Machined versus cast abutments for single dental implants: a 5-year within-patient multicentre randomised controlled trial
Purpose. To compare clinical outcomes of titanium machined abutments (machined group) versus cobalt-chrome cast abutments (cast group).
Materials and Methods. Thirty-one partially edentulous subjects received two single non-adjacent implant-supported crowns at three centres. Three months and half after implant placement, at impression taking, implants were randomised to receive a machined and a cast abutment according to a within-patient study design. Four patients dropped out and one patient lost one implant before randomization, so only 26 patients had their implants randomised. Outcome measures were: prosthesis and implant failures, any complications, and radiographic peri-implant marginal bone level changes. Patients were followed to 5-year after loading.
Conclusions. The present clinical data suggests that up to 5 years after loading implant prognosis may not be affected by using machined or cast abutments.
Introduction
One of the questions often debated in implant dentistry is the potential effect of marginal bacterial leaking coming from the implant-abutment junction on the occurrence of peri-implant inflammation, marginal bone loss and peri-implantitis. Theoretically it would be logical to think that any procedure able to minimise this leakage could improve the long-term prognosis of implant supported prostheses. There are different options to tackle this problem, such as the use of different connection types. Unfortunately, recent results from a long-term RCT did not validate this hypothesis. An alternative approach could be to maximise the abutment-implant fit using more precise pre-machined (milled) abutments instead of fully cast abutments which are believed to be a bit less precise. Obviously this has to be evaluated first in vitro to see if this difference in fit exists and could be quantified and then in long-term RCTs to evaluate whether a higher degree of fit could improve to long-term prognosis of dental implants.
One in vitro study evaluating external hexagon connections showed no difference in vertical misfit but a higher degree of horizontal misfit of about 66 μm at machined titanium than at cast cobalt-chromium abutments. Two in vitro studies by the same group evaluating external hexagon connections, showed no difference in bacterial leakage between machined or fully cast cobalt-chromium abutments. Similar results were reported in other in vitro studies.
All the above mentioned in vitro studies showed no better fit of machined versus fully cast abutments on implants with the same type of external connection. Nevertheless, it could be of interest to evaluate also implants with an internal connection both in vitro and in real clinical conditions. Unfortunately, there are no in vitro studies nor RCTs evaluating machined versus fully cast abutments on implants with an internal connection, therefore, in vitro studies and long-term RCTs are needed to understand how facts actually are.
This study originally had two aims:
to compare in vitro the implant abutment fit of machined titanium abutments (machined group) versus cobalt-chrome fully cast abutments (cast group). This data were previously presented and showed no difference for tight fit/gaps between the two abutment types;
to compare, in a randomised controlled trial (RCT) of within-patient design, the clinical outcome of titanium abutments (machined group) versus cobalt-chrome fully cast abutments (cast group). The test hypothesis was that there was no difference in clinical outcomes between machined and fully cast abutments, against the alternative hypothesis of a difference.
This report presents the clinical results up to 5 years after loading, the in vitro data were previously published together with the 1-year clinical data and the 3-year data were published in another article8. At protocol stage, it was planned to follow the patients up to 10 years after loading. The present article is reported according to the CONSORT statement (http://www.con-sort-statement.org/) and its extension checklist for reporting within person randomised trials (http://www.consort-statement.org/extensions/overview/withinperson) to improve the quality of reports of within person randomised controlled trials.
Materials and methods
Study design
This trial was designed as a multicentre RCT of within-patient design with radiographic blind assessment. Complications and failures were reported by the treating dentists in an unblinded fashion. Each patient provided two non-adjacent implants which received a randomly allocated machined or cast abutment, respectively. Random allocation was made at the time of impression taking.
Inclusion/exclusion criteria
Any partially edentulous patient, requiring at least two non-adjacent single implant-supported crowns, being at least 18-year-old and able to understand and sign an informed consent was screened for eligibility. Broad inclusion criteria were used including any type of bone, any location, smokers, etc. Bone volumes allowed placement of two implants at least 8 mm long and 3.75 mm wide. Implants could also placed in previous post-extractive sockets or in augmented bone, if at least 3 months have passed from the extraction and 6 months from augmentation procedure.
Patients were not admitted in the study if any of the following exclusion criteria was present:
- general contraindications to implant surgery;
- subjected to irradiation in the head and neck area;
- immunosuppressed or immunocompromised patients;
- treated or under treatment with intravenous amino-bisphosphonates;
- affected by untreated periodontitis;
- having poor oral hygiene and motivation;
- uncontrolled diabetes;
- pregnant or lactating;
- substance abusers;
- psychiatric problems;
- unrealistic expectations;
- acute/chronic infection/inflammation in the area intended for implant placement;
- needing any form of tissue augmentation at implant placement;
- patients participating in other trials, if the present protocol could not be properly adhered to;
- referred only for implant placement and cannot be followed at the treating centre;
- extraction sites with less than 3 months of healing;
- unable to commit to a 10-year post-loading follow-up.
Patients were categorised into three groups according to what they declared: non-smokers, moderate smokers (up to 10 cigarettes per day), and heavy smokers (more than 10 cigarettes per day). Patients were to be recruited and treated in four different centres using similar procedures and each centre was supposed to recruit and treat 15 patients, however it was soon obvious that one centre was not willing to recruit any patient. The three remaining centres were all private practices, two located in Italy (Dr. Marco Tallarico in Rome and Dr. Silvio Mario Meloni in Arzachena) and one in Albania (Dr. Etha Xhanari in Tirana).
Patients were assessed to establish their eligibility for the study. Preoperative radiographs were obtained for every potentially eligible patient to quantify bone volumes at the planned implant sites. Patients having sufficient bone volumes to receive two non-adjacent single implants were invited to join the study and were informed of its nature. Only after they fully understood the nature of the study, they were asked to join signing a written informed consent. For patients with more than two suitable implant sites, operators were free to chose those sites with more similar characteristics at the screening visit. The selected study implant site were then coded as number 1 and number 2.
Clinical procedures
About 10 days prior to implant placement, all patients were subjected to professionally delivered oral hygiene including debridement as required.
All patients received prophylactic antibiotic therapy: 2 g of amoxicillin 1 hour prior to the intervention. Patients allergic to penicillin were given clindamycin 600 mg 1 hour before implant placement. All patients rinsed with chlorhexidine mouthwash 0.2% for 1 minute prior to any surgical procedure and were treated under local anaesthesia using articaine with adrenaline 1:100.000. After crestal or slightly palatal incisions and full-thickness flaps elevation, the two non-adjacent implant sites were prepared under prosthetic guidance using a surgical template. Both implants were placed in the same surgical session. The standard implant placement procedure as recommended by the manufacturer was adopted. Drills with increasing diameters were used to prepare the implant sites with a speed of 800 to 1000 round per minute under copious saline irrigation. The following drilling sequence was used: locator drill, 2 mm drill, 3 mm drill, 3.3 drill and profile drill for 3.75 mm implants. In case of hard bone, also the 3.5 mm drill was used, followed, if necessary by the 3.75 mm bone tapping. For the 4.25 mm implants it was used the same procedure, adding the 3.8 mm drill and the profile drill for 4.25 diameter implants, followed, in the presence of hard bone only, by the 4.1 mm and, if necessary, by the 4.25 mm bone tapping. Bone quality was subjectively reported as hard, medium and soft and implant lengths and diameters were recorded.
The implants used were self-tapping Ticare Inhex implants (Mozo-Grau, Valladolid, Spain) with internal conical connection of 11° and a prosthetic indexation in their apical part, having a RBM (Resorbable Blast Media) titanium surface. Operators were free to choose implant lengths (8, 10, 11.5, 13 and 15 mm) and diameters (3.75 and 4.25 mm) according to clinical indications and their preferences. Implants were placed at crestal bone level with their coronal portion flush to the surrounding bone, ideally with a torque of 35 to 45 Ncm. Cover screws were placed, implants were submerged and flaps closed with vicryl 4.0 sutures. Baseline periapical radiographs of the study implants were taken using the paralleling technique. If the peri-implant marginal bone levels were not readable or difficult to be estimated, a new radiograph was taken.
Ibuprofen 600 mg was prescribed to be taken two to four times a day during meals, as long as required. In case of allergy or stomach problem 1 g paracetamol was prescribed instead. Patients were instructed to use 0.12% chlorhexidine mouthwash for one minute twice a day for 2 weeks, to have a soft diet for one week, and to avoid brushing and trauma on the surgical sites. Sutures were removed after seven to 10 days.
After 3 months of submerged healing, implants were exposed, manually tested for stability delivering a torque of 10 Ncm, and standard Ticare-Inhex healing abutments (Mozo-Grau) were placed. Sutures were placed if needed.
Two weeks after, impressions at implant level were taken using standard screw-retained Ti- care-Inhex impression copings (Mozo-Grau), a polyether impression material (ImpregumTM, 3M ESPE, Seefeld, Germany), and customised open impression trays. Healing abutments were placed and implants were randomised according to a within patient study design to receive either a titanium pre-machined Standard Ticare-Inhex hex titanium preparable abutments (Mozo-Grau) with a neck of 3 mm height (machined group; Figs. 1A-G) or an identical chromium-cobalt cast abutment derived from the fully castable hex UCLA abutment (Mozo-Grau) (cast group; Figs. 2A-G) by opening the sequentially numbered envelope corresponding to the patient recruitment number. All fully cast abutments were casted in a single Spanish laboratory (Laboratorio Viloria, Valladolid, Spain) using an induction casting machine (Ally Digital, Manfredi Reddish Stone, Pinerolo, Italy). Operators had then the abutments prepared at their own laboratory. Either 4 or 5 mm diameter abutments were used according to clinical indications and operator preferences.
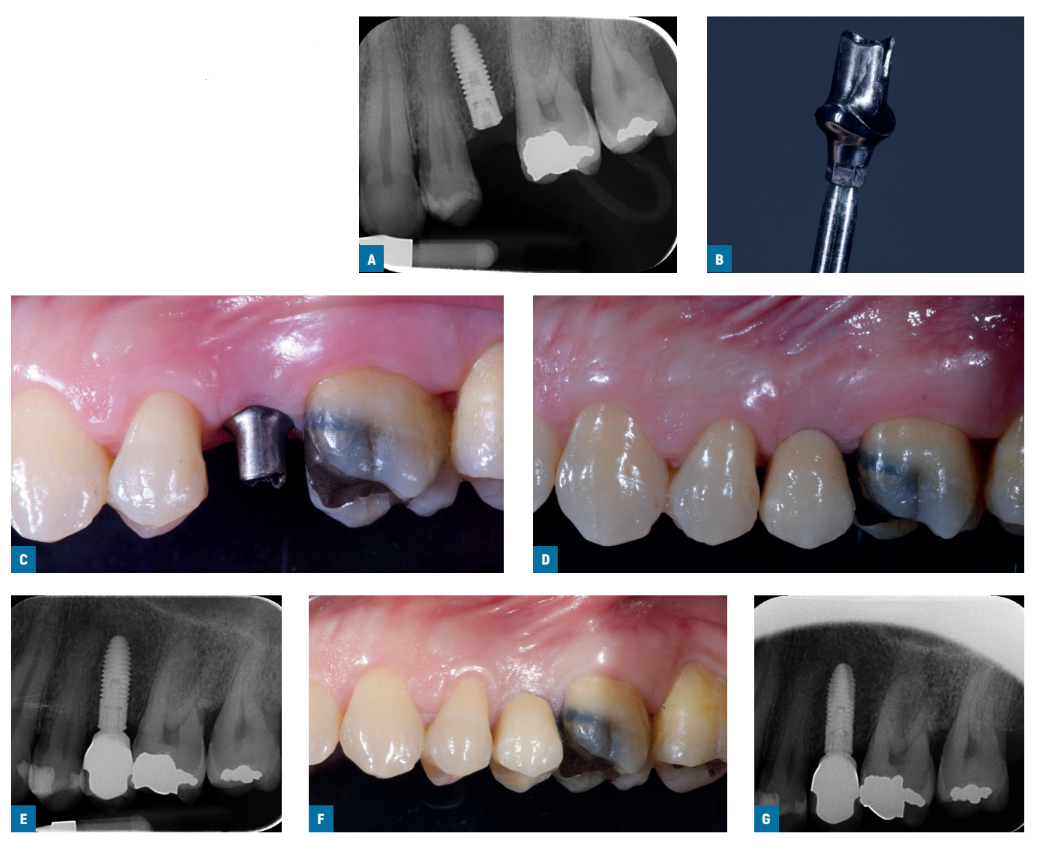
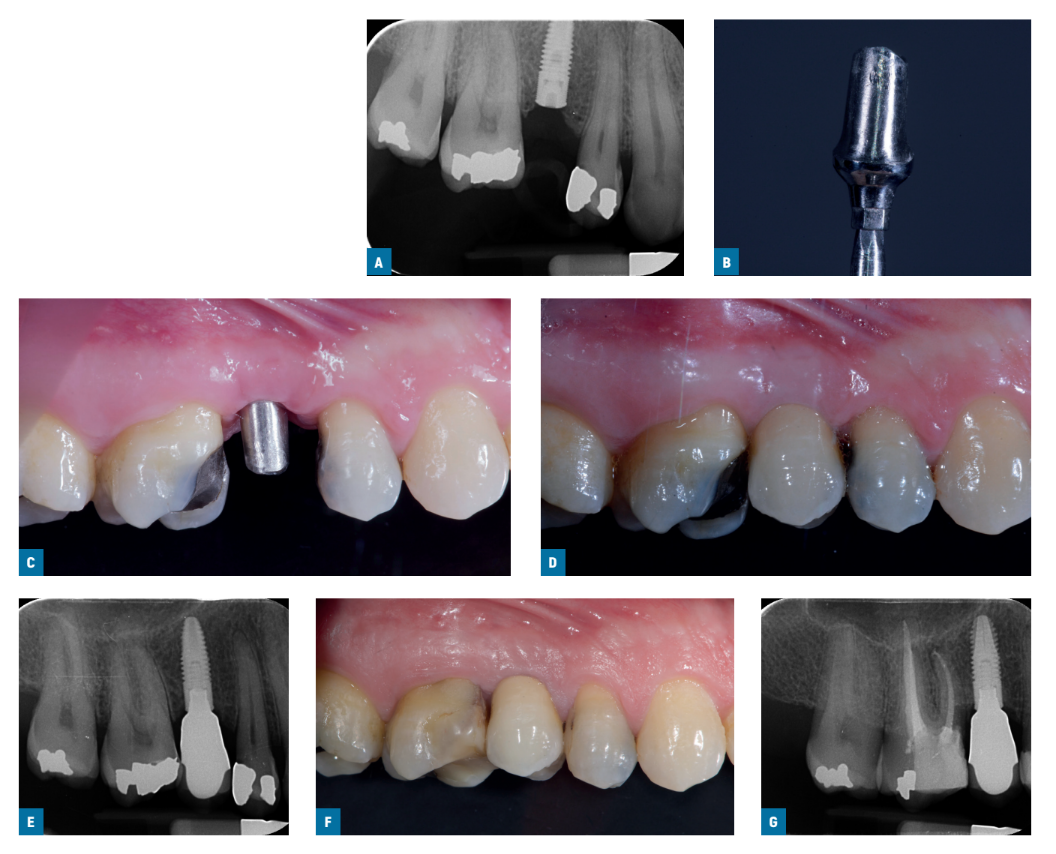
Within one month, after having tested the stability of the individual implants, the prepared abutments were screwed with a 30 Ncm torque into the study implants, according to the random allocation, and definitive cement-retained metal-ceramic crowns were cemented with radiopaque provisional cement (ImplaCem Automix, Dentalica, Milan, Italy) on the study abutments. The occlusal surfaces were in slight contact with the opposite dentition. Periapical radiographs of the study implants were taken. If the peri-implant marginal bone levels were not readable, a new radiograph was taken. Oral hygiene instructions were delivered. One week after initial loading, occlusion was checked and oral hygiene reinforced, if necessary. Patients were enrolled in an oral hygiene program with recall visits at least every 6 months for the entire duration of the study. Dental occlusion was evaluated at each follow-up visit. Follow-ups were conducted by local blind outcome assessors together with main operators.
Outcome measures
Outcome measures were:
- Сrown failures: loss of the crown secondary to implant failure, or replacement of the crown for any reasons.
- Implant failures: implant mobility, removal of stable implants dictated by progressive marginal bone loss or infection, or any mechanical failure rendering the implant unusable, such as implant fracture or deformation of the implant-abutment connection. Stability of individual implants was measured by local independent assessors who were not informed of the nature of the study manually tightening the screws with a torque of 10 Ncm at abutment connection (3 months after implant placement) and with a torque of 30 Ncm at initial loading (delivery of definitive crowns). At 1-, 3- and 5-year after loading, the single crown stability was assessed by rocking the crown with the metal handles of two dental instruments.
- Any biological or prosthetic complications was reported.
Peri-implant marginal bone level changes evaluated on digital intraoral radiographs taken with the paralleling technique at implant placement, at initial loading (baseline), 1, 3 and 5 years after loading. In the case of not properly readable radiographs, new radiographs were to be taken. A trained centralised outcome assessor measured peri-implant marginal bone levels using the Image J (National Institutes of Health, Bethesda, Maryland, USA) software. The software was calibrated for every single image using the known implant length. In case the full implant length was not represented in the radiograph, the diameter at the implant neck was used for the calibration. Measurements of the mesial and distal bone crest level adjacent to each implant was made to the nearest 0.01 mm. Reference points for the linear measurements were the coronal margin of the implant collar and the estimated most coronal point of bone-to-implant contact. Implants with bone up to the coronal margin of the implant collar were given the zero value. Mesial and distal measurements of each implant were averaged and means were calculated at group level.
One independent assessor at each centre masked to the interventions evaluated implant stability. Complications were managed and reported directly by the treating dentist. One single centralised outcome assessor (Dr. Caroline Bolle), not involved in the treatment of the patients nor aware of the scope of the study, measured all peri-implant marginal bone levels, blindly up to 3-year after loading. The bone level assessment at 5-year after loading were made by Riccardo Visconti. One of the clinicians noticed cast cobalt-chrome abutments appeared on radiographs a bit more radiopaque than machined titanium abutments, however the outcome assessors did not notice that possible difference.
No sample size was calculated since there are no previous trials evaluating this matter, however, it was decided to include only 60 patients (15 patients per centre), since that was our realistic recruitment capacity over a 2-year recruitment period. Four computer generated restricted randomisation lists were created. Only one of the investigators (Dr. Marco Esposito), not involved in the selection and treatment of the patients, was aware of the randomisation sequence and could have access to the randomisation lists stored in his password protected portable computer. The randomised codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially after impression taking; therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients.
Statistical analysis
All data analyses were carried out according to a pre-established analysis plan. The abutment was the statistical unit of the analyses. A dental student (Riccardo Visconti) specifically trained in statistics analysed the data without knowing group allocation. A comparison of the characteristics at implant placement between groups was presented. Differences in the proportion for dichotomous outcomes (crown/implant failures and complications) were compared between the groups using a McNemar chi-square test. Differences between groups for continuous outcomes (mean marginal bone level changes) were compared using a paired t-test. Comparisons between the various follow-up endpoints and implant placement and loading (baseline) measurements were made by paired t-tests, to detect any changes in mean marginal bone level for each study group. A mixed effect model using treatment group and centre as fixed effects, baseline (loading) radiographic bone levels as covariate and patient as random effect was created to compare changes between groups and centres of marginal bone levels between implant loading (baseline), 1- and 3-year follow-ups. Differences among centres for dichotomous outcomes were calculated using the chi-squared test or the Freeman-Halton extension of Fisher Exact test (when cell count <5). All statistical comparisons were conducted at the 0.05 level of significance. A modified intention to treat analysis was applied.
Results
Sixty-five patients were screened for eligibility but only 31 patients were consecutively enrolled in the trial by the three participating centres. Reasons for not including 34 patients were: unable to commit to 10-year follow-up (12 patients), need of bone augmentation at implant placement (nine patients); needed two adjacent implants (eight patients); requested immediate loading (three patients); refused to participate in the trials (two patients).
Each centre was supposed to enrol 15 patients, however not a single centre managed to achieve this goal and in particular: Dr Tallarico recruited 13 patients, Dr Meloni five patients, and Dr Xhanari 13 patients.
Unfortunately, five patients dropped out after implant placement but before randomisation and loading for the following reasons:
- Patient #2 (Roma centre) died for heart attack 6 weeks after implant placement
- Patient #3 (Roma centre) refused to continue with the treatment for family problems and then for the COVID-19.
- Patient #5 (Roma centre) had breast cancer diagnosed 2 months after implant placement, she stopped dental treatment for cancer treatment first and then for fear of COVID-19.
- Patient #11 (Roma centre) lost implant #26 1 week after second stage surgery but before random allocation at impression taking. Unfortunately, patient preferred to finalize his treatment with a partial fixed prosthesis on natural teeth (#25 to #27), avoiding a new implant.
- Patient #3 (Arzachena centre) moved to Panama 3 months after implant placement.
After randomization and more precisely at the 5-year follow-up, five drop-outs occurred:
- Patient #6 (Roma centre) moved to another country after the 1-year follow-up.
- Patient #4 (Tirana centre) moved to another country after the 2-year follow-up.
- Patient #9 (Tirana centre) moved to another country before the 1-year follow-up.
- Patient #10 (Tirana centre) moved to another country after the 3-year follow-up.
- Patient #13 (Roma centre) become unreachable after the 3-year follow-up.
The following protocol deviations were recorded:
Dentists delivered post-operatively 600 mg Ibuprofen instead of 400 mg as per protocol. Patients #4, #7, #9, #12 (Roma centre) received two adjacent implants while per protocol should have not.
Patient #6 (Roma centre) periapical radiographs at implant placement of both implants were lost. Patient #2 (Arzachena centre) the study implant 45 (cast group) was connected under the same prosthesis to implant in position 46 which was not in the study.
Patient #8 (Tirana centre) periapical radiographs at implant placement of both implants were lost. Patient #3 (Tirana centre) the crown was remade screw-retained, instead of cemented, after mucositis caused by cement retention.
Patients were recruited and treated from April 2017 to January 2019. The follow-up of all remaining patients was of 5 years after implant loading.
The main patient characteristics of the 26 patients having their implants randomly allocated were: 15 females and 11 males, 45 years old mean age (range from 21 to 83), 22 non-smokers and four smoking up to 10 cigarettes per day.
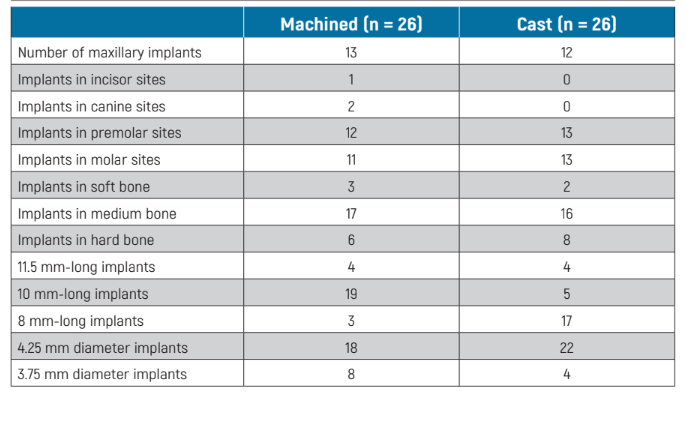
The main baseline characteristics of the implant which were actually randomised are presented in Table 1. There were no apparent significant baseline imbalances between the two groups with the possible exception of more 10 mm long implants in the machined group and more 8 mm long implants in the cast group.
Prosthesis failures: three crowns of the cast group failed versus none of the machined group. The difference was not statistically different (difference in proportions = 0.143 95% CI not estimated, McNemar test P = 0.248). One cemented crown on cast abutment (#46) in patient #3 (Tirana) was replaced by a screw-retained crown because the implant was affected by peri-implant mucositis, 1 month after loading, due to cement retention. Patient #5 (Arzachena) presented himself at the clinic holding in his hand the implant which was placed in position #15 with its own crown, 17 months after loading. Patient #2 (Roma) had his implant removed 4 years and half after loading due to recurrent peri-implantitis.
Implant failures: two implants failed from the cast group (see above) versus none from the machine group. The difference was not statistically different (difference in proportions = 0.095; 95% CI not estimated, McNemar test P = 0.480).
In addition, two implants failed before randomisation:
- Patient #7 (Tirana): during surgery implant #24 was placed too near the adjacent tooth. It was immediately removed and replaced after 2 months. The patient remained in the trial.
- Patient #11 (Roma): implant #26 failed 1 week after second stage surgery. The patient preferred a traditional fixed tooth borne prostheses on natural teeth, avoiding a new implant, therefore, exited the trial.
Complications: three complications occurred in the cast group versus one in the machined group, the difference being not statistically different (difference in proportions = 0.095; 95% CI -0.077 to 0.259, McNemar test P = 0.480). The complications in the cast group (patient #3, Tirana) consisted in a peri-implant mucositis affecting #46, 1 month after loading, caused by cement retention. It was resolved by changing the crown from cemented to screw retained. Patient #12 (Roma) was affected by a minor ceramic chipping at the margin for the crown, noticed at the 3-year follow-up, treated with some polishing. Patient #2 (Roma) was affected by peri-implantitis with repeated abscesses about every second month starting around the fourth year in function, unsuccessfully treated with systemic antibiotics and debridement.
The complication in the machined group (patient #1 Roma) was a minor ceramic chipping at the margin for the crown, noticed at the 1-year follow-up, which required no treatment.
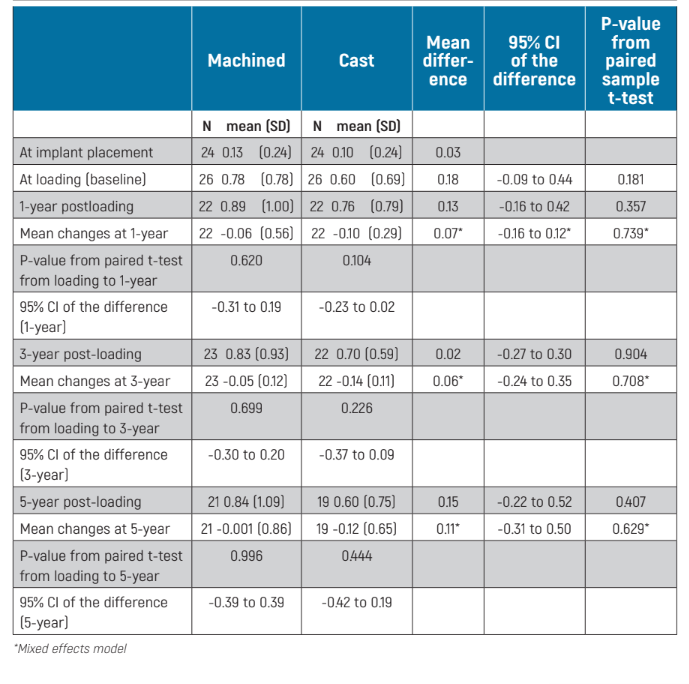
Peri-implant marginal bone level changes could be measured at all implant surfaces of the periapical radiographs. There were no statistically significant differences for bone levels at loading (baseline), 1-, 3- and 5-year after loading between the two groups (Table 2). Both groups gradually lost marginal peri-implant bone and this was statistically significant (P = 0.001 for machined abutments and <0.001 for cast abutments), but not from implant loading (baseline): P = 0.699 for machined abutments and P = 0.226 for cast abutments (Table 2). At 5-year post-loading, patients with machined abutments lost -0.76 ± 1.09 mm compared with -0.56 ± 0.77 mm for cast abutments, from implant placement, with no statistically significant differences between the two groups (mean difference -0.19 mm; 95% CI -0.54 to 0.25; P = 0.454). Whereas from implant loading (baseline), at 5-year post-loading, patients with machined abutments lost -0.001 ± 0.86 mm compared with -0.12 ± 0.65 mm for cast abutments, the difference between groups being not statistically significant (mean difference 0.11 mm; 95% CI –0.31 to 0.50, mixed effects model P = 0.629; Table 2).
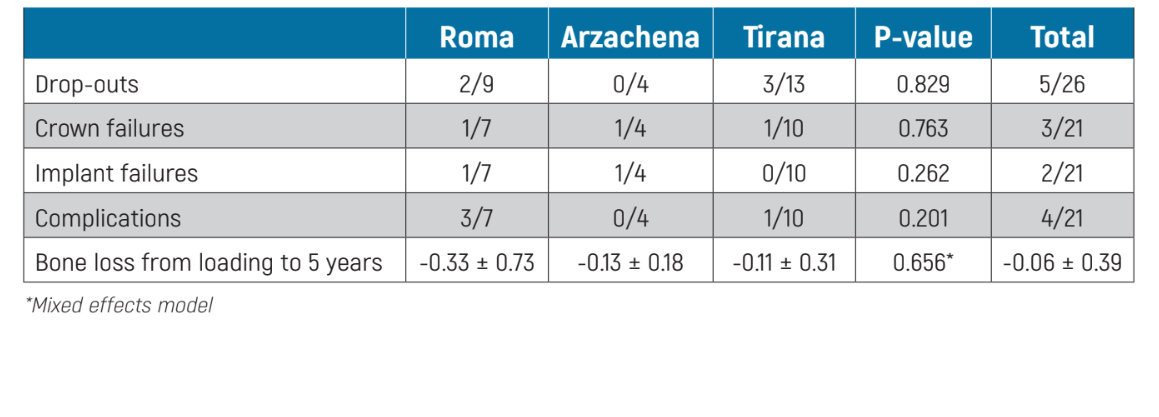
Comparison between difference centres: there were no differences for any of the outcome measures between the three centres (Table 3).
Discussion
This trial was designed to have some preliminary data on whether it would be more advisable to use machined or cast abutments with the aim of reducing possible bacterial leakage from the implant-abutment junction characterised by an internal connection, in order to minimise bone loss and the risk of peri-implantitis. Our preliminary results based on a small study population suggest very similar clinical medium-term results for both types of abutments. Obviously our preliminary results have to be confirmed by longer follow-ups (at least of 10 years in function) and by more trials with larger sample sizes.
It is difficult to compare the current results with those of other similar RCTs since there are no other trials testing this hypothesis.
The main limitations of the present trial are the small sample size, the lost radiographs, that further reduced the sample size for the radiographic evaluation, and the limited duration of this trial. Unfortunately, the planned sampled size was not achieved since one centre did not provide any data and the three remaining centres did not recruit the number of patients agreed a priori. In addition, some patients died or had implant failures after implant placement but before being randomised and finally travel restrictions imposed by the COVID-19 pandemics also affected the number of patients being able to attend the 1-year follow-up, though two patients reappeared for the 3-year follow-up.
If data from other RCTs will be available, it could be possible to combine the present data with those of similar trials in meta-analyses obtaining larger samples sizes to have a more precise estimate of possible differences, if any. Regarding the duration of the follow-up, it is hoped that all centres will continue to monitor these cohorts of patients up to the 10-year follow-up, since, if some differences between the two abutment types exist, they might appear only after several years in function.
Both abutments were evaluated in real clinical conditions and patient inclusion criteria were rather broad, therefore the results of the present investigation can be generalised with confidence to a wider population with similar characteristics. Further, the in vitro data obtained in a previous publication of this study found no differences in the fit of the cast and machined abutments. These results were in good agreement with findings from previous in vitro studies and match well the clinical observations of the present study.
Conclusions
The present clinical data suggests that up to 5 years after loading implant prognosis is not affected by using machined or cast abutments. Therefore, dentists can choose whatever they prefer. However, these preliminary results need to be confirmed by larger trials with follow-ups of at least 10 years.
Marco Tallarico, Katia Greco, Riccardo Federico Visconti, Erta Xhanari, Silvio Mario Meloni, Marco Esposito
References
- Esposito M, Maghaireh H, Pistilli R, Grusovin MG, Lee ST, et al. Dental implants with internal versus external connections: 10-year post-loading results from a pragmatic multicentre randomised controlled trial. Clinical Trials in Dentistry. 2020;2:5-19.
- Kano SC, Binon PP, Curtis DA. A classification system to measure the implant-abutment microgap. Int J Oral Maxillofac Implants. 2007;22(6):879-85.
- do Nascimento C, Barbosa RE, Issa JP, Watanabe E, Ito IY, et al. Bacterial leakage along the implant-abutment interface of premachined or cast components. Int J Oral Maxillofac Surg. 2008;37(2):177-80.
- do Nascimento C, Barbosa RE, Issa JP, Watanabe E, Ito IY, et al. Use of checkerboard DNA-DNA hybridization to evaluate the internal contamination of dental implants and comparison of bacterial leakage with cast or pre-machined abutments. Clin Oral Implants Res. 2009;20(6):571-7.
- Malaguti G, Denti L, Bassoli E, Franchi I, Bortolini S, et al. Dimensional tolerances and assembly accuracy of dental implants and machined versus cast-on abutments. Clin Implant Dent Relat Res. 2011;13(2):134-40.
- Atzenia E, Bassolib E, Dentib L, Gattob A, Iulianoa L, et al. Tolerance analysis for cast vs machined dental implants. Procedia CIRP 33. 2015;263-8.
- Esposito M, Aparicio C, Xhanari E, Meloni SM, Bolle C, et al. Machined versus cast abutments for dental implants: a 1-year within patients multicentre randomised controlled trial on single crowns assessing marginal seal capacity and outcomes. Clinical Trials in Dentistry. 2021;3:19-31.
- Xhanari E, Tallarico M, Bolle C, Buti J, Meloni SM, et al. Machined versus cast abutments for single dental implants: a 3-year within-patient multicentre randomised controlled trial. Clinical Trials in Dentistry. 2023;5:5-16.
