Periodontal Disease and Age-Related Maculopathy: A Case Report
Abstract
Age-related macular degeneration (AMD) is a leading cause of significant vision impairment in individuals aged 50 and older, primarily impacting central vision. This study seeks to investigate potential associations between periodontal disease and age-related maculopathy in the analyzed clinical case. A male subject of 66 years old, with age-related degenerative maculopathy and severe periodontal disease, was evaluated and treated. After an initial phase of non-surgical causal periodontal therapy, the periodontal indices were re-evaluated. In addition, the extraction of hopeless teeth replaced by dental implants was performed. A retinal topography was used to assess possible regression of the pathology, and proper anti-VEGF therapy was administered. The results showed a regression of periodontal disease and an improvement of the degenerative maculopathy. These preliminary results, even if encouraging, should be supported by larger prospective trials.
Introduction
Periodontal diseases encompass a range of inflammatory disorders that compromise the structural integrity of the teeth’s supporting tissues, including the gingiva, alveolar bone, and periodontal ligament. These conditions can result in tooth loss and may also contribute to systemic inflammation. An imbalance in the oral microbiota, which interacts with the host’s immune system to trigger inflammation and pathological processes, serves as a fundamental factor in the initiation and progression of periodontal disease. Periodontal disease severity is influenced by environmental and host risk factors including factors that may be modified like smoking and others that cannot modified like hereditary predisposition. Different therapies are available for the treatment of periodontitis. Professional scaling and root planing represent the primary non-surgical approach for managing periodontal disease, involving the thorough removal of dental plaque and calculus from both supragingival and subgingival surfaces. Periodontal disease is responsible for a multitude of problems such as tooth loss, bone loss, gum recession with consequent esthetic and functional problems. When periodontal disease has stopped the restoration of esthetic and masticatory function can be performed with many therapies including perioprostheses. Multiple risk factors contribute to the onset and progression of periodontal disease, including smoking, diabetes, and inadequate oral hygiene. An additional factor currently under investigation is age-related macular degeneration (AMD). AMD is a complex, multifactorial disease that frequently results in progressive and bilateral damage to the macula. The macula, the most functionally significant region of the retina, contains the highest density of photoreceptor cells responsible for central vision. In AMD, pathological changes develop in the macula of an otherwise structurally normal eye, ultimately leading to irreversible vision loss, particularly in the elderly population.
Periodontally compromised patients have a higher risk of AMD, but the causal relationship between PD and AMD has not been confirmed. A possible mechanism to affect systemic diseases is that periodontal bacteria and their metabolites (such as endotoxin) enter the systemic circulation, and when they reach other parts of the body, they activate monocytes/macrophages and produce a large number of inflammatory factors, which in turn causes inflammation in other organs.
The present study aimed to investigate the role of possible correlations between degenerative maculopathy and periodontal disease, evaluating a possible correlation, and discussing and analyzing the results obtained with the following clinical case. This study was reported according to the CARE Case Report Guidelines (https://www.care-statement.org/).
Case Report
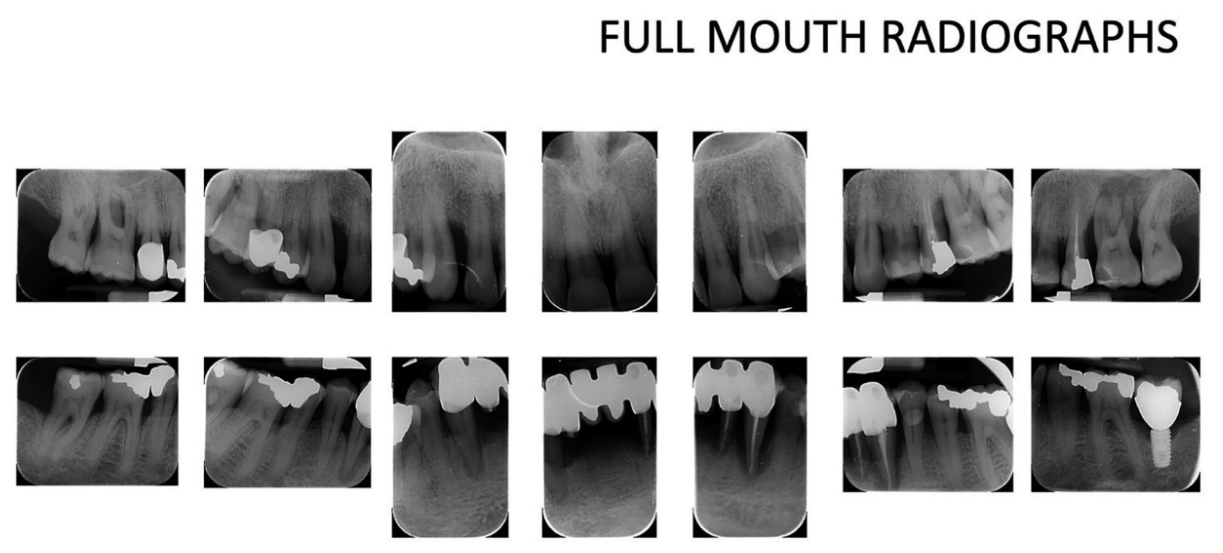
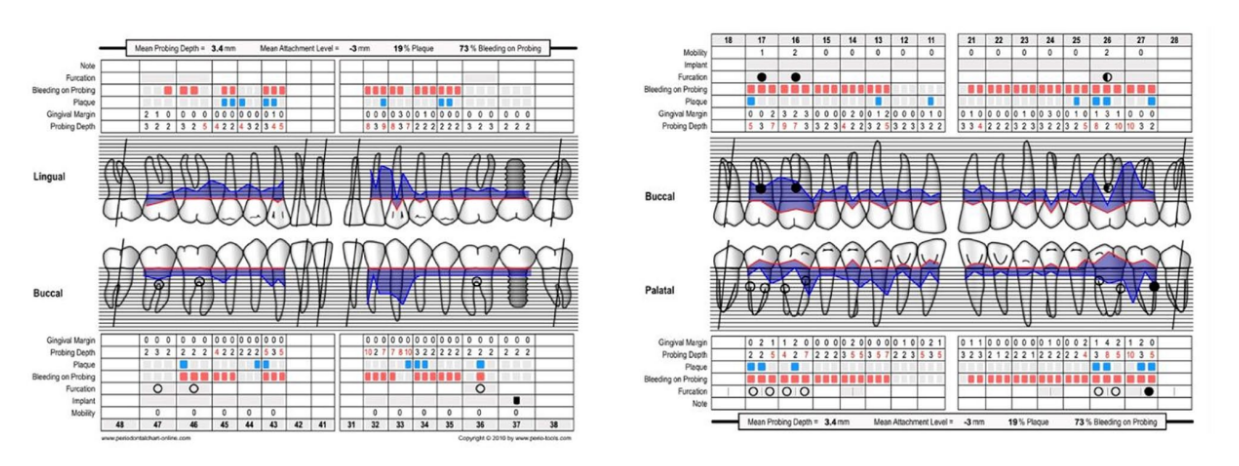
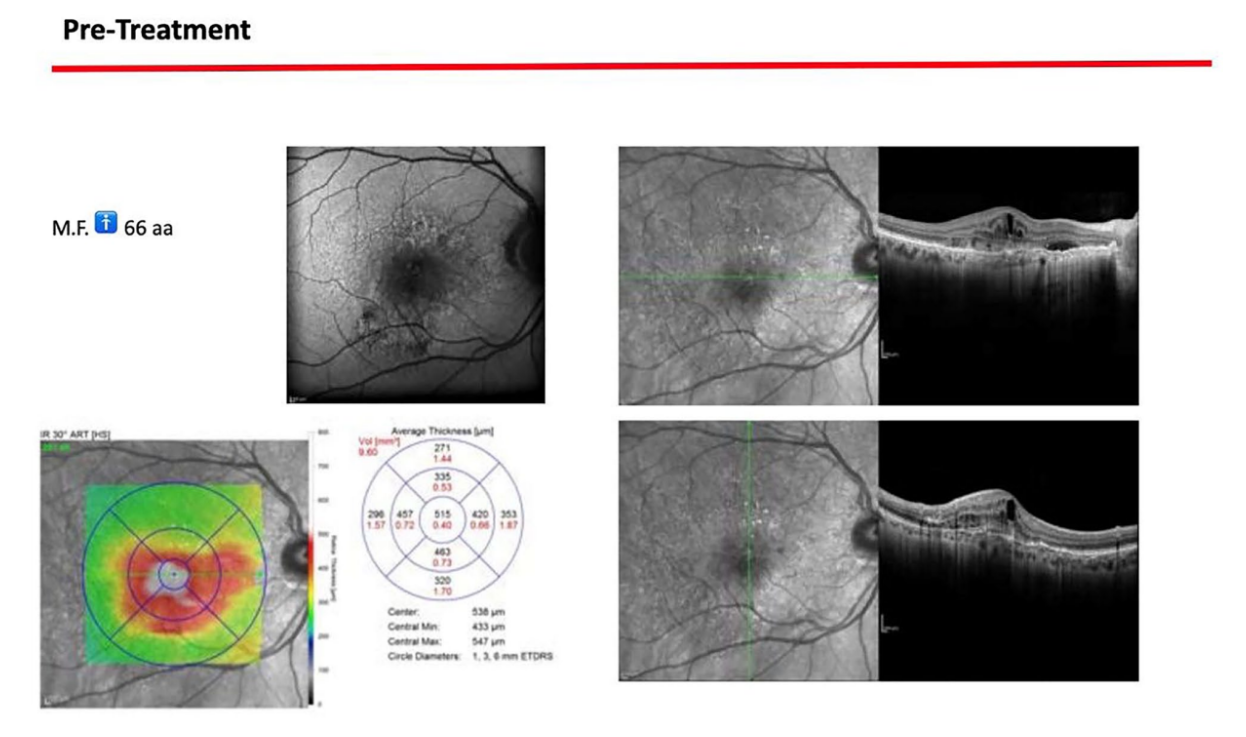
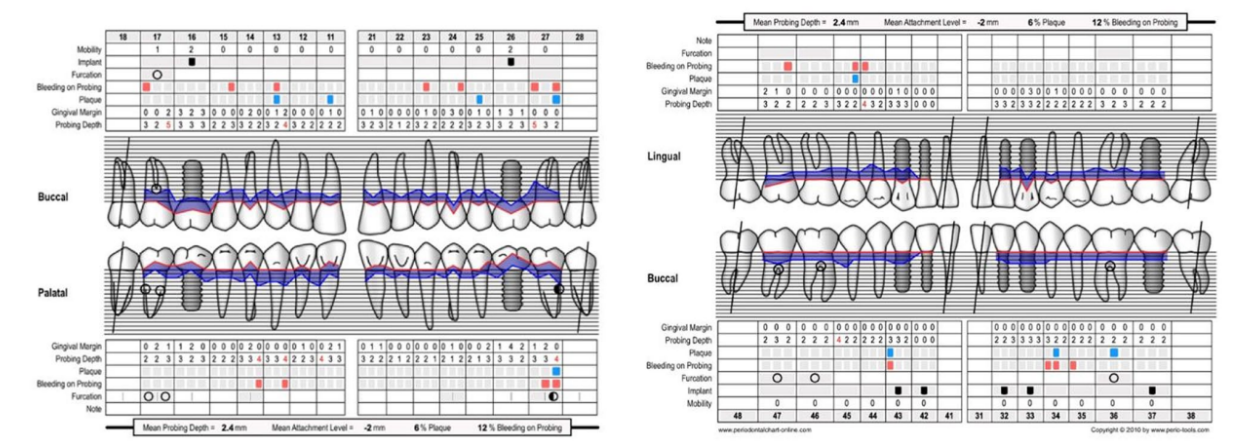
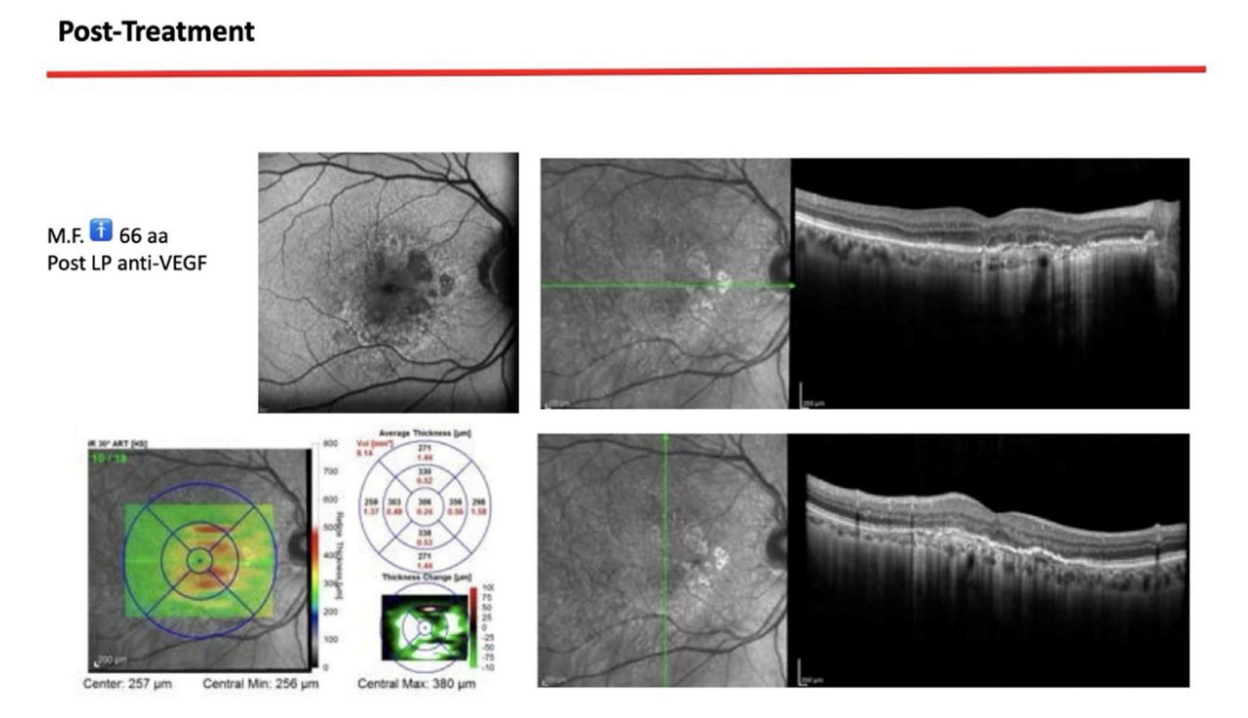
A 66-year-old patient was referred to a private clinic due to spontaneous gingival bleeding, pain while eating, and the presence of an abscess in the region of the left mandibular canine (Figures 1 and 2). His medical history revealed a familiarity with periodontal disease, previous retinal pathology, smoking (less than 15 cigarettes a day), and hypertension controlled with medication. At the time of the visit, the patient had started 3 months of treatment with anti-VEGF therapy. The periodontal diagnosis made was stage IV and grade C periodontitis, according to the Italian Society of Periodontology (SIDP), in addition to the severe alveolar bone resorption (>33%), the patient presented local complexity parameters, such as masticatory dysfunction, 2° mobility on several dental elements, and involvement of the furcation up to grade 3°. A grade C was diagnosed because of the relationship between the radiographic bone loss /RBL) at the site, and age (RBL%/AGE) was >1 (Figure 3). A periodontal chart was compiled (Figure 4). At baseline, the patient had more than 42 pockets ⩾4 mm an average probing depth of 3.4 mm with an average attachment loss of 3 mm. The periodontal assessment revealed a full-mouth plaque score (FMPS) of 19% and a full-mouth bleeding score (FMBS) of 73%. Once the periodontal data were analyzed, the most appropriate treatment was planned for the patient. Nonsurgical periodontal therapy (initial periodontal therapy) aimed to improve the periodontal index through scaling and root planing; extraction of hopeless teeth (1.6; 2.6; V sextant), followed by implant placement, 4 months later, after periodontal and radiographic evaluation. The patient was also given a topographical macular examination before and after non-surgical periodontal therapy to assess possible changes in his pathological picture (Figure 5).
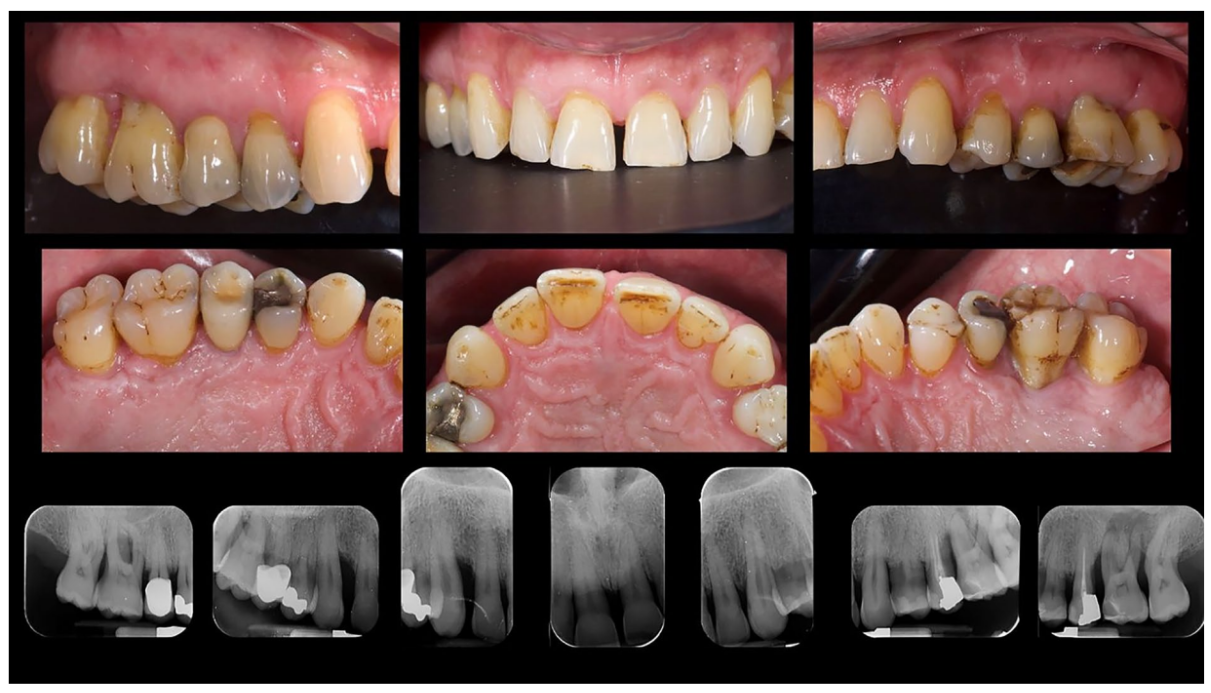
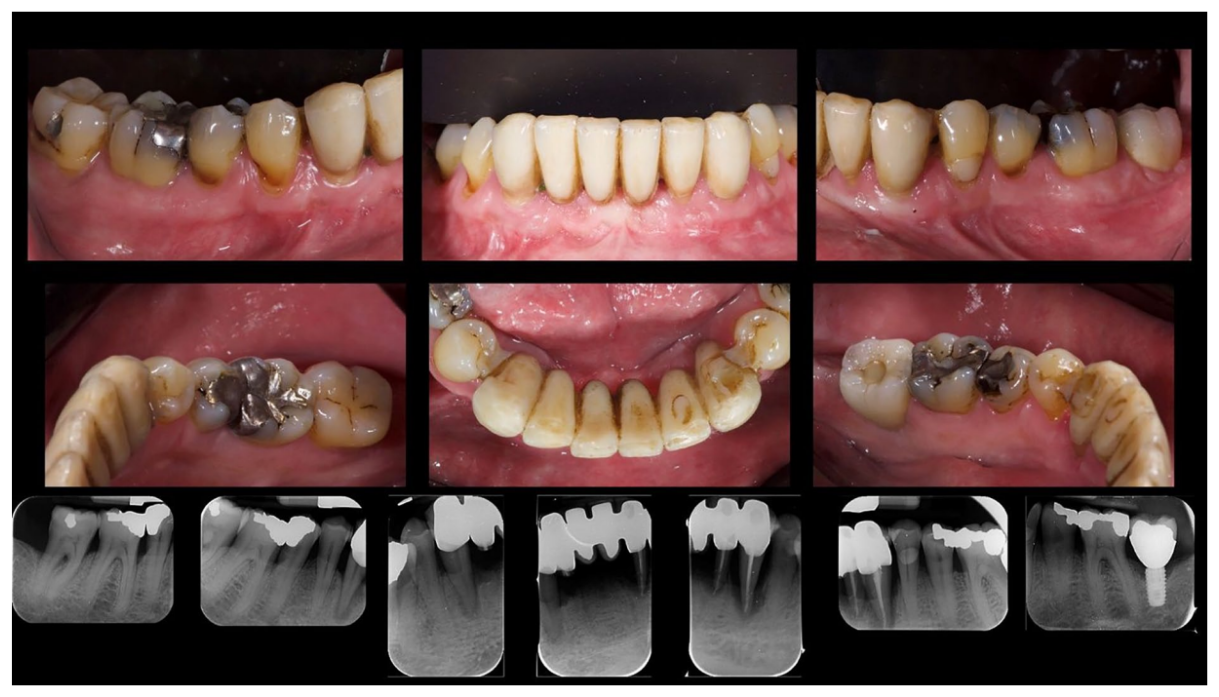
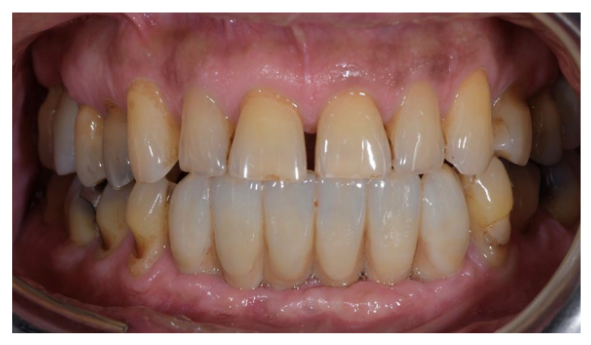
After an osseointegration period of 6 months, the definitive prostheses, made on monolithic zirconia, bonded on titanium link, were carried out (Figure 6). After that, the periodontal status and the retinal pathology of the patient were constantly monitored with periodontal maintenance therapy every 4 months, and anti-VEGF therapy according to the specialist. The post-treatment clinical and medical conditions are shown in Figures 6 to 9.
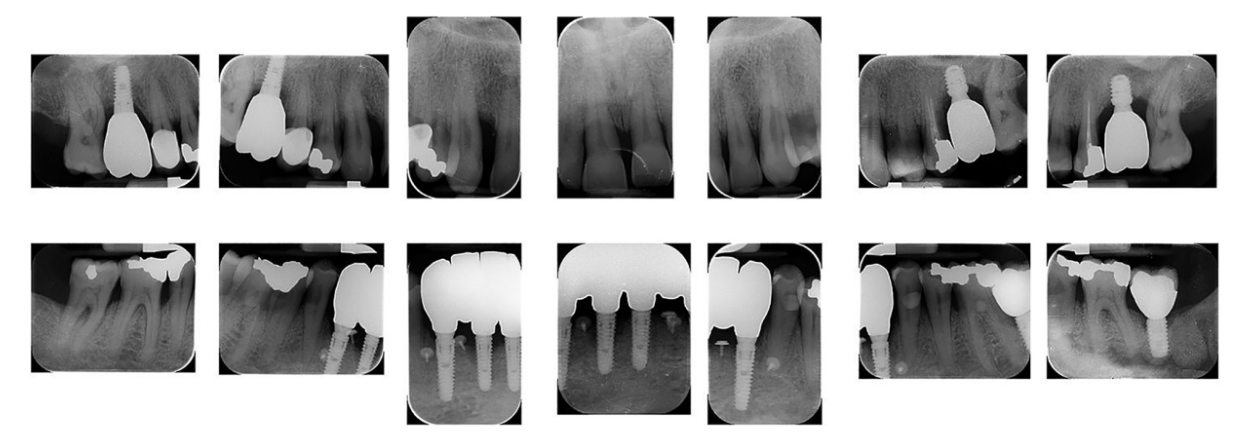
The survival rates and complications of implants and prostheses were assessed. Implant failure was defined as the removal of an implant due to factors such as implant mobility, progressive marginal bone loss, infection, or implant fracture. The stability of individual implants was evaluated by the prosthodontist at the time of definitive crown placement using a removal torque of 35 Ncm. Following loading, implant stability was further assessed manually by the same prosthodontist using 2 dental mirror handles. Prosthesis failure was considered any prosthesis which has been redone for any reason. Variation in probing depth (PPD) is defined as a reduction in gingival sulcus depth from baseline to last follow-up. Variation in bleeding on probing (BOP) is understood as a reduction in bleeding sites from baseline to last follow-up. Presence of gingival margin recessions from delivery of final prosthesis to last follow-up. Complications: understood as prosthetic lining fractures, prosthetic framework fractures, and periodontal complications.
Upon completion of the treatment, the periodontal evaluation revealed an average probing depth of 2.4 mm and an average attachment loss of 2 mm. In addition, a topographical examination of the macular region performed before periodontal treatment, with a series of horizontal and vertical scans, showed in the right eye the persistence of an atrophicocicatricial maculopathy. The left eye showed a reduction of sub-retinal areas with high reflectivity superior to the foveal regions. A topographic examination of the macular area was performed after periodontal causal therapy and after re-evaluation, showed in the right eye an anatomical picture unchanged from the previous one. The left eye showed an improved anatomical picture with the persistence of a slight sub-retinal exudation in the foveal region. This exudation was reduced at the next check-up. At the level of the right eye, intraretinal exudates are no longer evident, although atrophic-cicatricial maculopathy persists. From these examinations, it can be concluded that the scarring status persists, as expected. However, there was a complete remission of the related exudate phenomena, indicative of a decrease in the inflammatory picture.
At the 2-year follow-up, all the symptoms related to the pathologist remain constant. The patient was satisfied with the results and the patient understood the need for maintenance therapy.
Discussion
This study aimed to assess a potential correlation between age-related degenerative maculopathy and periodontal disease. Periodontitis is a chronic, multifactorial inflammatory condition linked to a dysbiotic plaque biofilm, leading to the progressive destruction of the tooth-supporting structures. Clinically, it is characterized by clinical attachment loss, while radiographic evaluation reveals alveolar bone resorption. Periodontal disease is a significant public health concern due to its high prevalence and potential consequences, including tooth loss, which negatively affects masticatory function and esthetics. Additionally, it can contribute to systemic health complications, further underscoring its clinical relevance. The bacteria implicated in periodontitis may contribute to the pathogenesis of degenerative retinal disorders, potentially influencing their development and progression. The pathological microbiota of the oral cavity and one of the main organisms causing periodontal disease is Porphyromonas gingivalis and researchers have discovered its ability to invade epithelial cells epithelial cells, fibroblasts and dendritic cells Age-related macular degeneration (AMD) causes irreversible visual loss in elderly individuals by causing lesions of the macula, the center of the retina, on an eye that was previously normal. Unfortunately, there is currently no accepted cause of AMD and no effective treatment for the condition. Nevertheless, the identification of risk factors and the implementation of palliative therapies—such as laser treatment, dynamic phototherapy, surgical interventions, and anti-vascular endothelial growth factor (anti-VEGF) therapy—form the cornerstone of AMD management. Recent research has demonstrated the involvement of systemic inflammatory indicators in the pathophysiology of AMD, in addition to the risk factors already recognized to be involved in its pathogenesis (old age, tobacco use, obesity, complement factor polymorphism, diet). At this time, the correlation between periodontal disease and AMD is not entirely clear, but there are numerous reviews analyzing this possible correlation. In addition to this, other authors, such as Karesvuo have demonstrated the possible association between PD and AMD. It was observed that individuals with AMD exhibited greater periodontal pocket depth (PD) compared to those without AMD (P = .031). Additionally, individuals with AMD had a lower number of remaining teeth (P < .001) and experienced more significant alveolar bone loss (P = .004) than those without the condition. Similarly, it was observed that individuals with periodontitis may have an increased risk of developing AMD compared to those without periodontal disease. According to previous literature reviews, a potential association between AMD and periodontitis has been suggested; however, further studies are required to validate these findings and establish a clearer causal relationship. The research connecting gut dysbiosis to AMD is explored, along with pre-clinical animal models and methods suitable for investigating the influence of gut microbiota on AMD development. This includes its interactions with systemic inflammation, immune regulation, chorioretinal gene expression, and dietary factors. As knowledge of the gutretina axis expands, so will the potential for more accessible and effective strategies for preventing and treating this sight-threatening disease.
Within the limitation of the present case report such as the low number of treated patients, the periodontal disease represents a plausible additional risk factor for age-related maculopathy. The link between the diseases remains unclear. In the clinical case presented, once the periodontal indices were met, we saw a decrease in inflammatory processes in the eyes. The present study had some limitations including a single-patient sample and a lack of a control group. Further studies are needed to establish any possible corrections between periodontal disease and age-related maculopathy. In addition, larger-scale trials exploring the biological pathways connecting systemic inflammation and microbiota dysbiosis are needed to better understand the correlations between the pathologies.
Conclusion
The present case report highlights a potential correlation between periodontal disease and age-related maculopathy (AMD). The case of a 66-year-old male with severe periodontal disease and degenerative maculopathy was evaluated to explore this possible link. Following an initial phase of non-surgical periodontal therapy, significant improvements were observed in both periodontal indices and the patient’s macular condition. Specifically, there was a notable reduction in inflammatory processes in the eyes, as evidenced by improved anatomical pictures and a decrease in sub-retinal exudation.
Francesco Mattia Ceruso, Stephany Gabriela Zambrano Leon, Luca Fiorillo, Gabriele Cervino, Marco Cicciù, Artak Heboyan, Francesco Pernice, Silvio Meloni and Marco Tallarico
References
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038.
- Jeffcoat MK, Hauth JC, Geurs NC, et al. Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol. 2003;74(8):1214-1218.
- Kinane DF, Peterson M, Stathopoulou PG. Environmental and other modifying factors of the periodontal diseases. Periodontol 2000. 2006;40(1):107-119.
- Axelsson P, Lindhe J, Nyström B. On the prevention of caries and periodontal disease: results of a 15-year longitudinal study in adults. J Clin Periodontol. 1991;18(3):182-189.
- Tunkel J, Heinecke A, Flemmig TF. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol. 2002;29:72-81.
- Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy: II. Severely advanced periodontitis. J Clin Periodontol. 1984;11(1):63-76.
- Sheiham A, Steele JG, Marcenes W, et al. Prevalence of impacts of dental and oral disorders and their effects on eating among older people; a national survey in Great Britain. Community Dent Oral Epidemiol. 2001;29(3):195-203.
- Coleman HR, Chan C-C, Ferris FL III, Chew EY. Age-related macular degeneration. Lancet. 2008;372(9652):1835-1845.
- Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137(3):486-495.
- Srimaneepong V, Heboyan A, Zafar MS, et al. Fixed prosthetic restorations and periodontal health: a narrative review. J Funct Biomater. 2022;13(1):15.
- Lv X, Li W, Fang Z, Xue X, Pan C. Periodontal disease and age-related macular degeneration: a meta-analysis of 112,240 participants. Biomed Res Int. 2020;2020:4753645.
- Kalhan AC, Wong ML, Allen F, Gao X. Periodontal disease and systemic health: an update for medical practitioners. Ann Acad Med Singapore. 2022;51(9):567-574.
- Beck JD, Papapanou PN, Philips KH, Offenbacher S. Periodontal medicine: 100 years of progress. J Dent Res. 2019;98(10):1053-1062.
- Di Spirito F, La Rocca M, De Bernardo M, et al. Possible association of periodontal disease and macular degeneration: a case control study. Dent J. 2020;9(1):1.
- Hajishengallis G, Maekawa T, Abe T, Hajishengallis E, Lambris JD. Complement involvement in periodontitis: molecular mechanisms and rational therapeutic approaches. Adv Exp Med Biol. 2015;865:57-74.
- Cook HL, Patel PJ, Tufail A. Age-related macular degeneration: diagnosis and management. Br Med Bull. 2008;85(1):127-149.
- Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39(5):367-374.
- Pockpa ZAD, Struillou X, Kone D, et al. Periodontal diseases and age-related macular degeneration: is there a link? Perm J. 2019;23:18-260.
- Javed F, Sculean A, Romanos GE. Association between age-related macular degeneration and periodontal and peri-implant diseases: a systematic review. Acta Ophthalmol. 2021;99(4):351-356.
- Arigbede AO, Babatope BO, Bamidele MK. Periodontitis and systemic diseases: a literature review. J Indian Soc Periodontol. 2012;16(4):487-491.
- Meduri A, Frisina R, Rechichi M, Oliverio GW. Prevalence of meibomian gland dysfunction and its effect on quality of life and ocular discomfort in patients with prosthetic eyes. Prosthesis. 2020;2(2):91-99.
- Garrido-Hermosilla AM, Martínez-Alberquilla I, Díaz-Ruiz MC, et al. Experience using a new high-density polyethylene-based porous orbital implant: explorative 1-year performance and safety results. Prosthesis. 2024;6(6):1400-1409.
- Xiao J, Zhang JY, Luo W, He PC, Skondra D. The emerging role of gut microbiota in age-related macular degeneration. Am J Pathol. 2023;193(11):1627-1637.
- Lin P, McClintic SM, Nadeem U, Skondra D. A review of the role of the intestinal microbiota in age-related macular degeneration. J Clin Med. 2021;10(10):2072.
