Influence of reciprocating single-file and rotary instrumentation on bacterial reduction on infected root canals
Abstract
Aim: To compare the bacterial reduction achieved with reciprocating and rotary systems during root canal preparation.
Methodology: Sixty distobuccal root canals of maxillary molars were contaminated with Enterococcus faecalis broth culture. After an incubation period of 21 days, bacterial samples were collected and cultured on m-Enterococcus agar plates. The root canals were divided into five groups, according to the system used for instrumentation: WaveOne, Reciproc, ProTaper, Mtwo and manual instrumentation. The negative controls consisted of five uncontaminated root canals that were subjected to the same instrumentations as each of the experimental groups. Bacterial samples were collected immediately and 7 days after instrumentation. Statistical analysis was performed by paired t-tests and ANOVA tests.
Results: Compared with the samples before instrumentation, the bacterial count was significantly reduced after instrumentation in all groups, with no significant difference in bacterial count reduction amongst the reciprocating, rotary and manual techniques. However, the samples tested 7 days after instrumentation showed significantly higher bacterial counts than the samples tested immediately after instrumentation.
Introduction
The presence of bacteria is the main cause of periodontal infection and apical periodontitis development (Kakehashi et al. 1965). Amongst the diverse bacteria species found in endodontic infections, Enterococcus faecalis stands out for its resistance, survival in nutrient-poor environments and association with persistent apical periodontitis (Sedgley et al. 2005, Sakamoto et al. 2007). Although chemical agents are important in root canal instrumentation, some agents are not completely efficacious against all of the bacterial species in biofilms (Pappen et al. 2010). Moreover, mature biofilms are more resistant to the actions of chemicals (Shen et al. 2011).
Mechanical removal by instrumentation is particularly effective in disrupting the bacterial biofilm and reducing the presence of bacteria in the main root canal (Aydin et al. 2007, Machado et al. 2010, Gorduysus et al. 2011, Matos Neto et al. 2012). Although manual instrumentation is commonly used by practitioners, automated rotary systems are associated with several advantages compared with manual techniques, including more rapid procedures (Guelzow et al. 2005, Yin et al. 2010), more centred preparations (Taşdemir et al. 2005, Aguiar et al. 2009) and less apical extrusion of debris (Madhusudhana et al. 2010). In particular, the ProTaper (Dentsply Maillefer, Ballaigues, Switzerland) and Mtwo (VDW, Munich, Germany) systems have been shown to provide adequate geometry (Yang et al. 2011) and substantial bacterial reduction in the root canal (Machado et al. 2010).
Recently, instrumentation with a reciprocating single-file has been proposed. Systems using this approach include WaveOne (Dentsply Maillefer) and Reciproc (VDW), which essentially differ in their cross section, but are similar to ProTaper and Mtwo, respectively. Initial studies have shown that the use of Reciproc or Mtwo results in cleaner canals in the apical third compared with the use of WaveOne or ProTaper (Bürklein et al. 2012). Moreover, the use of WaveOne was shown to reduce the morphological modification of the canal compared with the use of ProTaper (Berutti et al. 2012). However, few studies have evaluated the mechanical action of these systems in infected canals.
The aim of this study was to compare the bacterial reduction achieved by reciprocating and rotary systems in root canals contaminated with E. faecalis. The manual technique was used as reference for comparison. The null hypothesis tested was that there are no differences in the bacterial reduction promoted by reciprocating and rotary systems.
Material and methods
Preparation of samples
Approval for this study was obtained from the Ethical Committee of the School of Dentistry of the University of São Paulo (194/2010). Sixty-five distobuccal root canals of the maxillary molars were standardized to 12 mm and instrumented to a working length of 11 mm up to a size 15 K-file (Dentsply Maillefer) under irrigation with distilled water. The root canals were filled with 17% EDTA (Fórmula & Acão, São Paulo, SP, Brazil) for 3 min to remove the smear layer and washed with 5 mL of distilled water. The apex was covered with composite resin (3M, Saint Paul, MN, USA), and the external root surface was sealed with epoxy resin (Araldite; Brascola, Joinvile, SC, Brazil). The specimens were fixed onto 24-well polystyrene microtitre plates by acrylic resin and sterilized by ethylene oxide (Acecil, Campinas, SP, Brazil).
Contamination of samples
A suspension of E. faecalis (ATCC 29212) in tryptic soy broth (TSB; Difco, Le Pont-de-Claix, RA, France) was prepared and standardized to 4 on the McFarland scale. Sixty root canals were contaminated with the E. faecalis suspension by an insulin syringe. The five remaining root canals were filled with TSB. The specimens were incubated at 37 °C for 21 days. The root canal contents were replaced with fresh TSB every 48 h.
After the incubation period, the root canals were filled with distilled water. Samples (S1) were collected with three sterilized size 15 paper points (Dentsply Maillefer), which were inserted into the root canals for 1 min each. The points were stored in tubes containing 500 IL of peptone water, and serial dilutions were prepared. Different dilutions were plated in triplicate on m-Enterococcus agar culture medium (Difco). The plates were incubated at 37 °C for 48 h, and the bacterial count was measured (in CFU mL—1).
Instrumentation of samples
The contaminated specimens were divided into five groups, as described below.
- Group 1 was prepared with the WaveOne Primary file (tipsize 25, 0.08 taper) (Dentsply Maillefer). With the motor in reciprocating motion (Dentsply Maillefer), the file was gently inserted into the cervical third and withdrawn. The file was inserted into the middle third and withdrawn. Lastly, the file was inserted into the apical third up to the working length with a brushing action performed against walls.
- Group 2 was prepared with the Reciproc R25 (tipsize 25, 0.08 taper) (VDW) in a similar manner as group 1.
- Group 3 was prepared with the ProTaper system (Dentsply Maillefer). The cervical third was enlarged with Gates-Glidden drills 1, 2 and 3 (Dentsply Maillefer) and then with the instrument sequence SX and S2. The middle and apical thirds were instrumented with S1, S2, F1 and F2 (tip-size 25, 0.08 taper).
- Group 4 was prepared with the Mtwo system (VDW). The cervical third was enlarged with Gates-Glidden drills 1, 2 and 3. The files were introduced directly to working length with slight back-and-forth movements, whilst the pressure in the apical direction was gradually increased, and then brushing action was performed against walls. The sequence of the files was size 10, 0.04 taper, size 15, 0.05 taper, size 20, 0.06 taper and size 25, 0.06 taper.
- Group 5 (positive control) was prepared by crown-down manual technique. The cervical and middle thirds were enlarged with Gates-Glidden drills 1, 2 and 3, and then a size 20 K-file was inserted gently up to working length using quarter turn and filing movements against the walls. This movement was repeated until the instrument no longer met resistance, and the file was free. These manoeuvres were subsequently repeated with size 25, 30 and 35 K-files.
The negative controls consisted of five uncontaminated specimens that were instrumented according to each group.
Irrigation during instrumentation was performed with a total of 10 mL of distilled water using a syringe and a 29-gauge NaviTip (Ultradent Products, South Jordan, UT, USA), which was taken up to 3 mm short of the working length through an in-and-out motion for better flow. In groups 1 and 2, irrigation and exploration with a size 15 K-file were performed each time after the instrument was withdrawn. In groups 3, 4 and 5, the irrigation was repeated with each exchange of an instrument.
Data collection after instrumentation
To determine the bacterial count (in CFU mL—1) immediately after instrumentation (S2), an additional 5 mL of distilled water was introduced after the final irrigation. Filing was then performed with a size 25 Hedström file, introducing it into the canal up to the working length with circumferential filing strokes on all of the root canal surfaces. The file was sectioned below the handle and dropped into a tube containing 500 IL of peptone water. Three sterilized size 15 paper points were inserted into the root canal for 1 min each and were stored in the same tube as the file.
The root canals were filled with TSB and incubated at 37°C for 7 days. A third sampling was performed in the same manner as for S2 to determine the bacterial count at 7 days after instrumentation (S3).
Statistical analysis
Each bacterial count was log-transformed for statistical analysis. The paired t-test was used for intragroup analysis, and analysis of variance (ANOVA) was used for intergroup analysis. The level of significance for all analyses was P < 0.05.
Results
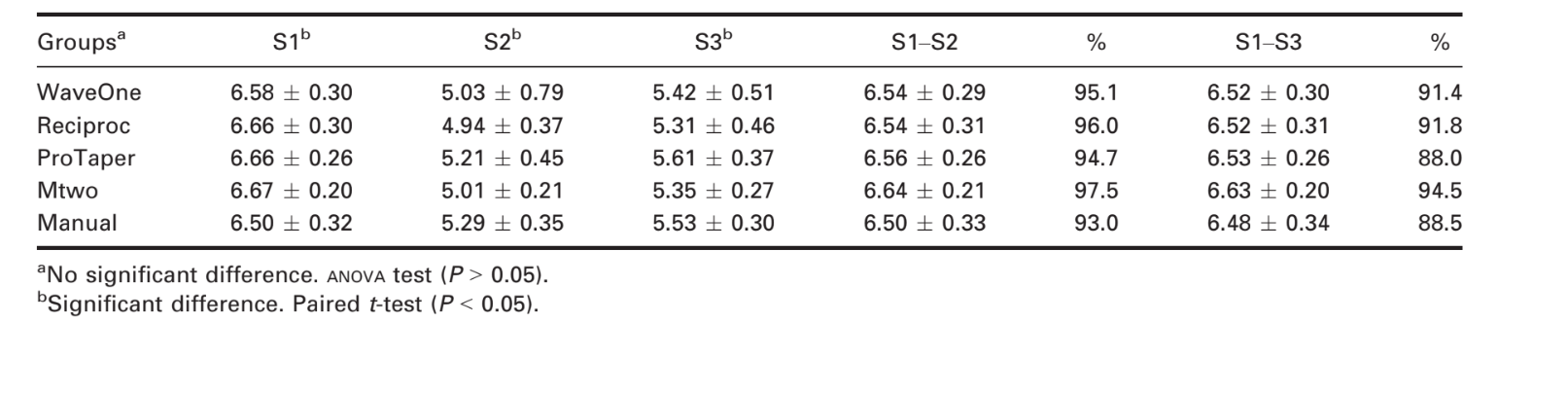
Table 1 shows the results for bacterial reduction. Bacterial counts in S2 and S3 were reduced compared with S1 for all groups (P < 0.0001 by t-test). This result demonstrated that all of the preparation techniques were effective at reducing the bacterial count. However, a comparison of the bacterial counts at S2 and S3 revealed bacterial growth in the 7 days after instrumentation (P < 0.01 by t-test). The negative control did not show any bacterial growth, which indicated that aseptic conditions were maintained during the experiment. The reciprocating, rotary and manual techniques had similar results immediately and 7 days after instrumentation (P = 0.78 and P = 0.76, respectively, by ANOVA).
Discussion
Microbiological elimination is of utmost importance in endodontic therapy, because the presence of bacteria is the main cause of endodontic failure (Kakehashi et al. 1965, Sakamoto et al. 2007). Many methodologies have been used to study this concept. In the present study, the roots of the molars were examined. The WaveOne Primary, Reciproc R25 and ProTaper F2 have the same tip size and taper, which is a limitation in terms of disinfecting canals of large diameter. Manual instrumentation was performed up to a size 35, 0.02 taper K-file. However, from a microbiological perspective, the use of different tip sizes and tapers has been reported to result in similar bacterial counts (Machado et al. 2010).
Similar to many previous studies (Siqueira et al. 1999, Aydin et al. 2007, Machado et al. 2010, Singla et al. 2010, Siqueira et al. 2010, Alves et al. 2011, Gorduysus et al. 2011, Alves et al. 2012, Matos Neto et al. 2012, Paranjpe et al. 2012), the plate-culture method was utilized. Alves et al. (2011) observed similar results with the polymerase chain reaction technique as they did with the plate-culture method. Although other authors have compared bacterial growth before and after instrumentation of the root canal without filing (Siqueira et al. 1999, Machado et al. 2010, Singla et al. 2010, Gorduysus et al. 2011, Matos Neto et al. 2012), but the filing is necessary due to the presence of a smear layer, remnants of bio- film and non-instrumented areas that could influence the between-group comparisons (Aydin et al. 2007, Siqueira et al. 2010, Alves et al. 2011, 2012, Paranjpe et al. 2012).
The third sampling at 7 days (S3) was used to verify bacterial growth in the root canal between appointments (Siqueira et al. 2007). The bacterial counts showed significant bacterial reduction in all instrumentation techniques at both S2 and S3 compared with controls. However, the comparison between S2 and S3 revealed significant bacterial growth in the main canal. This finding contradicts the results found by Siqueira et al. (2007), who used intracanal medication between the appointments. The remaining bacteria within the dentinal tubules in our study could have multiplied and entered the main canal. The use of nutrient-rich culture broth in the root canal over 7 days obviously favoured bacterial growth.
No statistically significant differences were observed between techniques and systems, consistent with the results of assays comparing manual techniques to rotary systems (Dalton et al. 1998, Siqueira et al. 1999, Chuste-Guillot et al. 2006, Matos Neto et al. 2012). Previous studies have observed mean bacterial reductions of 81.94% for ProTaper and 84.29% for Mtwo (P > 0.05) (Machado et al. 2010). Although the present study also found no difference between these systems, the mean reductions were 94.71% for ProTaper and 97.43% for Mtwo.
In the present report, none of the samples was totally free of bacteria. In contrast, Coldero et al. (2002) observed that 81% of the samples prepared by the manual technique were free of bacteria. This difference should be considered in the light of the fact that very small bacterial growth cannot be detected by traditional culture methods (Siqueira & Rôcas 2005).
In the present study, bacterial count reductions of 95.1% immediately after and 91.4% at 7 days after instrumentation were achieved by the reciprocating systems without the use of chemical agents. These results were comparable to those obtained with conventional techniques (i.e. 94.7% and 88%, respectively, for rotary systems and 93% and 88.5%, respectively, for manual systems). Therefore, the single-file reciprocating systems resulted in similar bacterial reductions compared with those obtained with rotary systems or with the manual technique.
Conclusion
It can be concluded that all systems tested reduced bacterial counts to a similar level.
M. E. L. Machado, C. K. Nabeshima, M. F. P. Leonardo, F. A. S. Reis, M. L. B. Britto & S. Cai
References:
- Aguiar CM, Mendes DA, Câmara AC, Figueiredo JAP (2009) Evaluation of the centreing ability of the ProTaper Universal rotary system in curved roots in comparison to Nitiflex files. Australian Endodontic Journal 35, 174–9.
- Alves FRF, Almeida BM, Neves MAS, Rôças IN, Siqueira JFJr (2011) Time-dependent antibacterial effects of the self-adjusting file used with two sodium hypochlorite concentrations. Journal of Endodontics 37, 1451–5.
- Alves FRF, Rôças IN, Almeida BM, Neves MAS, Zoffoli J,Siqueira JF Jr (2012) Quantitative molecular and culture analyses of bacterial elimination in oval-shaped root canals by a single-file instrumentation technique. International Endodontic Journal 45, 871–7.
- Aydin C, Tunca YM, Senses Z, Baysallar M, Kayaoglu G, Ørstavik D (2007) Bacterial reduction by extensive versus conservative root canal instrumentation in vitro. Acta Odontologica Scandinavica 65, 167–70.
- Berutti E, Chiandussi G, Paolino DS et al. (2012) Canal shaping with WaveOne Primary reciprocating files and ProTaper system: a comparative study. Journal of Endodontics 38, 505–9.
- Bürklein S, Hinschitza K, Dammaschke T, Schäfer E (2012) Shaping ability and cleaning effectiveness of two single-file systems in severely curved root canals of extracted teeth: Reciproc and WaveOne versus Mtwo and ProTaper. International Endodontic Journal 45, 449–61.
- Chuste-Guillot M-P, Badet C, Peli J-F, Perez F (2006) Effect of three nickel-titanium rotary file techniques on infected root dentin reduction. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 102, 254–8.
- Coldero LG, McHugh S, Mackenzie D, Saunders WP (2002) Reduction in intracanal bacteria during root canal preparation with and without apical enlargement. International Endodontic Journal 35, 437–46.
- Dalton BC, Ørstavik D, Pettiette M, Trope M (1998) Bacterial reduction with nickel-titanium rotary instrumentation. Journal of Endodontics 24, 763–7.
- Gorduysus M, Nagas E, Torun OY, Gorduysus O (2011) A comparison of three rotary systems and hand instrumentation technique for the elimination of Enterococcus faecalis from the root canal. Australian Endodontic Journal 37, 128–33.
- Guelzow A, Stamm O, Martus P, Kielbassa AM (2005) Comparative study of six rotary nickel-titanium systems and hand instrumentation for root canal preparation. International Endodontic Journal 38, 743–52.
- Kakehashi S, Stanley HR, Fitzgerald RJ (1965) The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surgery, Oral Medicine, Oral Pathology 20, 340–9.
- Machado MEL, Sapia LAB, Cai S, Martins GHR, Nabeshima CK (2010) Comparison of two rotary systems in root canal preparation regarding disinfection. Journal of Endodontics 36, 1238–40.
- Madhusudhana K, Mathew VB, Reddy NM (2010) Apical extrusion of debris and irrigants using hand and three rotary instrumentation systems – an in vitro study. Contemporary Clinical Dentistry 1, 234–6.
- Matos Neto M, Santos SSF, Leão MVP, Habitante SM, Rodrigues JRDD, Jorge AOC (2012) Effectiveness of three instrumentation systems to remove Enterococcus faecalis from root canals. International Endodontic Journal 45, 435–8.
- Pappen FG, Shen Y, Qian W, Leonardo MR, Giardino L, Haapasalo M (2010) In vitro antibacterial action of Tetraclean, MTAD and five experimental irrigation solutions. International Endodontic Journal 43, 528–35.
- Paranjpe A, Gregorio C, Gonzalez AM et al. (2012) Efficacy of the self-adjusting file system on cleaning and shaping oval canals: a microbiological and microscopic evaluation. Journal of Endodontics 38, 226–31.
- Sakamoto M, Siqueira JF Jr, Rôças IN, Benno Y (2007) Bacterial reduction and persistence after endodontic treatment procedures. Oral Microbiology and Immunology 22, 19–23.
- Sedgley CM, Lennan SL, Appelbe OK (2005) Survival of Enterococcus faecalis in root canals ex vivo. International Endodontic Journal 38, 735–42.
- Shen Y, Stojicic S, Haapasalo M (2011) Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. Journal of Endodontics 37, 657–61.
- Singla M, Aggarwal V, Logani A, Shah N (2010) Comparative evaluation of rotary ProTaper, Profile, and conventional stepback technique on reduction in Enterococcus faecalis colony-forming units and vertical root fracture resistance of root canals. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 109, e105–10.
- Siqueira JF Jr, Rôças IN (2005) Exploiting molecular methods to explore endodontic infections: part 1: current molecular technologies for microbiological diagnosis. Journal of Endodontics 31, 411–23.
- Siqueira JF Jr, Lima KC, Magalhães FAC, Lopes HP, Uzeda M (1999) Mechanical reduction of the bacterial population in the root canal by three instrumentation techniques. Journal of Endodontics 25, 332–5.
- Siqueira JF Jr, Paiva SSM, Rôças IN (2007) Reduction in the cultivable bacterial population in infected root canals by a chlorhexidine-based antimicrobial protocol. Journal of Endodontics 33, 541–7.
- Siqueira JF Jr, Alves FRF, Almeida BM, Oliveira JCM, Rôças IN (2010) Ability of chemomechanical preparation with either rotary instruments or Self-adjusting file to disinfect oval-shaped root canals. Journal of Endodontics 36, 1860–5.
- Taşdemir T, Aydemir H, Inan U, Ünal O (2005) Canal preparation with Hero 642 rotary Ni-Ti instruments compared with stainless steel hand K-file assessed using computed tomography. International Endodontic Journal 38, 402–8.
- Yang G, Yuan G, Yun X, Zhou X, Liu B, Wu H (2011) Effects of two nickel-titanium instrument systems, Mtwo versus ProTaper universal, on root canal geometry assessed by micro-computed tomography. Journal of Endodontics 37, 1412–6.
- Yin X, Cheung GS, Zhang C, Masuda YM, Kimura Y, Matsumoto K (2010) Micro-computed tomographic comparison of nickel-titanium rotary versus traditional instruments in C-shaped root canal system. Journal of Endodontics 36, 708–12.
