to our clinic for full-arch rehabilitation of the maxilla. The patient was in good general health and a non-smoker. His primary complaints included difficulty in chewing, maxillary pain and occasional halitosis. Clinical and radiographic evaluation revealed a short-span fixed metal–ceramic prosthesis supported by seven anterior maxillary teeth. The prosthesis had debonded, and four of the abutment teeth were structurally compromised. The remaining three showed varying degrees of caries and periodontal problems. A diagnosis of failing dentition was established (Figs. 1 & 2).
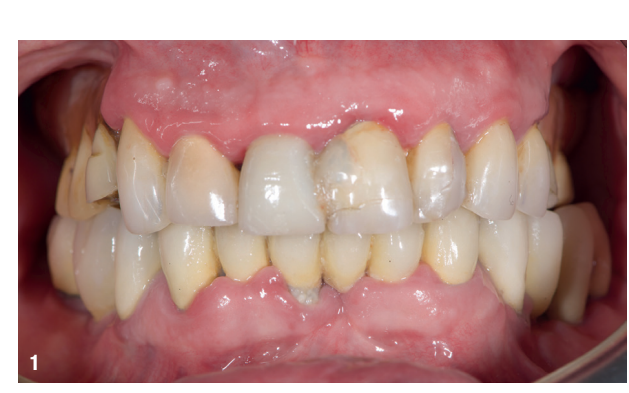
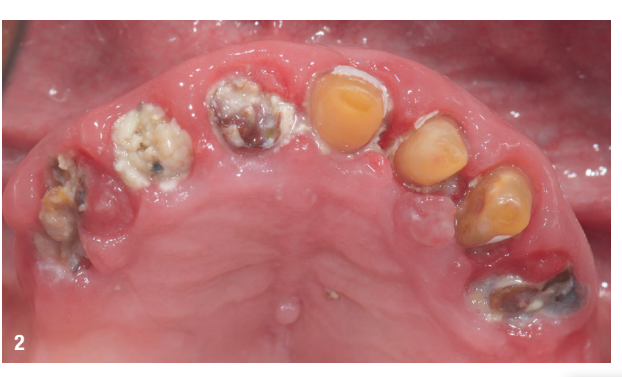
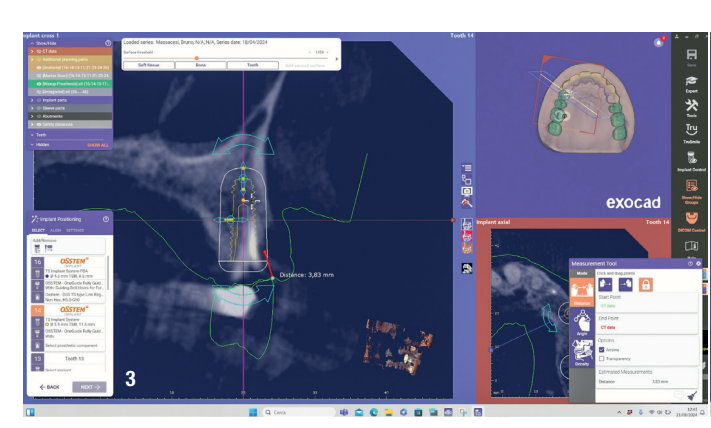
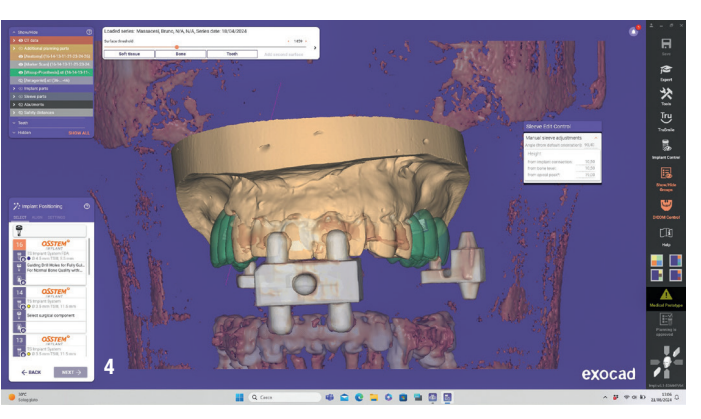
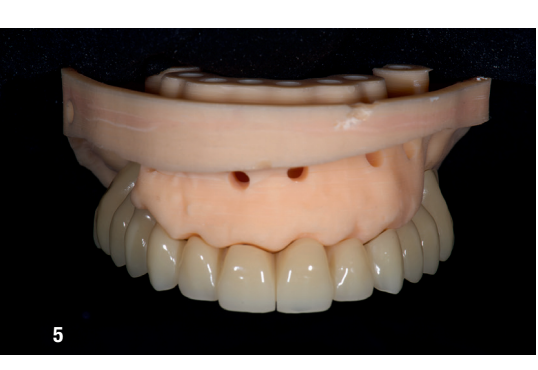
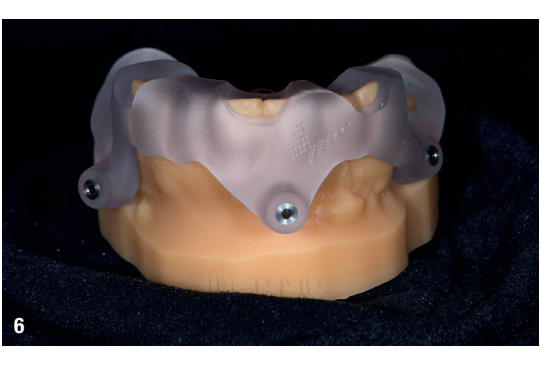
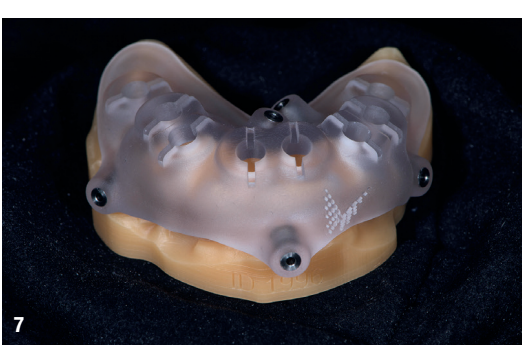
At the initial visit, digital impressions were captured using the Medit i700 scanner to document the residual teeth, soft tissue, existing prosthesis and opposing arch. The occlusal records were obtained at the current vertical dimension of occlusion and in centric relation. Mandibular movements were recorded with the zebris JMA jaw registration system (zebris Medical). Intra- and extra-oral photographs were also taken. A virtual diagnostic wax-up and a digital smile simulation were created based on these records (Smile Creator, exocad). Finally, eight implants were planned (Figs. 3 & 4) according to the new wax-up (exoplan 3.1, exocad), and both a surgical guide and a metal-reinforced temporary prosthesis were fabricated in advance (Figs. 5–7).
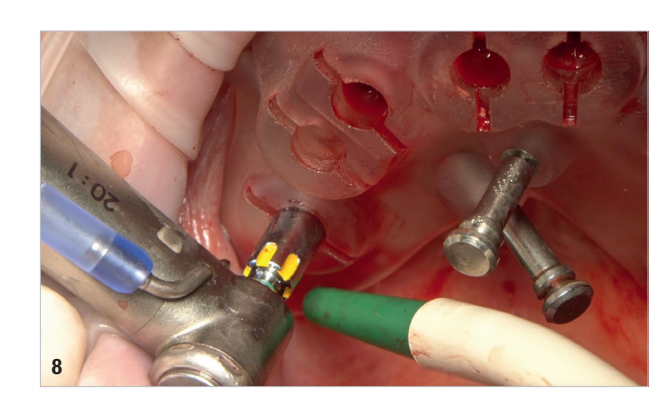
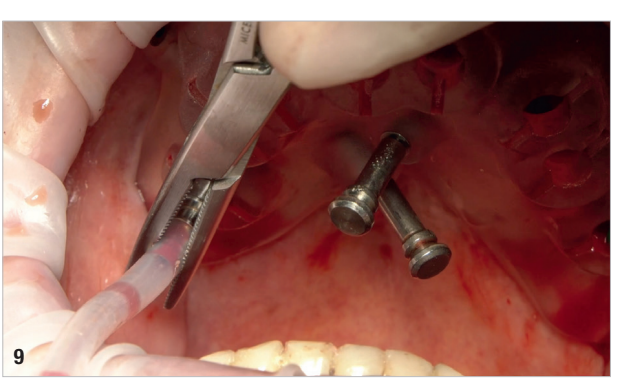
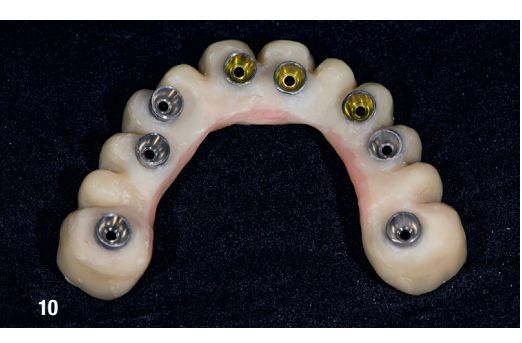
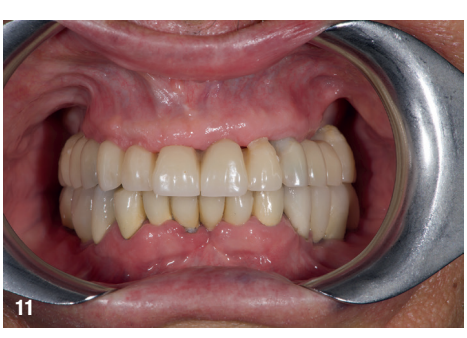
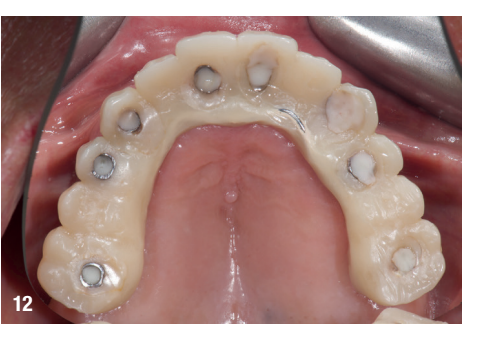
On the day of surgery, local anaesthesia was administered, and the first surgical guide was stabilised on the remaining teeth to prepare five anchor pin sites. The compromised teeth were then extracted, and a second surgical guide was secured at the prepared pin sites. Eight implants (Osstem TSIII SOI, Osstem Implant) were placed, five in immediate post-extraction sites and three in previously healed areas. All the placements were fully guided using a surgical guide without metallic sleeves and a dedicated surgical kit (OneGuide KIT, Osstem Implant; Fig. 8). One implant required a crestal sinus lift, which was performed using a fully guided approach (OneCAS KIT, Osstem Implant; Fig. 9). Xenograft bone material (A-Oss, Osstem Implant) was used to fill the extraction sockets and the sinus cavity. Multi-unit abutments and temporary abutments (Osstem Implant) were immediately placed on all the implants, according to the one abutment, one time concept. A prefabricated PMMA-based temporary prosthesis with metal reinforcement was delivered and immediately fixed in the mouth. Chairside adjustments were made to refine the prosthesis and ensure proper occlusion (Figs. 10–12). The patient received postoperative instructions and medication.
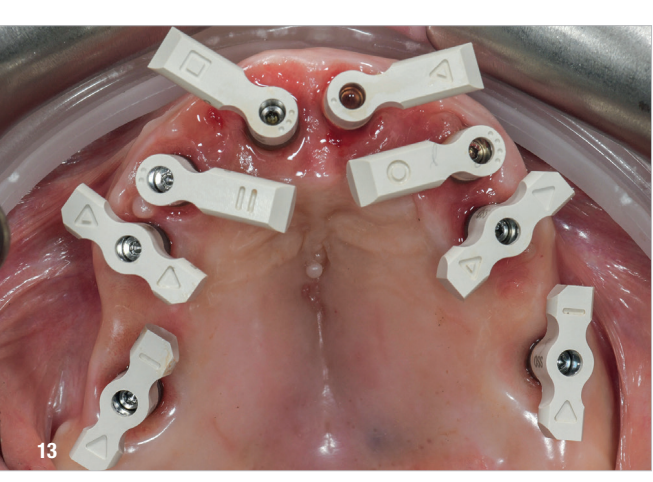
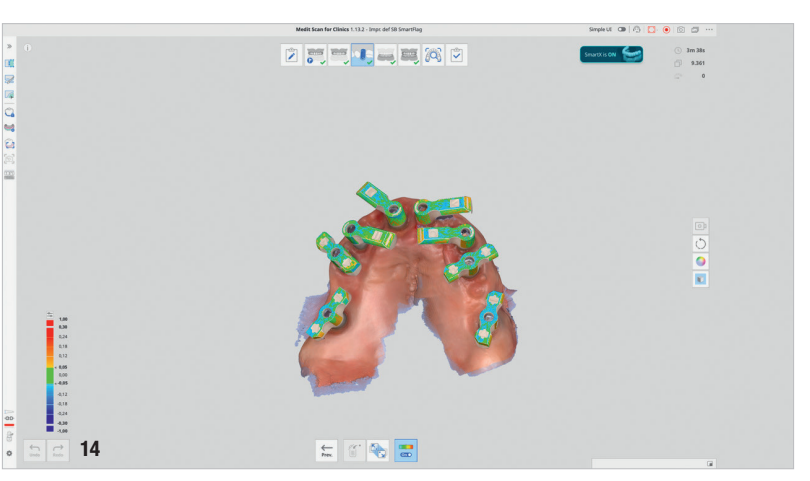
Four months later, digital impressions were taken using the Medit i900. These included an impression of the functional temporary prosthesis, an impression of the soft-tissue anatomy and an impression with custom-designed scan bodies, featuring lateral extensions to enhance intra-oral scan accuracy (SmartFlag, APOLLO; Fig. 13). A novel digital workflow, named Medit SmartX (Medit Link Version 3.4.2), was utilised (Fig. 14). This system enables real-time recognition and alignment of scan body libraries, enhancing predictability, efficiency and safety of full-arch digital impressions. A combination of scanning techniques was applied to increase the final accuracy: straight and zigzag in the anterior regions and straight in the posterior regions.
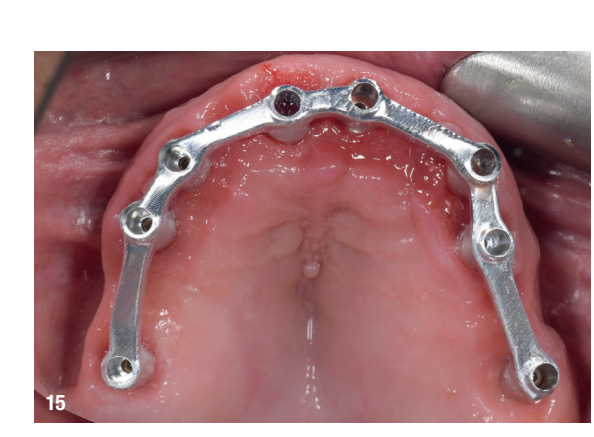
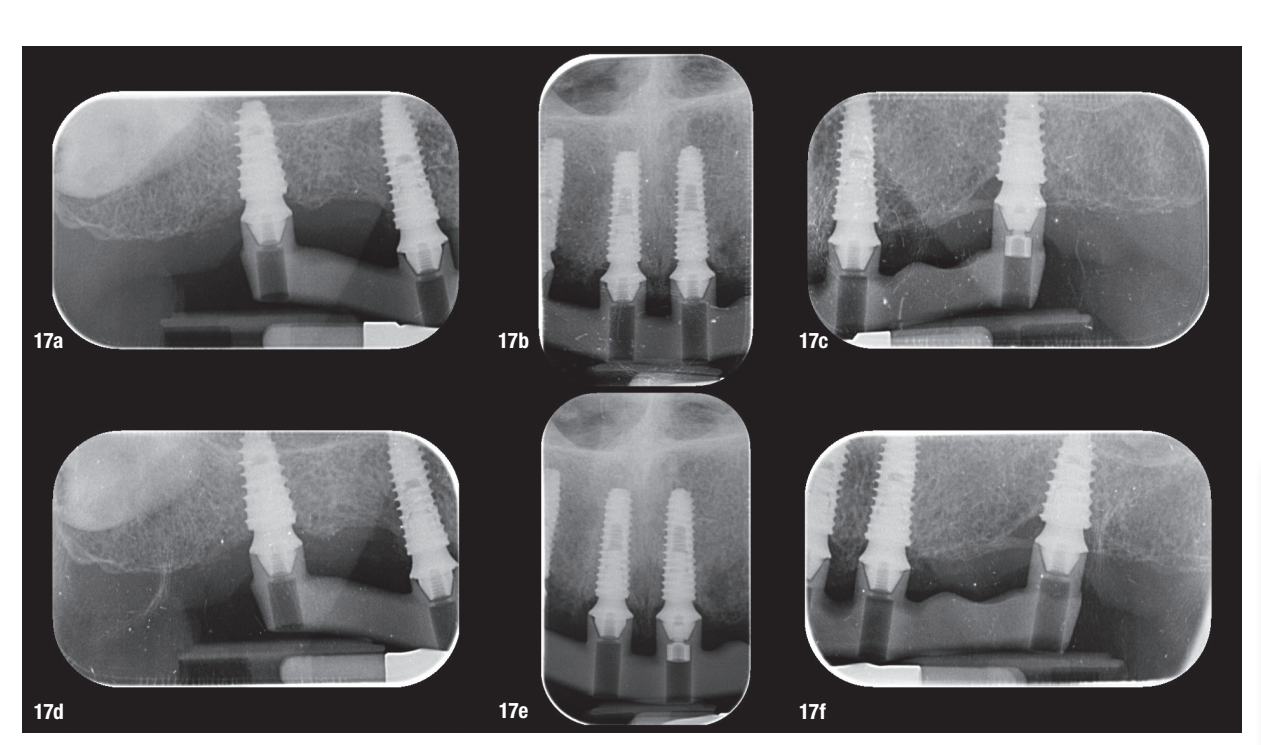
At the second appointment, a PMMA prototype supported by a metal bar was tested for aesthetic and functional accuracy. Passive fit of the metal framework was assessed using the one-screw (Sheffield) test and tactile verification with a dental explorer (Figs. 15–17f).
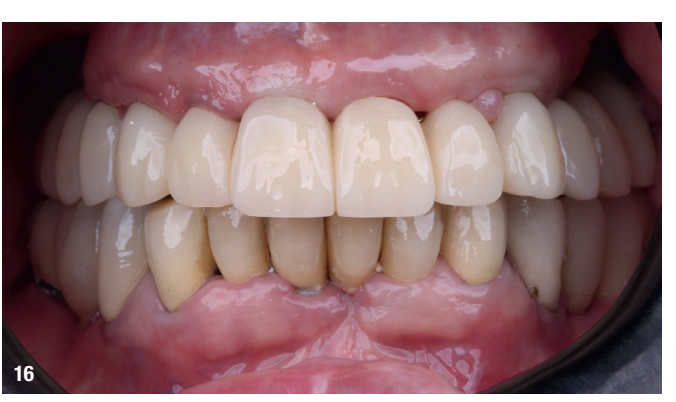
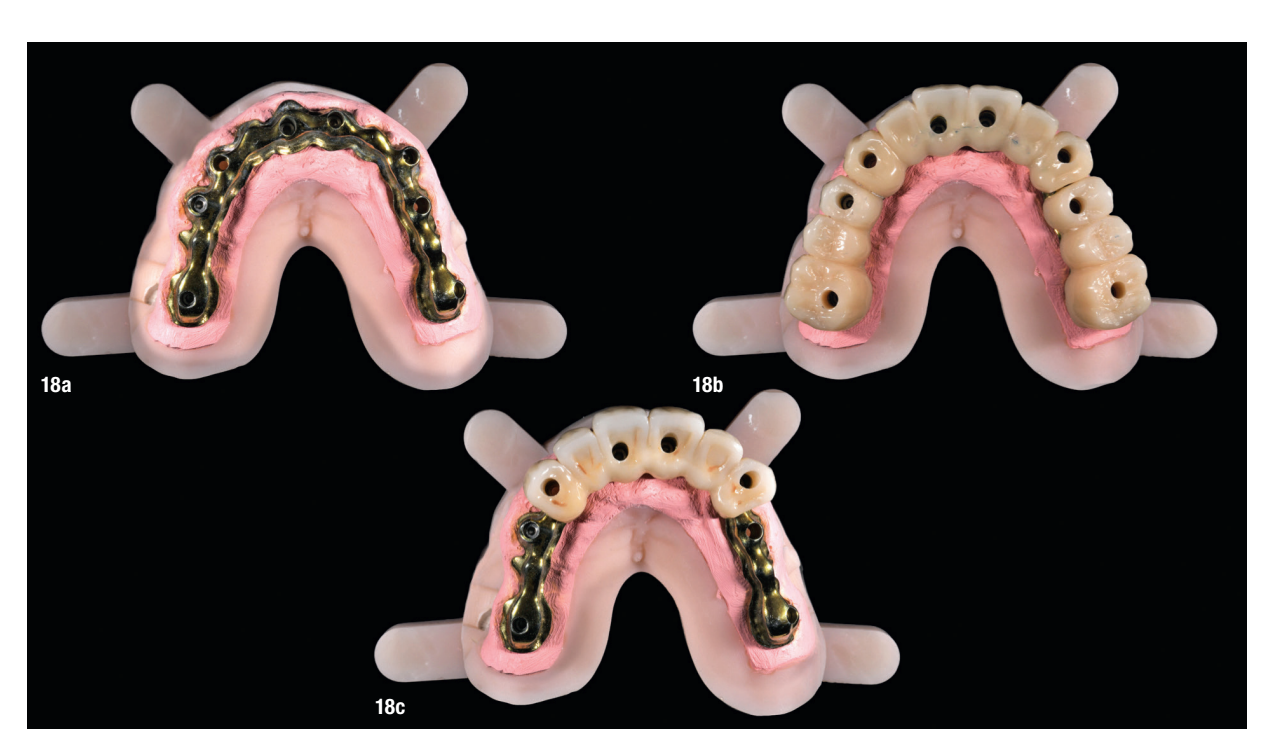
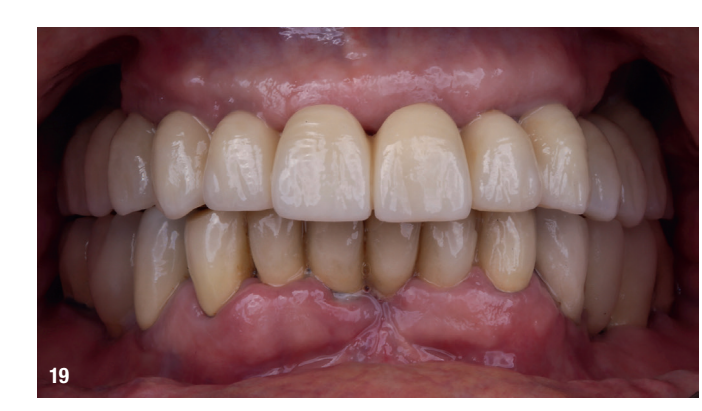
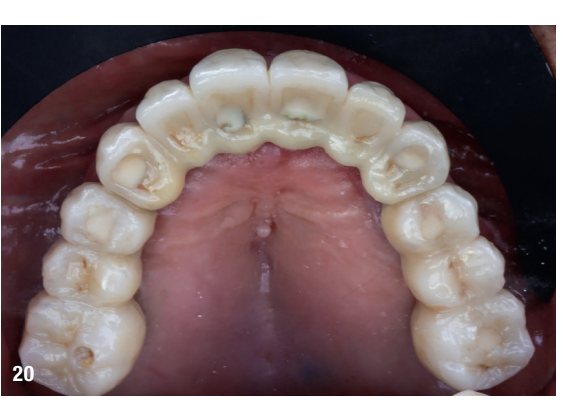
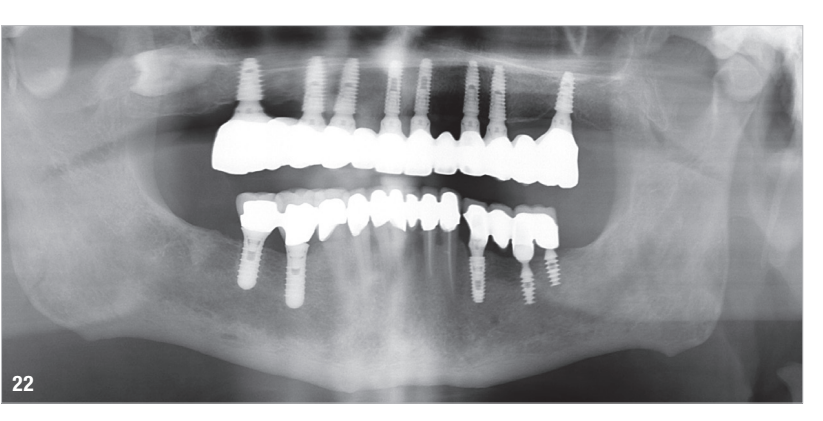
At the final appointment, the definitive hybrid screw-retained prosthesis was delivered. The definitive prosthesis consisted of a CAD/CAM titanium framework screwed on to all of the implants and three monolithic zirconia prosthetic segments bonded on top (Figs. 18a–c). The occlusion was evaluated, and the patient was enrolled in a four-month maintenance programme. At the last follow-up (one year after implant placement), all of the implants were successful and the patient was fully satisfied with the new prosthesis (Figs. 19–22).

Discussion
This case highlights the evolving role of digital technologies in achieving high precision and predictability in full-arch implant rehabilitation. One of the key determinants of long-term success in such rehabilitation is the passive fit of the definitive prosthesis. A truly passive fit minimises mechanical stress on the implants and surrounding bone, reducing the risk of biological or technical complications such as peri-implantitis, screw loosening or framework fracture. In this case, a combination of multi-unit abutments, intra-oral scanning and a CAD/CAM metal framework contributed to verifying passive fit using the one-screw test and tactile evaluation.
Another critical factor that influences long-term clinical outcomes is the 3D positioning of implants. Accurate implant placement in terms of depth, angulation and mesiodistal spacing directly affects prosthetic fit, occlusal harmony and load distribution. In the edentulous maxilla—where anatomical constraints and soft-tissue mobility challenge manual techniques—the use of computer-guided surgery proves especially valuable. In this case, fully guided implant placement was facilitated by digital planning and a 3D-printed surgical guide anchored by pins. This protocol enabled a minimally invasive approach and the execution of a crestal sinus lift, illustrating how guided surgery can manage anatomical complexity with minimal invasiveness. The precision afforded by virtual planning translates directly into surgical accuracy, improving both prosthetic predictability and patient outcomes.
The integration of intra-oral scanning technologies and CBCT imaging allowed for a seamless, non-invasive digital workflow from diagnostic evaluation to prosthesis delivery. This approach enabled precise implant planning, pros- thetic design and the fabrication of a customised surgical guide, all aligned with the patient’s anatomy and prosthetic requirements. In contrast to traditional workflows, digital tools reduce the margin for manual error and offer significant advantages in terms of patient comfort, efficiency and reproducibility.
A key innovation in this case was the use of Medit SmartX, an AI-driven platform that enhances the accuracy of intra-oral scanning by optimising scan body recognition and alignment. The system leverages AI to reduce potential mismatches during full-arch data acquisition—one of the most critical phases in digital implantology. Medit SmartX provided a streamlined scan protocol, combining straight and zigzag patterns in the anterior regions and straight scans in the posterior regions, ultimately resulting in higher-quality datasets and better prosthetic fit. These improvements were especially beneficial in managing a fully edentulous maxilla, where the lack of anatomical landmarks can compromise data integrity.
The Medit SmartX workflow may optimise scan data alignment principles and scanning settings to achieve more precise data compared with conventional processes. This would help better highlight the technical advantages of Medit SmartX.
The integration of precise and predictable surgical kits, such as those used in this clinical case report, combined with implants engineered with advanced surface technologies designed to enhance osseointegration— Osstem Implant’s SOI surface—plays a vital role in achieving reliable and consistent clinical outcomes. These innovations not only streamline surgical procedures, but also support long-term success in implant dentistry, making them indispensable tools for modern dental practitioners.
Despite the successful outcome, this case report presents certain limitations. As a single case, the results cannot be generalised across all clinical scenarios. The patient had favourable bone volume and good systemic health, conditions that may not be present in more compromised individuals. Additionally, while the Medit SmartX system showed promising results, its long-term performance, reproducibility across different operators and integration with other software ecosystems warrant further investigation. The assessment of passive fit relied on clinical methods that, although widely accepted, remain partially subjective without verification via digital stress analysis or industrial-level measurements. Moreover, the time and effort required to learn to effectively employ AI-assisted tools and fully digital workflows remains significant. Successful application requires not only access to advanced equipment but also a deep understanding of digital planning, software logic and potential intra-operative adjustments.
Conclusion
This case underscores the clinical value of fully digital workflows supported by AI-assisted technologies in achieving accurate, efficient and patient-centred full-arch rehabilitation. The combination of guided surgery, precise 3D implant positioning and digital verification proto- cols enables clinicians to deliver prostheses with high functional and aesthetic predictability. While the integration of systems like Medit SmartX can enhance scan fidelity and prosthetic fit, further studies—including clinical trials and multi-operator analyses—are needed to fully validate these innovations and determine their broader applicability.
Marco Tallarico, Carlotta Cacciò, Barbara Massaccesi & Andrea Pedetta
