Efficacy of long-term low-dose macrolide therapy in preventing early recurrence of nasal polyps a er endoscopic sinus surgery
Background: This study assessed efficacy of clarithromycin “long-term” macrolide therapy as an adjunct to maintenance therapy with nasal corticosteroids to prevent recurrence of nasal polyps (NP) afier functional endoscopic sinus surgery (FESS).
Methods: A total of 66 patients with chronic rhinosinusitis and bilateral NP were randomized into 3 study arms, 22 patients in each arm. Afier FESS, patients in the first and second groups were treated with clarithromycin 250 mg/day for 12 and 24 weeks, respectively, whereas patients in the third group did not receive any clarithromycin. Patients in all 3 groups received maintenance therapy with mometasone furoate 400 μg/day. Patient assessment was conducted before the surgery and 6, 12, and 24 weeks after surgery, using a visual analogue scale (VAS), 20-item SinoNasal Outcome Test (SNOT-20), acoustic rhinometry, rhinomanometry, saccharin transit time, nasal endoscopy, computed tomography (CT) of paranasal sinuses, and measurement of the level of eosinophil cationic protein (ECP) in their nasal secretions.
Results: The study confirmed efficacy of “long-term” macrolide therapy, resulting in significant improvement of all parameters except acoustic rhinometry and VAS in both clarithromycin groups as compared to the control. Concentration of ECP in the nasal secretions increased dramatically afier surgery, then returned to baseline levels after 12 and 24 weeks of treatment with clarithromycin. In the control group, ECP level continued to increase and was significantly higher at the endpoint. Both groups with clarithromycin showed significantly be5er endoscopic and CT scores than the control group at the end point.
Conclusion: “Long-term” low-dose clarithromycin 250 mg/day is able to control eosinophilic inflammation and prevent early relapse of NP after FESS 2014 ARS-AAOA, LLC.
Chronic rhinosinusitis (CRS) often occurs in association with nasal polyps (NP). In 1 study CRS with NP (CRSwNP) was diagnosed in 4% of an entire population and is associated with bronchial asthma (BA) in 7% to 13% of cases. In addition, aspirin-exacerbated respiratory disease (AERD) and NP are present in a large number of patients (ranging, 36-96%). In CRSwNP the predominate inflammatory cell is the eosinophil, which is found in both the tissue and in the airway mucus in almost all patients with CRSwNP. Although the role of infection (neutrophilic inflammation) has been investigated it does not seem to be a primary factor in the development of CR-SwNP although CRSwNP is associated with BA. Allergy seems to be a comorbid condition and not a primary factor in the development of CRSwNP. At present, neither medical nor surgical treatment can ensure permanent control or enduring cure. Currently the only proven treatment for effective control of CRSwNP is topical nasal steroid sprays with or without systemic glucocorticosteroids (GCS). Recurrent CRSwNP is not always prevented even with systemic GCS and the side effects can be serious, including cataracts and vertebral collapse. Because disease control can be difficult even with systemic GCS we decided to study treatment with “long-term” therapy (3-6 months and more) using low dose macrolide antibiotics. Apparently it is the non-antimicrobial properties of the macrolides (erythromycin, roxithromycin, clarithromycin) that contribute to their anti-inflammatory effects, which includes inhibition of both neutrophilic and eosinophilic inflammation. The macrolides are capable of modulating the immune response, inhibiting polyp growth, destroying biofilms, and enhancing the protective properties of the respiratory tract mucosa.
Macrolide effectiveness in patients with CRS without NP has been confirmed; however, their efficacy in CRSwNP patients has not been thoroughly investigated. Therefore, our prospective randomized controlled study was designed to evaluate the efficacy and safety of a long-term course (3 and 6 months) of low-dose clarithromycin therapy in patients with CRSwNP after functional endoscopic sinus surgery (FESS).
Patients and methods
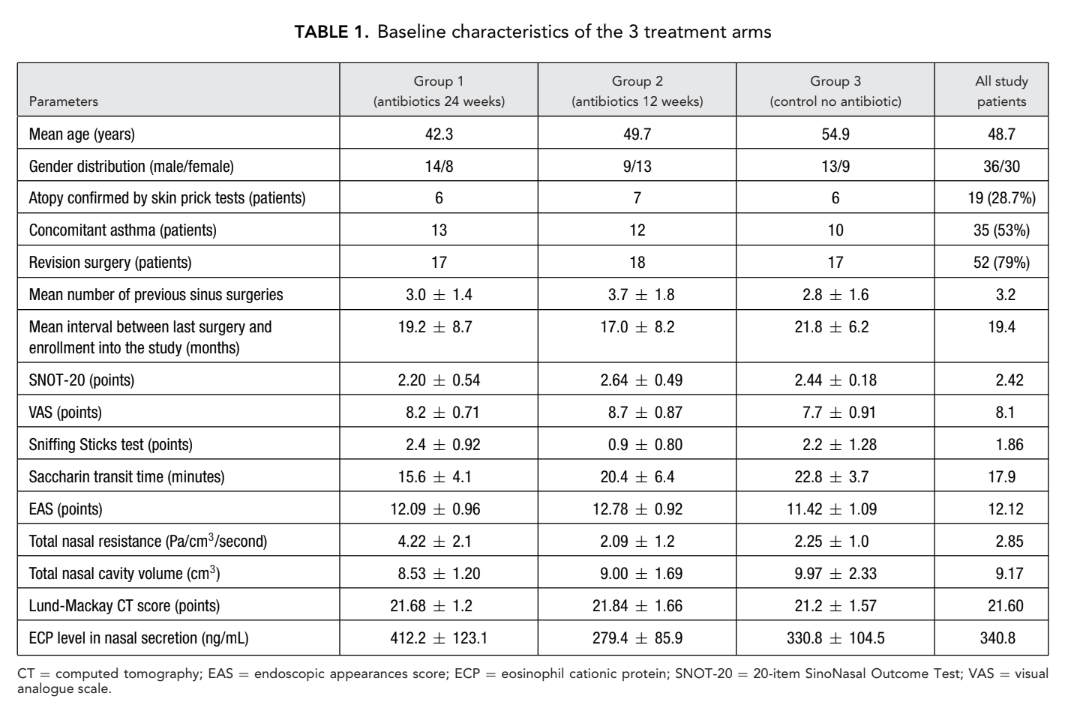
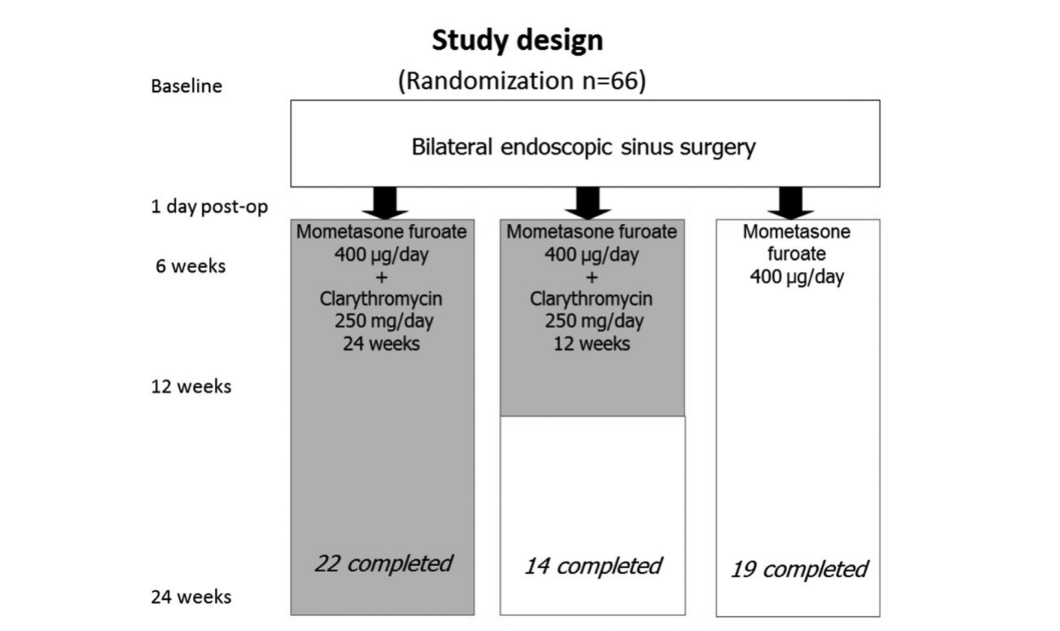
A total of 66 patients (36 men and 30 women) aged from 18 to 77 (average, 48.7) years with bilateral CRSwNP confirmed by endoscopy were recruited. The study period was from January 2008 to March 2011. All 66 patients were randomly assigned (sealed envelope system) to 1 of 3 study groups (22 patients per group) as follows: group 1 (antibiotics for 24 weeks); group 2 (antibiotics for 12 weeks); and group 3 (control, no antibiotics).
All 3 groups received topical nasal steroid spray (mometasone furoate), 400 μg/day for 24 weeks after FESS. The macrolide antibiotic used in both groups 1 and 2 was clarithromycin 250 mg/day (Klacid; Abbott Laboratories, Abbott Park, Illinois, USA). The majority of patients (79%) had at least 1 previous sinus surgery without long-term success; the mean number of previous surgeries in all 66 patients was 3.2 (Table 1).
Exclusion criteria included the following: unilateral CR-SwNP, macrolide intolerance, use of systemic steroids, pregnancy, lactation, and severe somatic diseases. We excluded all patients who were on systemic steroids because in Russia, systemic steroid therapy is reserved only for severe BA patients. It would be unethical to discontinue systemic steroids in these BA patients; in addition, systemic steroids could have a negative impact on reliability of our study results. The Ethics Committee of the Sechenov First Moscow State Medical University, required that all patients in all 3 groups maintain use of topical nasal steroids after FESS otherwise NP can rapidly reoccur.
After recruitment, all 66 patients underwent bilateral FESS performed by the same surgeon (senior author Lopatin A.). Patients in group 1 (antibiotics for 24 weeks) and group 2 (antibiotics for 12 weeks) began long-term therapy with clarithromycin 250 mg/day on the first post-operative day and over 24 weeks (6 months) or 12 weeks (3 months), respectively. On the 7th day after surgery, all patients began maintenance therapy with mometasone furoate nasal spray (Nasonex, MSD, Merck & Co., Inc., NJ, USA) 400 μg/day for a full 6 months, including group 3 (control no antibiotics) patients. The Ethics Committee approved the final study protocol with all enrolled 66 patients signing consent forms.
Patients were carefully followed and seen at 6 weeks, 12 weeks, and 24 weeks after FESS (Fig. 1). Results of treatment were based on evaluation of the following tests.
20-item SinoNasal Outcome Test (SNOT-20) The 20-item SinoNasal Outcome Test (SNOT-20) is a questionnaire for evaluating the quality of life (QoL) in rhinosinusitis patients.
Visual analogue scale
The disease severity was assessed by the patients’ subjective evaluation on the 10-cm visual analogue scale (VAS); 1 cm on the scale reflected 1 point of the patient’s assessment; 0 to 3 points corresponded to mild disease, 3 to 7 points corresponded to moderate disease, and 7 to 10 points corresponded to severe disease.
Olfactory test
The “Sniffin Sticks test,” extended version (Burghart Messtechnik GmbH, Wedel, Germany) was used for each patient. Odor identification was assessed by means of 16 test tubes with different odors. The number of correct answers was related to the degree of olfactory disturbance, with 16 being the maximum score possible. A score from 0 to 6 reflects anosmia, a score from 7 to 12 is termed hyposmia, whereas a score ranging from 13 to 16 is normal.
Saccharin transit time test
This test measures the transit time for a saccharin particle to pass from the anterior head of the inferior nasal turbinate to the pharynx when the patient first experiences the sensation of a sweet taste.
Nasal endoscopy
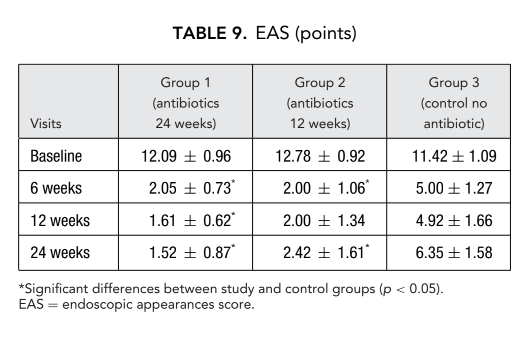
Endoscopy was performed with a rigid 2.7-mm endoscope (Karl Storz, Tutlingen, Germany) without decongestion. Semiquantitative scores were recorded for polyps, edema, discharge, crusting, and scarring at baseline, 6 weeks, 12 weeks, and at the final assessment 24 weeks after FESS. These results were evaluated using an endoscopic appearance score (EAS). Polyps were graded by size from 0 to 3 points; an absence of polyps was scored as 0, polyps appearing only in middle meatus were scored as 1; polyps extending beyond the middle meatus but not obstructing the nose were scored a 2; and polyps completely obstructing the nose were scored a 3. Discharge was scored as follows: 0 = no discharge; 1 = clear, thin discharge; and 2 = thick, purulent discharge. The mucosal edema was scored as follows: 0 = absent; 1 = mild; 2 = severe. Crusting and scarring in the postoperative cavity was scored as follows: 0 = absent; 1 = mild; and 2 = severe; these findings were evaluated for the right and left nasal cavity separately.
Active anterior rhinomanometry and acoustic rhinometry
The active anterior rhinomanometry (AAR) and acoustic rhinometry (AR) studies were carried out with the SRE 2000 device (Rhinometrics, Lynge, Denmark), which allowed performing both tests: AAR objectively assessed both nasal resistance and nasal airflow whereas AR reflected the geometry and the volume of the entire nasal cavity.
Multislice computed tomography Multislice computed tomography (CT) of the nose and paranasal sinuses was performed prior to and 6 months after FESS on all patients. One-half-centimeter (5 mm) slices in both the axial and coronal planes were obtained. The degree of opacification of a particular sinus (0-2) and the ostiomeatal complex (0 = intact, 2 = occluded) were calculated using the Lund-Mackay scoring system.
Eosinophil cationic protein
Eosinophil cationic protein (ECP) contents in the nasal discharge was measured using a collection kit with data generated by the automatic chemiluminescent analyzer IMMULITE 1000 (Siemens Healthcare Diagnostics Inc, NY, USA). To collect the mucus, a piece of sterile foam-rubber sponge measuring 20 × 20 × 5 mm was introduced into the middle meatus for 20 minutes. After removal, the sponge was placed in a 10-mL test tube and centrifuged for 3 minutes at a velocity of 4000 rpm allowing for collection of 0.2 to 1.0 mL of mucus for further analysis. The test sensitivity was 0.2 ng/mL.
Microbiological testing
Nasal swab for microbiological testing was collected from the middle nasal meatus prior to FESS and at all postoperative visits. Culture and sensitivity (resistance) testing was performed using bacteriological analyzer Walk Away-40 (Dade Behring, Marburg, Germany) and the disk diffusion method (Becton Dickinson discs with clarithromycin, USA). Results were estimated according to the CLSI (clinical and laboratory standards institute) recommendations.
Skin prick tests
Skin prick tests for indoor and outdoor allergens were performed in all patients using standard methods.
Final assessment of treatment results
Final assessment of treatment results was carried out using the changes of EAS and Lund-Mackay CT scores at 24 weeks after FESS.
Statistical analysis
After consulting a medical statistician, the results obtained were entered into a computerized database and processed using the statistical software package SPSS version 17.0 for Windows. Wilcoxon signed rank tests were performed to evaluate treatment effects at various time points. Values were presented as means ± standard deviations. Changes within and between groups were considered statistically significant when p values were <0.05.
Results
Eleven patients were dropped from the study for various reasons: 8 patients in group 2 (antibiotics for 12 weeks) were withdrawn. One patient developed abdominal pain after starting clarithromycin therapy; a second patient was withdrawn because of nightmares beginning 3 days after starting clarithromycin treatment. One female in the same group developed an exacerbation of erosive duodenitis 2 months after enrollment and was withdrawn whereas 5 others were withdrawn because of noncompliance. One female patient in group 3 (control no antibiotics) was excluded because of pregnancy and 2 others in this group were withdrawn because of noncompliance. Therefore, 55 patients completed the study and at the last visit all 22 patients in group 1 (antibiotics for 24 weeks) completed the study, 14 patients remained in group 2 (antibiotics for 12 weeks), and 19 patients remained in group 3 (control no antibiotics).
Thirty-five patients had BA, and 27 of these 35 patients presented with AERD. Atopy was confirmed by skin prick tests in 19 of the 66 initial patients. There were no significant differences between the 3 groups regarding age, sex, presence of atopy, severity of the disease, and number of previous surgeries, as well as all the other initial parameters that we examined. Baseline characteristics of each of the 3 treatment arms: group 1 (antibiotics for 24 weeks), group 2 (antibiotics for 12 weeks), and group 3 (control no antibiotics) are presented in Table 1.
Treatment results were better for patients completing the course of long-term clarithromycin treatment in group 1 (antibiotics for 24 weeks) and group 2 (antibiotics for 12 weeks) compared to patients in group 3 (control no antibiotics).
Statistically significant differences (p < 0.05) were obtained for all parameters (but not at every visit) between the study medication groups 1 and 2 and group 3 (control no antibiotics) with the only exception being for VAS and AR where statistically significant evidence was not achieved.
SNOT-20
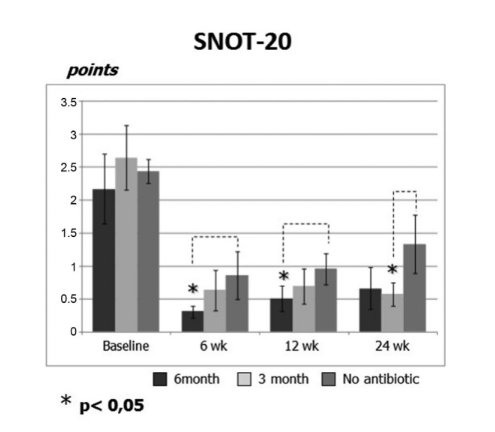
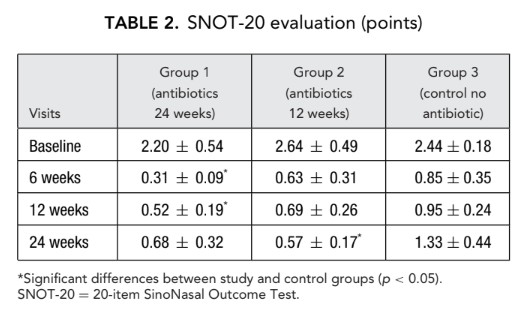
The initial SNOT-20 scores were 2.20 ± 0.54 in group 1 (antibiotics for 24 weeks), 2.64 ± 0.49 in group 2 (antibiotics for 12 weeks), and 2.44 ± 0.18 in group 3 (control no antibiotics). After the FESS, QoL improved and the severity of the rhinosinusitis symptoms was relieved in all study subjects. However, SNOT-20 scores of patients in group 1 (antibiotics for 24 weeks) at visits 6 weeks and 12 weeks after FESS (0.31 ± 0.09 and 0.52 ± 0.19, respectively) as well as SNOT-20 scores of patients in group 2 (antibiotics for 12 weeks) at the final visit (0.57 ± 0.17) were significantly better than the patients in group 3 (control no antibiotics) at the same visits; 0.85 ± 0.35, 0.95 ± 0.24, and 1.33 ± 0.44, respectively (p < 0.05), (Fig. 2, Table 2).
VAS scores
Differences in changes of VAS scores between the study (groups 1 and 2) and the control group (group 3 no antibiotics) did not reach statistical significance (Table 3).
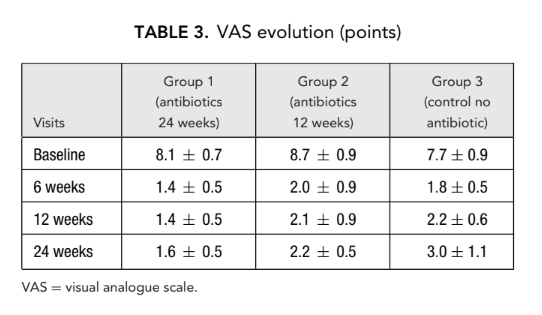
Olfactory test
Severe olfactory dysfunction was detected in all study subjects prior to FESS. Complete or almost complete loss of the ability to identify odors (anosmia) was noted when the nose was totally obstructed (blocked) by diffuse nasal polyps. None of these 66 patients received systemic steroids prior to FESS. At the second and third visits, olfaction significantly improved in all 3 patient groups, although no patients reached normal values. Statistically significant difference in the mean number of correct answers (p < 0.05) was revealed only between group 1 (antibiotics for 24 weeks) (9.5 ± 1.3) and group 3 (control no antibiotics) (4.4 ± 0.5) at the second visit 6 weeks after surgery (Table 4).
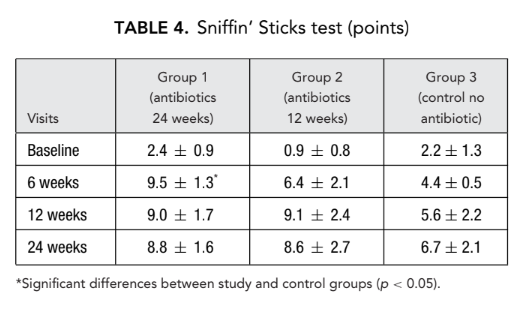
Saccharin transit time
Similarly, 12 weeks after surgery significant reduction (improvement) of the saccharin transit time was observed in group 1 (antibiotics for 24 weeks) (13.6 ± 3.0 minutes) as compared with group 3 (control no antibiotics) (21.0 ± 4.1 minutes) (Table 5).
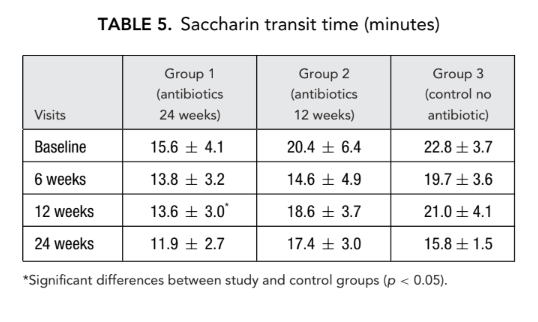
AR and AAR
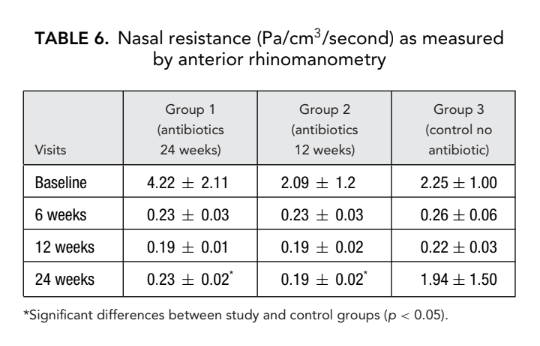
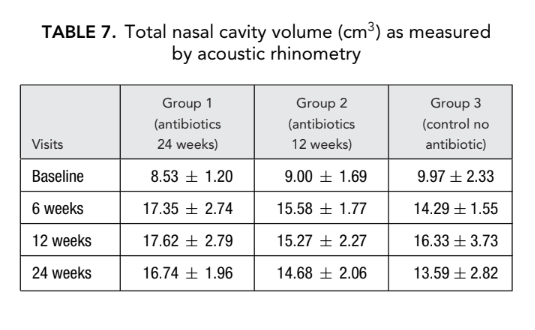
There was no significant difference in the acoustic rhinometry parameters (nasal cavity volume, minimal cross-sectional area) between all groups. However, nasal resistance measured by anterior rhinomanometry in group 1 (antibiotics for 24 weeks) and group 2 (antibiotics for 12 weeks) was significantly lower (better breathing) (0.23 ± 0.02 and 0.19 ± 0.02 Pa/cm3/second, respectively) than in group 3 (control no antibiotics) (1.94 ± 1.50 Pa/cm3/second) at endpoint (Tables 6 and 7).
CT scans
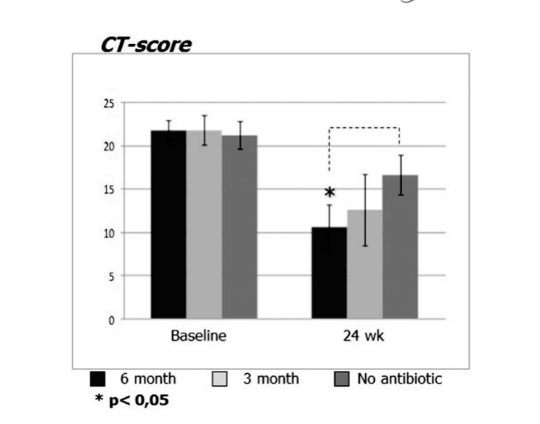
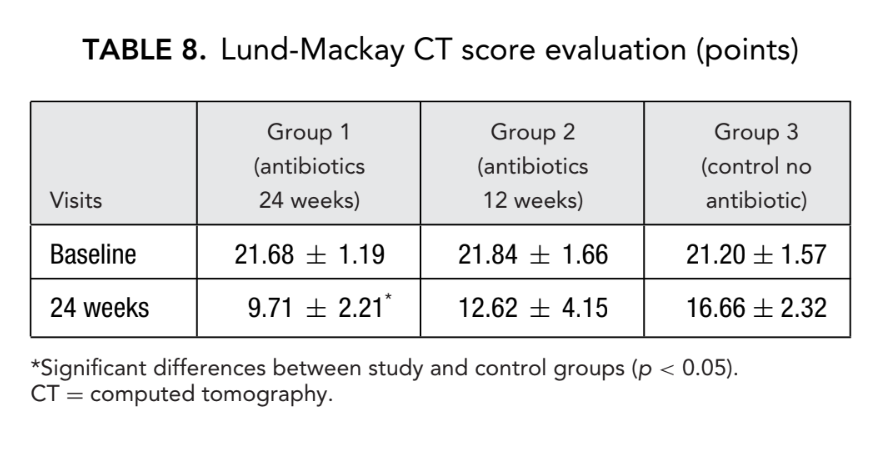
Before initiation of treatment, mean values for the Lund-Mackay score in the first, second, and third patient groups did not differ significantly, being 21.68 ± 1.20, 21.84 ± 1.66, and 21.2 ± 1.57, respectively. The mean score of paranasal sinus opacification on CT scans dramatically decreased (improved) in all 3 groups 6 months after FESS. However, a significant difference was observed only between group 1 (antibiotics for 24 weeks) with a mean score of 9.71 ± 2.21, and group 3 (control no antibiotics) with a mean score of 16.66 ± 2.32 (p < 0.05). In group 2 (antibiotics for 12 weeks) the mean score was 12.62 ± 4.15, but this difference did not reach statistical significance (Fig. 3, Table 8).
Nasal endoscopy
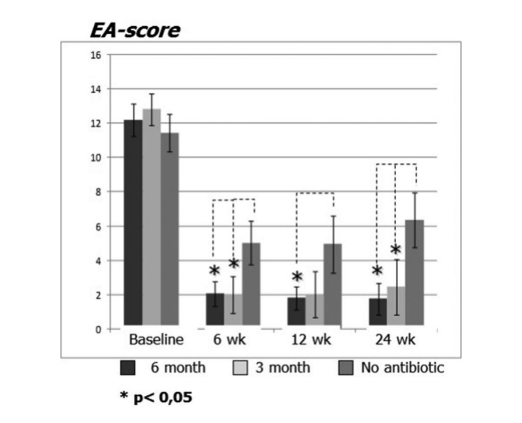
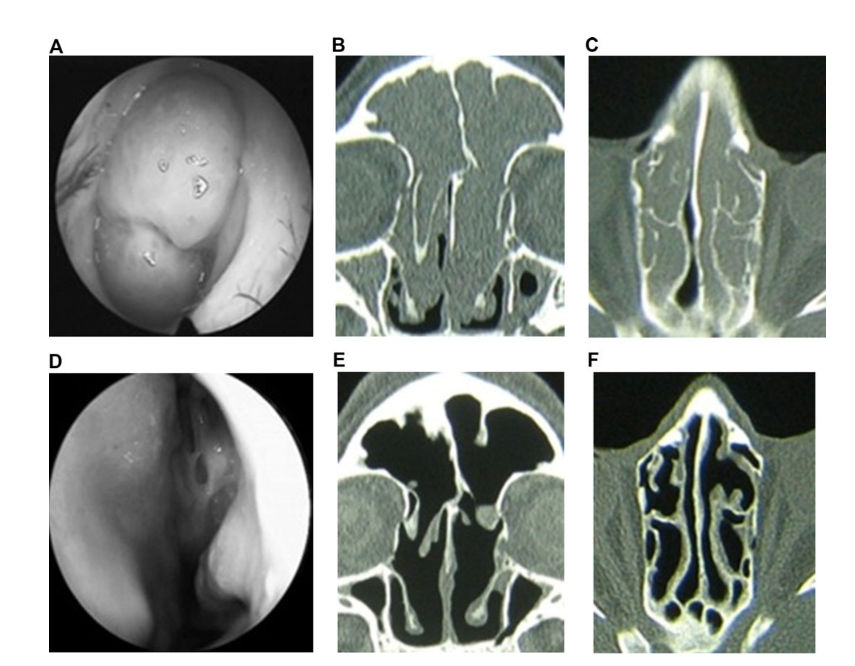
Patients on clarithromycin therapy in group 1 (antibiotics for 24 weeks) and group 2 (antibiotics for 12 weeks) showed better EAS at each visit when compared to patients in group 3 who did not take antibiotics (Fig. 4, Table 9). Twenty-four weeks after surgery mean EAS were: 1.52 ± 0.87 in group 1 (antibiotics for 24 weeks) and 2.42 ± 1.61 in group 2 (antibiotics for 12 weeks), and these results were significantly better than group 3 (control no antibiotics) 6.35 ± 1.58 (p < 0.05) (Fig. 4, Table 9). One of the most impressive cases demonstrating efficacy of postoperative long-term macrolide therapy is presented in Figure 5.
The most remarkable results occurred in the evaluation of ECP concentration postoperatively. Before the surgery, median values of ECP concentrations in all 3 patients groups did not differ significantly, being 412.2 ± 123.1, 279.4 ± 85.9, and 330.8 ± 104.5, respectively. Six weeks after surgery, the ECP level in the nasal discharge increased in all study patients, being 553.2 ± 115.5, 604.0 ± 173.2, and 660.0 ± 171.6 ng/mL in groups 1, 2, and 3, respectively. Twelve weeks after FESS, a significant decrease of the ECP level in the nasal discharge was clearly observed in group 1 (antibiotics for 24 weeks): 153.6 ± 98.8 ng/mL (p = 0.028), and in group 2 (antibiotics for 12 weeks): 290.4 ± 77.2 ng/mL (p = 0.036). ECP level in the nasal discharge in group 3 (control no antibiotics) patients did not change significantly and was recorded as 654.0 ± 184.9 ng/mL (p = 0.25). Only in group 1 (antibiotics for 24 weeks) did the ECP concentration remain at the same low level (154.8 ± 89.8 ng/mL) at 24 weeks. In group 2 (antibiotics for 12 weeks) there was a slight increase of the ECP levels up to 338.1 ± 83.1 ng/mL (p = 0.084) when these patients were studied at 24 weeks (3 months after stopping the antibiotics); however, with a p value of 0.084, the difference was not statistically significant. The mean ECP level in the nasal discharge in group 3 (control no antibiotics) rose significantly to 1000.0 ± 222.7 ng/mL (p = 0.041) (Fig. 6, Table 10).
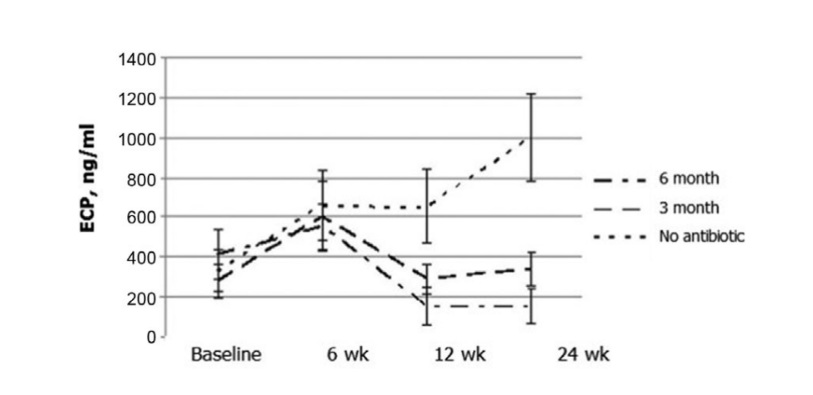
It is important to note that the ECP level in patients treated with the macrolides over a full 6 months (group 1) was significantly lower than in those patients in group 2, who stopped the antibiotic therapy after 3 months of treatment.
Side effects were uncommon with discontinuation of antibiotic therapy required in only 3 patients. Liver enzymes levels (alanine transaminase [ALT], aspartate aminotransferase [AST], and alkaline phosphatase [ALP]) remained normal in all patients.
Microbiological study of the swabs from the middle meatus revealed a wide spectrum of bacteria. The most common organism was Staphylococcus aureus (30% of patients), followed by Staphylococcus epidermidis (25%), Streptococcus haemolyticus (11%), Escherichia coli (9%), Pseudomonas aeruginosa (6%), and Enterobacter aerogenes (6%). Bacterial spectrum changed significantly after the surgery, but the proportion between clarithromycin-resistant strains (13%) and clarithromycin-sensitive strains (87%) remained the same. At the study conclusion, some macrolide-sensitive bacterial strains did acquired resistance to clarithromycin. Interestingly, an opposite phenomenon occurred as well; initially some bacterial strains resistant to macrolides were replaced by some bacterial strains sensitive to macrolides. In general, the number of clarithromycin-resistant strains (13%) remained constant throughout the course of long-term clarithromycin treatment.
Discussion
The current evidence supports the idea that long-term low-dose treatment with macrolides is effective when reserved for recalcitrant nonatopic CRS patients in whom topical nasal steroids and saline irrigations have failed to control symptoms. In atopic CRSwNP patients, BA and AERD macrolide therapy has not been useful.
In the more recent literature long-term low-dose macrolide therapy has been reported to be effective in CRS, including those patients with elevated immunoglobulin E (IgE) levels and BA. A recent prospective study demonstrated that an 8-week course of clarithromycin therapy was equally effective in both atopic and nonatopic patients with nasal polyposis. In a retrospective study, CRS patients with atopy responded well to long-term macrolide treatment, whereas patients who smoked had the poorest treatment outcome.
The results of our study demonstrated that long-term low-dose macrolide therapy prevents early recurrence of nasal polyps after FESS, including patients with atopy and BA. There was a clear correlation between atopy and the severity of disease because our atopic patients had higher VAS scores (Spearman’s rank correlation coefficient 0.332; p = 0.01). The presence of atopy correlated with higher ECP levels in the nasal discharge (0.834; p = 0.01). These findings suggest that symptom severity is directly related to the intensity of the eosinophilic inflammation. Unfortunately, we did not investigate total IgE levels in a majority of patients; therefore, subgroup analysis of those patients with low IgE levels was not possible.
A most remarkable study finding was the changing ECP levels in the nasal secretions after FESS and during the post-operative period with macrolide therapy. In group 3 (control no antibiotics) we noted an almost 3-fold increase of the mean ECP level 6 weeks after surgery. This finding of an elevated ECP level after FESS in these group 3 patients reflects an exacerbation of the eosinophilic inflammation caused by the surgery that could not be adequately controlled with intranasal topical steroids alone. On the other hand, in treatment groups 1 (antibiotics for 24 weeks) and 2 (antibiotics for 12 weeks) there was a gradual decrease of the ECP levels with long-term low-dose clarithromycin treatment, reflecting control and reduction of the eosinophilic inflammation.
One goal of this work was to study the efficacy of a longer (6 months) treatment course when compared to the relatively short (3 months) course of low-dose macrolide therapy. The data suggested some benefit from a longer antibiotic course but the difference between group 1 (antibiotic 24 weeks) and group 2 (antibiotic for 12 weeks) failed to reach statistical significance in the majority of patients. Nonetheless, there is some evidence that a longer duration of treatment group 1 (antibiotics 24 weeks) appears to be more effective than a shorter course seen in group 2 (antibiotics for 12 weeks).
The CT scores for group 1 (antibiotics 24 weeks) patients was 9.71 ± 2.21, which was significantly lower than in group 3 (control no antibiotics), with readings of 16.66 ± 2.32 at endpoint. The difference between group 2 (antibiotics for 12 weeks) and group 3 (control no antibiotics) did not reach statistical significance (Table 8). There were significant differences in the mean ECP levels in groups
1 and 2 at the endpoint, indirectly confirming that a 6-month antibiotic course reduces the eosinophilic inflammation, thereby preventing early recurrence of nasal polyps. Obviously, oral steroid use would certainly be considered the standard of care in the United Sates and some other Western countries, especially in the setting of postoperative eosinophilic inflammation flare-up. However, in countries such as Russia where systemic corticosteroid therapy in CRSwNP is extremely uncommon, long-term macrolides therapy might be an alternative option because it carries less risk of systemic side effects.
We did not find an increase in macrolide resistant bacterial cultures from the middle meatus after a long-term, low-dose course 250 mg/day of clarithromycin therapy, agreeing with previous studies that also failed to find resistant microorganisms after long-term treatment with azithromycin and erythromycin. Of course, the risk of developing antibiotic-resistant strains of bacteria induced by the long-term macrolide therapy is always possible.
Although a placebo arm was not designed into our protocol and patients were not blinded when receiving additional therapy, we evaluated 6 different objective methods in all 3 study groups. All investigators were blinded when evaluating and grading the results of nasal endoscopy, CT scans, and all other tests. A future randomized double-blind placebo-controlled study, with a large sample size, would be required to determine the efficacy of long-term macrolide therapy, particularly in preventing the recurrence of nasal polyps after FESS. In addition, such a study could hopefully predict which CRSwNP patients would benefit from long- term antibiotic treatment and if this treatment increases the risk of inducing significant bacterial resistance.
Conclusion
Results of this study demonstrated the efficacy and relative safety of long-term (6 months) low-dose (250 mg/day) macrolide (clarithromycin) therapy for preventing early recurrence of nasal polyps in patients with CRSwNP after FESS. Despite limited clinical data, our evidence suggests that patients with recurrent CRSwNP (surgical failures) deserve a trial of low-dose clarithromycin treatment (250 mg daily for 3-6 months), which may be initiated immediately after FESS along with maintenance therapy using topical nasal steroids.
References
- Hedman J, Kaprio J, Poussa T, Nieminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717–722.
- Settipane G. Epidemiology of nasal polyps. In: Settipane G, Lund VJ, Bernstein JM, Tos M, eds. Nasal Polyps: Epidemiology, Pathogenesis and Treatment. Providence, RI: Oceanside Publications; 1997:17– 24.
- Ogino S, Harada T, Okawachi I, Irifune M, Matsunaga T, Nagano T. Aspirin-induced asthma and nasal polyps. Acta Otolaryngol Suppl. 1986;430:21– 27.
- Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps group. European Position Paper on Rhinosinusitis and Nasal Polyps 2007. Rhinol Suppl. 2007;(20):1–136.
- Desrosiers MY, Kilty SJ. Treatment alternatives for chronic rhinosinusitis persisting after ESS: what to do when antibiotics, steroids and surgery fail. Rhinology. 2008;46:3–14.
- Kudoh S, Uetake T, Hagiwara K, et al. Clinical effect of low-dose, long-term erythromycin chemotherapy on diffuse panbronchiolitis (English Abstract). Jpn J Thorac Dis. 1984;25:632–642.
- Kudoh S, Asuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998;157:1829– 1832.
- Cervin A, Wallwork B. Macrolide therapy of chronic rhinosinusitis. Rhinology. 2007;45:259– 267.
- Cervin A, Wallwork B, Mackay-Sim A, Coman WB, Greiff L. Effects of long-term clarithromycin treatment on lavage-fluid markers of inflammation in chronic rhinosinusitis. Clin Physiol Funct Imaging. 2009;29:136–142.
- Stjarne P, Ollson P, Alenius M. Use of mometasone furoate to prevent polyp relapse after endoscopic sinus surgery. Arch Otolaryngol Head Neck Surg. 2009;135:296–302.
- Piccirillo J, Merritt M, Richards M. Psychometric and clinimetric validity of the 20-item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg. 2001;126:41–47.
- Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–243.
- Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184.
- Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012.
- Rhinol Suppl. 2012;(23):3 p preceding table of contents, 1–298.
- Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006;116:189– 193.
- Haruna S, Shimada C, Ozawa M, Fukami S, Moriyama H. A study of poor responders for long-term, low-dose macrolide administration for chronic sinusitis. Rhinоlоgy. 2009;47:66–71.
- Videler WJ, Badia L, Harvey RJ, et al. Lack of efficacy of long term, low-dose azithromycin in chronic rhinosinusitis: randomized controlled trial. Allergy. 2011;66:1457–1468.
- Peric A, Vojvodic D, Baletic N, Peric A, Miljanovic O. Influence of allergy on the immunomodulatory and clinical effects of long-term low-dose macrolide treatment of nasal polyposis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154:327–334.
- Videler WJ, van Hee SM, Reinartz C, Georgalas FW, Meulen K, Fokkens WJ. Long term, low-dose antibiotics in recalcitrant chronic rhinosinusitis: a retrospective analysis. Rhinology. 2012;50:45–55.
- Cervin A, Kalm O, Sandkull P, Lindberg S. One-year low-dose erythromycin treatment of persistent chronic sinusitis after sinus surgery: clinical outcome and effects on mucociliary parameters and nasal nitric oxide. Otolaryngol Head Neck Surg. 2002;126:481–489.
