The Effect of Regional Anesthetic Sphenopalatine Ganglion Block on Self‐Reported Pain in Patients With Status Migrainosus
Background. Status migrainosus (SM) is defined as a debilitating migraine attack lasting more than 72 hours in patients previously known to suffer from migraine headache. Typically, these attacks fail to respond to over the counter and abortive medications. The sphenopalatine ganglion (SPG) plays a critical role in propagating both pain and the autonomic symptoms commonly associated with migraines. SPG block via transnasal lidocaine is moderately effective in reducing migraine symptoms, but this approach is often poorly tolerated and the results are inconsistent. We proposed that an SPG block using a suprazygomatic injection approach would be a safe and effective option to abort or alleviate pain and autonomic symptoms of SM.
Methods. Through a retrospective records review, we identified patients with a well‐established diagnosis of migraine based on the International Headache Society criteria. Patients selected for study inclusion were diagnosed with SM, had failed to respond to 2 or more abortive medications, and had received a suprazygomatic SPG block. Patients had also been asked to rate their pain on a 1–10 Likert scale, both before and 30 minutes after the injection.
Results. Eighty‐eight consecutive patients (20 men and 68 women) received a total of 252 suprazygomatic SPG block procedures in the outpatient headache clinic after traditional medications failed to abort their SM. At 30 minutes following the injections, there was a 67.2% (±26.6%) reduction in pain severity with a median reduction of 5 points (IQR= −6 to −3) on the Likert scale (ranging from 1 to 10). Overall, patients experienced a statistically significant reduction in pain severity (P < .0001).
Conclusion. The SPG is known to play an integral role in the pathophysiology of facial pain and the trigeminal autonomic cephalalgias, although its exact role in the generation and maintenance of migraine headache remains unclear. Regional anesthetic suprazygomatic SPG block is potentially effective for immediate relief of SM. We believe the procedure is simple to perform and has minimal risk.
Introduction
Approximately 15% of the general population suffer from migraine headache. Status migrainosus (SM) is defined as a debilitating migraine attack lasting more than 72 hours in patients previously known to suffer from migraine headache. Typically, these attacks fail to respond to standard over the counter medications, triptans, intravenous (IV) steroids, parenteral benzodiazepines, or neuroleptic medication, which provide short‐term relief typically followed by recurrent headache within 24 hours. In the United States, visits to the emergency department (ED) for SM cost approximately $700 million per year. These patients are frequently hospitalized. In 2010, hospitalization cost for SM totaled approximately $375 million. In one report, the average hospital length of stay for a patient with SM was 2.7 days.
The sphenopalatine ganglion (SPG) is the largest extracranial parasympathetic ganglion in the body and is part of the autonomic nervous system that may play a role in the therapeutic treatment of SM. Specifically, the SPG is located within the pterygopalatine fossa, behind the nasopharynx, posterior to the middle turbinate, and inferior to the sphenoid sinus (Fig. 1). Since the SPG is anatomically located adjacent to facial sensory nerves and trigeminal nerve branches, it has a potential role in the pathophysiology of unilateral headache and idiopathic facial pain mediated through the trigeminovascular complex.
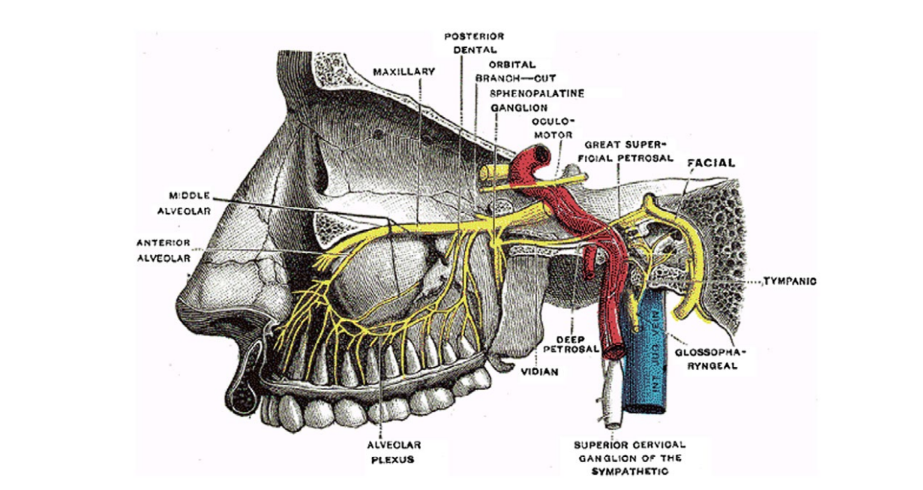
Regional anesthetic suprazygomatic SPG block (SSPGB) likely affects parasympathetic outflow to cranial vascular structures, although the mechanism is not completely understood. When a migraine is triggered, the trigeminal‐autonomic reflex is activated. Afferent trigeminal neurons stimulate the superior salivary nucleus through the pons, increasing the parasympathetic output of the SPG via the facial nerve. Outflow of the SPG mediates vasodilation of cerebral blood vessels within the meninges, which then facilitates extravasation of inflammatory mediators, leading to the pain experienced by patients. Migraine headache is commonly associated with autonomic symptoms including photophobia, phonophobia, nausea, and emesis and occasionally nasal congestion. Similar to cluster headache, it is theorized that the SPG plays a pivotal role in facilitating these autonomic symptoms when associated with migraine, and successful blockade of the SPG could therefore provide a benefit in relieving these symptoms. Transnasal SPG blocks with lidocaine‐soaked pledgets have been proven effective in reducing migraine pain but are often poorly tolerated, and overall pain reduction results are unimpressive and inconsistent. Recently, the transnasal delivery of anesthetic to the foot process of the SPG via the TX360 device has improved this approach considerably, but has been studied only in the setting of chronic migraine with repeat applications over 6 weeks. In this study, we hypothesized that an SSPGB would be tolerable and effective in aborting the pain of SM, a condition clinically more common in the general population than chronic migraine.
Methods
We conducted a retrospective study to evaluate the effect of the SSPGB on patients with SM treated at the outpatient headache clinic of a large, tertiary care hospital in eastern Pennsylvania. Patient records from January 2008 to December 2016 were reviewed to identify patients with a well‐established diagnosis of migraine and SM, using the International Headache Society criteria. Our headache clinic acute treatment algorithm for SM includes routinely given oral, intranasal, or subcutaneous triptans as a first line therapy. In triptan refractory patients, ergotamine is used either as Migranal nasal spray, oral Cafergot, or 1 mg of intramuscular dihydroergotamine 45 (DHE). Patients are instructed to take these medications early in the course of their migraine evolution. For headache persisting 3 days or longer, a dexamethasone taper is prescribed over 3 days consisting of 12, 8, and 4 mg in consecutive days. This is coupled with methergine 0.2 mg every 6 hours orally for 3 days. In patients who cannot tolerate triptans or ergotamine therapy, isometheptene/dichloralphenazone/acetaminophen is prescribed as a first line agent with codeine 30 mg given orally every 8 hours as rescue if the initial therapy was ineffective. Patients with SM unresponsive to the algorithm outlined above were referred for SPG block by all neurologists in the practice. All patients were evaluated consecutively with no exclusions from the analysis. Patients were asked to rate their pain on a 1–10 Likert scale, both before and 30 minutes after the injection procedure. The procedure was explained to the patients in detail and a written informed consent was obtained prior to the procedure.
Exclusion criteria included systemic anticoagulation, tension‐type headache, cluster headache, trigeminal neuralgia, post‐concussive headache, cervicogenic headache, and new daily persistent headache with migrainous features. Patients who had an inadequate response to SPG block and remained in severe pain were typically admitted for IV therapy that included normal saline, DHE 45, antiemetic therapy with metoclopramide, ondansetron, or chlorpromazine, and magnesium. IV lidocaine was rarely used.
This study did not request an ethics board approval as this procedure is commonly used in oral surgery, and transnasal and infrazygomatic SPG block has been shown to be effective in migraines. Our group followed the ethical guidelines outlined in the Declaration of Helsinki. This study was approved by our institutional review board, which contains some members of our ethics board.
The procedure: regional anesthetic spg block
The SSPGB was performed using a 5 mL mixture of dexamethasone (1 mL) and 0.5% ropivacaine (4 mL). With the patient in the lateral decubitus position, a 25‐gauge, 2‐inch needle was advanced at a 45‐degree angle to the skull, directing posteriorly at a 15‐degree angle from the ocular meatal line toward the posterior condyle of the ramus of the mandible (Fig. 2).
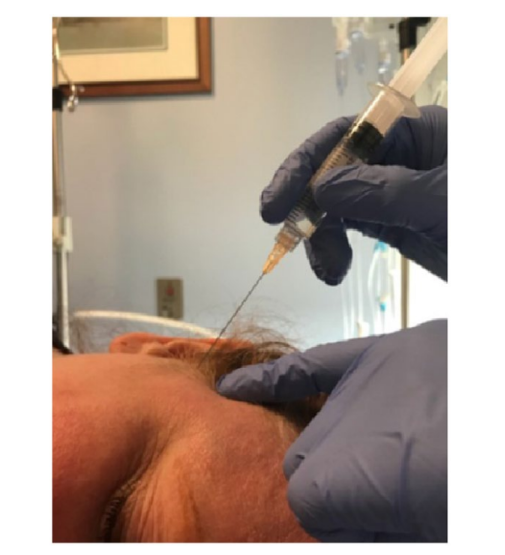
Accuracy of this procedure was determined by the presence of numbness in the posterior portion of the tongue as well as the cheek. Additionally, relief of photophobia and nausea was another measure to support the accuracy of the injection.
Outcome and statistical analysis
Descriptive statistics were generated for all variables. The mean and standard deviation are presented for the continuous variables. If the distribution of the continuous variables was skewed, the median and interquartile range (IQR) are presented instead. Skewness was assessed by visual inspection of histograms as well as the skewness statistic. Frequencies and percentages are used to describe the categorical variables.
There were 2 units of analysis for this study – patient and procedure – due to the fact that several patients had more than one SPG block performed during the study period. To test the hypothesis, only the initial procedure was used, as the Wilcoxon signed rank test assumes that all pairs are independent. However, the repeat procedures were still analyzed descriptively. The percent change in pain scores was calculated by subtracting the pre‐ and post‐procedure scores and dividing the difference by the baseline pain score.
The Wilcoxon signed rank test was used to test our hypothesis, due to the skewness of the distributions of pre‐ and post‐procedure pain scores as well as the ordinal nature of the scores. All statistical tests were 2‐tailed and a P value of .05 was considered statistically significant. The analysis was conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
A total of 88 Caucasian patients, 20 men and 68 women, were enrolled in this study. All patients were between the ages of 24-76 years. Patients underwent a total of 252 SSPGB procedures. The study included patients that had 1 or more episodes of intractable SM on different occasions.
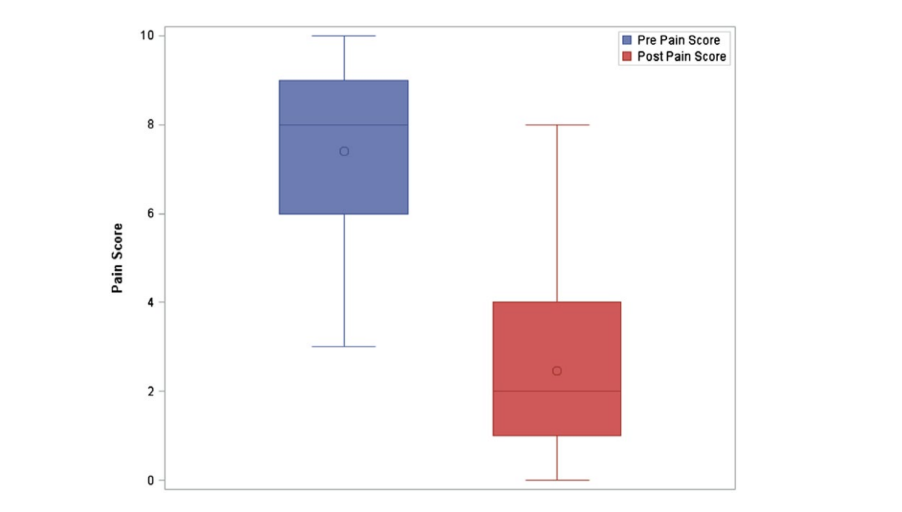
When analyzing each patient’s initial procedure only, the median Likert pain score pre‐procedure was 8 (IQR = 6‐9). The median post‐procedure Likert pain score was 2 (IQR = 1‐4), equating to an average of 67.2% (±26.6%) reduction in pain severity, across all patients for the initial SPG procedure. The mean improvement in Likert pain scale was 5 points (IQR = 6‐3). At 30 minutes following the injection, there was a statistically significant reduction in pain severity (P < .0001). Figure 3 shows the distributions of pre‐ and post‐procedure pain scores for initial procedures only. The boxplot reveals that pre‐procedure pain scores were higher than post‐procedure pain scores. Additionally, it shows that the distribution of pre‐procedure pain scores was skewed to the left, while post‐procedure pain scores were skewed to the right.
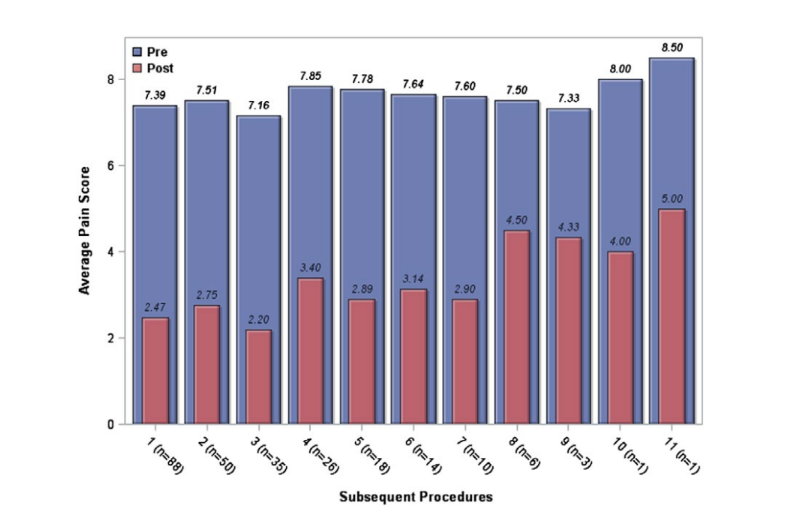
The majority of the sample (57%) received at least 2 blocks during the study period. Subsequent SSPGB were analyzed separately. When analyzing results of subsequent procedures, the median Likert pain score pre‐procedure in this group was 8. The median post‐procedure pain score was 3 (IQR = 1‐4). The median improvement in Likert pain scale was 4 points (IQR = −6 to −3), which resulted in an average improvement in pain of 64.1% (±27). Figure 4 shows that the efficacy of the SPG block did not diminish with repeated injections. Patients had a comparable reduction of pain severity with repeated injections.
There were no significant complications of the procedure. One patient developed a transient cranial nerve VI palsy that resolved completely in approximately 6 hours.
Discussion
Regional anesthetic block of the SPG utilizing the suprazygomatic approach presents an innovative and important option to abort SM in patients that fail to respond to standard prescription anti-migraine medications. The SPG has been known to play an integral role in the pathophysiology of facial pain and trigeminal autonomic cephalalgias, but its exact role in migraines is not well understood. In this study, we measured pre‐ and post‐procedure pain scores in patients with SM who received an SSPGB. At 30 minutes following treatment, the overall reduction of pain among patients was statistically significant. When we first started to perform the SSPGB in our headache clinic, our headache specialists noted that it immediately became a treatment of choice in patients with SM, obviating the need for use of ED or inpatient services. Most patients experienced immediate significant pain relief and on recurrent episodes of SM returned to clinic for repeat SSPGB treatment.
Cady et al demonstrated that repeated stimulation of the SPG over a period of 6 weeks leads to a reduction in migraine severity and duration in patients suffering chronic migraine, with a distinct patient population and pain syndrome different from that of our study. Six weeks, however, is a prolonged time period to reach therapeutic relief, particularly in patients with severe pain.
With proper identification of landmarks, this procedure can easily be performed in fewer than 2 or 3 minutes. This approach is well established with oral maxillofacial surgeons and is used in oral procedures. Additionally, Radder and colleagues noted greater benefit when using a suprazygomatic approach for maxillary nerve block for dental extractions than when using the infrazygomatic approach. The majority of their patients did not experience any significant discomfort with this approach. An infrazygomatic transcoronoid approach under fluoroscopy has been utilized by Tepper and colleagues when implanting neural stimulators for migraine headache; however, patients experienced significant discomfort and an alternative approach was considered. The SSPGB method utilized in our study provided a fast, effective, and safe, regional anesthetic block to the SPG.
Schaffer et al treated patients with migraines with intranasal SPG block in the ED. Although the study showed no significant reduction in pain when compared to placebo, there was persistent pain reduction 24 hours following treatment. This demonstrated that anesthetic block of the SPG continued to be effective in pain reduction for a period longer than the half‐life of the agent used. In comparison, pain reduction in our study reached statistical significance.
IV‐DHE is currently accepted by the community as the gold standard for aborting SM. In the original study with IV‐DHE, 49 out of 55 patients were completely pain‐free at 48 hours post‐treatment. Although these data would suggest that IV‐DHE is more effective, many patients in our practice were not responsive to DHE initially but rather responded to the treatment only if given over 3 to 5 days. In another study using IV‐DHE in the ED for SM, only 3 of 32 patients experienced a relief of symptoms after treatment and 15 patients reported residual severe headache.
The average cost of inpatient hospital stay ranges from $5000 to $7000 in pediatric patients treated with IV‐DHE, which is presumed to be comparable to that in the adult population. The cost of an SSPGB injection in our headache clinic is approximately $160. In patients with vascular, renal, hepatic, and cardiac disease, DHE is strongly contraindicated, whereas the SSPGB has no such contraindications.
A major limitation of our study is the lack of long-term follow‐up data on pain scoring. Our study assessed only immediate (30 minutes post‐ procedure) effects of the SPG block. We did not assess longitudinal data on the duration of pain relief and whether these patients experienced recurrent migraine in the days and weeks post‐procedure. This restrospective study was designed to look at the safety and immediate efficacy in providing pain relief in the setting of SM. A prospective study to evaluate the long-term effect of the SPG block is warranted. The current study did not control for any confounding factors such as pre‐procedure autonomic symptoms.
Most patients reported immediate relief of autonomic symptoms following the procedure, even prior to complete control of pain. Because the SPG plays a role in mediating the trigeminal‐autonomic reflex, a block may potentially alleviate autonomic symptoms, which was not systematically evaluated in our study. A third limitation is that our study was not placebo‐controlled.
Practitioners should exercise caution when performing an SPG block as there are several potential complications including abducens or facial nerve palsies. Injury of the maxillary artery, which is anatomically located close to the SPG, was not observed in our study. Radder and colleagues reported that there is no risk of maxillary artery injury using the suprazygomatic approach.
In our study, there was one reported adverse reaction of transient abducens nerve palsy. In our headache clinic, nearly 1000 SPG blocks were performed over the past 8 years, but data of pain levels pre‐ and post‐ procedure in SM patients were available in only 25% of the cases, with no facial nerve palsy or maxillary artery injury reported in that subgroup. Adverse effects were assessed immediately post‐procedure, at 30 minutes, and during follow-up visits. Patients were also asked to contact the provider and report any adverse reactions after discharge.
We do not feel that this is an overly aggressive or difficult procedure. It is not more difficult or painful for patients than an occipital or auriculotemporal nerve block or the botulinum toxin injection protocol for chronic migraine. In this study, the frequency of patients that underwent repeat blocks suggests that this procedure is well tolerated and produces significant pain relief. Most patients who had immediate pain relief following the procedure reported lasting benefit.
We plan to continue evaluating this technique with a prospective, randomized placebo controlled study to evaluate long‐lasting pain response to the SSPGB.
Conclusion
Patients with SM who received an SSPGB in our headache clinic experienced a statistically significant reduction in self‐reported pain over 30 minutes. SSPGB may provide a relatively inexpensive, therapeutic abortive therapy in patients with SM, which in turn would reduce the number of patients’ visits to the ED and need for hospitalization. Lack of adverse reactions to this treatment also increases the benefit‐to‐risk ratio. Additional trials are warranted to support these findings.
Dev Mehta, Megan C. Leary, Hussam A. Yacoub, Mohammed El‐Hunjul, Hope Kincaid, Vitaliy Koss, Katrina Wachter, Don Malizia, Barry Glassman, John E. Castaldo
References
- Adelsmann A, Saccomani P, Dreyer E, da Costa AL. Treatment of status migrainosus by general anesthesia: A case report. Braz J Anesthesiol. 2015;65:407‐410.
- Insinga RP, Ng‐Mak DS, Hanson ME. Costs associated with outpatient, emergency room and inpatient care for migraine in the USA. Cephalalgia. 2011;31:1570‐1575.
- Lucado J, Paez K, Elixhauser A. Agency for healthcare research and quality. Headaches in U.S. hospitals and emergency departments, 2008. Bethesda, MD: Healthcare Cost and Utilization Project. Statistical Brief #111. https://www.hcup-us. ahrq.gov/reports/statbriefs/sb111.pdf. Published May 2011. Accessed March 14, 2018.
- Piagkou M, Demesticha T, Troupis T, et al. The pterygopalatine ganglion and its role in various pain syndromes: From anatomy to clinical practice. PainPract. 2012;12:399‐412.
- Siéssere S, Vitti M, De Sousa LG, Semprini M, Iyomasa MM, Regalo SC. Anatomic variation of cranial parasympathetic ganglia. Braz Oral Res. 2008;22:101‐105.
- Yin W. Spenopalatine ganglion radiofrequency lesions in the treatment of facial pain. Tech Reg Anest Pain Manag. 2004;8:25‐29.
- Yarnitsky D, Goor‐Aryeah I, Bajwa ZH, et al. 2003 Wolff Award: Possibleparasympathetic contributions to the peripheral and central sensitization during migraine. Headache. 2003;43:704‐714.
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine— current understanding and treatment. N Engl J Med. 2002;346:257‐270.
- Goadsby PJ, Holland PR, Martins‐Oliviera M et al. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97:553‐622.
- Maizels M, Geiger AM. Intranasal lidocaine for migraine: A randomized trial and open‐label follow‐ up. Headache. 1999;39:543‐551.
- Cady R, Saper J, Dexter K, Manley H. A double‐ blind, placebo‐controlled study of repetitive transnasal sphenopalatine ganglion blockade with Tx360® as acute treatment for chronic migraine. Headache. 2015;55:101‐116.
- GraphPad Software, Inc., Analysis checklist: Wilcoxon matched pairs. Retrieved from: https://www.graphpad.com/guides/prism/7/statistics/index.htm?the_results_of_a_wilcoxon_matched_pairs_ test.htm. Published in 2017. Accessed March 14, 2018.
- Chiono J, Raux O, Bringuier S, et al. Bilateral suprazygomatic maxillary nerve block for cleft palate repair in children: A prospective, randomized, double‐blind study versus placebo. Anesthesiology. 2014;120:1362‐1369.
- Radder K, Shah A, Fatima S, Kothari C, Zakaullah S, Siddiqua A. Efficacy and feasibility of frontozygomatic angle approach for extra oral maxillary nerve block in oral surgery: A descriptive clinical trial. J Maxillofac Oral Surg. 2014;13:231‐237.
- Tepper S, Rezai A, Narouze S, Steiner C, Mohajer P, Ansarinia M. Acute treatment of intractable migraine with sphenopalatine ganglion electrical stimulation. Headache. 2009;49:983‐989.
- Schaffer JT, Hunger BR, Ball KM, Weaver CS. Noninvasive sphenopalatine ganglion block for acute headache in the emergency department: A randomized placebo‐controlled trial. Ann Emerg Med. 2015;65:503‐510.
- Neil R. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36:995‐997.
- Gallagher RM. Emergency treatment of intractable migraine. Headache. 1986;26:74‐75.
- Nelson GR, Bale JF, Kerr LM. Outcome and cost of inpatient hospitalization for intravenous dihydroergotamine treatment of refractory pediatric headache. Pediatr Neurol. 2017;66:76‐81.
