Temporomandibular joint arterial variability
Abstract
The study aimed to investigate temporomandibular joint (TMJ) arterial variability. In this prospective study, the vasculature variability was studied using a 3D volume rendering CT angiography including random patients at two hospitals. A 16-quadrant (A1-D4) evaluation grid was developed using the Frankfurt plan as main reference. For each quadrant, the number of arterial ramus or branches was scored as clearly visible (2), partially visible (1), or not visible (0).
A total of 50 patients were enrolled (mean age of 62.9 ± 16.0); 21 (42%) were men, and 29 (58%) were women. The authors observed bilaterally higher arterial density in the posterior aspect of the ascending ramus of the mandible (p < 0.0001), corresponding to quadrants B2 (5.92 ± 2.27 and 6.14 ± 2.56), B3 (9.76 ± 2.97 and 11.18 ± 2.86) and B4 (7.38 ± 2.78 and 8.10 ± 2.42). A strong correlation was found between the number of vessels and the variability of the region (r ¼ 0.87, p ¼ 0.00001). No differences were observed between men and women.
Within the limitations of the study, arterial variability was observed in the TMJ territory. The posterior zone of the condyle and ramus is the most vascularized area, with great variability, representing an increased risk for surgical bleeding. Therefore, this knowledge seems to be particularly relevant for surgeons dedicated to TMJ and other facial surgery or facial/cerebral radiologic interventions. The authors encourage future studies to include larger samples and to identify thoroughly the arterial branches in this area.
Introduction
Anatomical vascular variations are an important part of medical knowledge, with special interest in surgical and medical specialties with interventional actions. In the head and neck context, those vascular variations can have clinical and surgical implications in different areas, including facial surgery, facial trauma, arteriovenous malformations management, interventional and vascular radiology procedures, facial reconstructions with possible vascular anastomosis and cosmetic interventions.
The temporomandibular joint (TMJ) anatomic region presents a complex arterial vascularization centered on the branches of the external carotid artery (ECA). Literature is not consensual regarding the normal arrangement of vascularization in this territory and the existence of arterial anatomical variations (Toure, 2018).
In 1978, Godlewski et al. (1978) found that the blood supply in the TMJ area is mostly carried out by the Superficial Temporal Artery (STA), the Anterior Tympanic Artery (ATA), and the Deep Posterior Temporal Artery (DPTA). The Deep Auricular Artery (DAA), the Transverse Facial Artery (TFA) and the Middle Meningeal Artery (MMA), presented greater variation (Godlewski et al., 1978). A recent article verified that the blood supply of the TMJ is circumferential, concluding that all arteries within a radius of 3 cm contributed to the vascularization of the TMJ through the emergence of secondary capillaries that branch out to surround the capsule joint (Cuccia et al., 2013). Moreover, it was also described that most of the vascular supply appeared to come from the lateral and medial aspect of the condylar head. The STA, the Internal Maxillary Artery (IMA), the Inferior Alveolar Artery (IAA), and MMA were depicted in all cases. The Transverse Facial Artery (TFA), the Masseterine Artery (MTA), the ATA, and the condylar branch of the STA were described with a frequency of 70%, 60%, 60%, and 50%, respectively (Cuccia et al., 2013).
A clinical case by Ezure et al. (2011), described a complete absence of the Facial Artery (FA), drawing attention to the implication of this anatomical variation in clinical practice. The FA is commonly used in chemotherapy treatments for head and neck cancer and in microvascularized flaps for complex facial reconstructions (Shimizu et al., 1990); thus, it is important to know the anatomical variations of this artery.
Knowledge of these anatomical variations has great importance in TMJ surgeries, parotid gland surgery, facial aesthetic surgeries, interventional radiology, imaging, and facial reconstructions, as well as in preventing vascular injuries associated with arterial trauma (Cillo et al., 2005; Vesnaver, 2020; Gerbino et al., 2021; Cooney et al., 2020; Mao et al., 2021).
Three-dimensional (3D) volume rendering is a promising non-invasive technique used to evaluate intracranial vascularity (Sparacia et al., 2007; Cascio et al., 2020). This technique successfully delineated the TMJ vascular supply anatomy with good resolution and detail (Cuccia et al., 2013).
The main purpose of this study was to analyze the arterial variability in the TMJ area. From our knowledge, no previous study has reviewed that topic.
Study design
A prospective study was designed to analyze the TMJ arterial variability of individuals submitted to contrasted angiography CT in the context of the Portuguese cerebral stroke program (“Via Verde do AVC”), during the period from December 1, 2019 to January 31, 2020 in the Neuroradiology departments of the following centers:
Centro Hospitalar e Universita´rio de Lisboa Central (CHULC) and São João University Hospital Center (CHSJ).
The study was authorized by the Ethics Committees and Boards of Directors of the different institutions involved (CHSJ-nº 427/19 and CHULC - 800/2019). Criteria for study inclusion and exclusion are represented in Table 1.
Imaging protocol
The first hospital, CHULC, used a GE LightSpeed 64 Scanner with a helicoidal scan type and a GE BrightSpeed 16 Scanner with an axial scan type. The contrast solution used during the procedure was a non-ionic iodinated agent: Iomeprol 175 g iodine. The second hospital, CHSJ, used a Philips Tomoscan Brilliance 16 scan and two contrast agents: Iohexol and Iomeprol.
Analysis of arterial variability in the TMJ territory
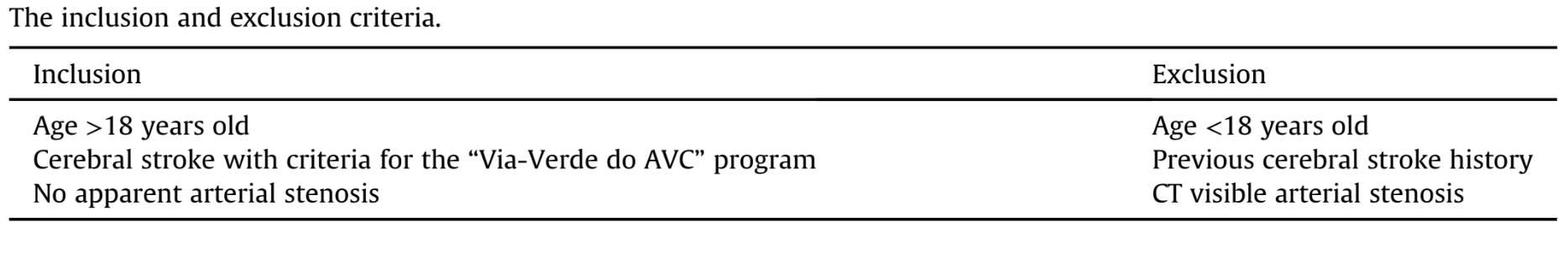
To assess the arterial anatomy of the temporomandibular region, the authors developed a grid with 16 quadrants. The Frankfurt Plan (FP) (Taub, 2007) was used as a main reference for these quadrants. A second horizontal line (b) was drawn parallel to the FP, using a tangent to the sigmoid notch as a reference. The third horizontal line (c), also parallel to the FP, presents a tangent to the lower edge mastoid part of the temporal bone. The fourth horizontal line (d) parallel to the FP follows a tangent to the upper surface of the C2 vertebra's spinous process. Finally, the last horizontal line (a) is a line parallel to the FP that is halfway between the FP and the second line (b). The vertical lines are all perpendicular to the FP. The first vertical line (e) is a tangent to the concavity of the anterior edge of the ascending mandible ramus, the second vertical line (f) is tangent to the maximum convexity of the mandibular notch, and the third vertical line (g) is tangent to the anterior ridge of the mandibular condyle. The fourth (h) and fifth lines (i) are tangent, respectively, with the anterior and posterior edge of the external auditory canal (Fig. 1).
The CT images, in DICOM format, were integrated on the Horos™ software and submitted to a 3D rendering with the following parameters: for the images obtained from the CHULC, it was set to a Window Level (WL) of 257, a Window Width (WW) of 296, the CLUT “VR Red Vessels (8-bit)”, opacity of inverse logarithmic table, standard shadows, without filter, parallel projection, and 0% background for red, green and blue; for the images obtained from CHSJ, it was set to a WL of 140, a WW of 120, the CLUT “VR Red Vessels (8- bit)”, opacity of inverse logarithmic table, standard shadows, without filter, parallel projection, and 0% background for red, green, and blue. These parameters were chosen to simulate an external light source. This made possible to obtain images with a more realistic view of the anatomical structures and easier to isolate the bone and vascular structures (Cuccia et al., 2013).
The different quadrants were drawn on the software using the references described and were subsequently analyzed individually, using the following classification system: each arterial branch found in a quadrant was categorized as clearly visible (2 points), partially visible/only visible for a short trace (1 point), or not visible (0 points) (Takagi et al., 1998). For each set of CT images of an individual, two tables were established: one for the right TMJ and another for the left TMJ. These tables correspond to the quadrants previously defined (Fig. 1).
Statistical analysis
After the arterial evaluation of each quadrant, a number was determined by calculating the sum of the values attributed to each vessel observed in that area. Then, an average value and a standard deviation (SD) were calculated for each quadrant on both sides. Normality of data was verified for all tests. All tests were performed for each hemiface. A comparison of quadrants’ average values was performed using a Kruskal-Wallis test. Since the variability is directly associated with the SD, for each one obtained, a 95% confidence interval (a ¼ 0.05) was calculated. Assuming that "s" is the SD, "n" is the number of observations (50 in each quadrant of each hemiface), and "a" is the level of significance, it was considered that the anatomical variability of a quadrant would be significant from a clinical point of view if the lower limit value of the 95% confidence interval for its SD was greater than 2. The value of 2 was equivalent to a clearly visible arterial branch or two partially visible vessels. This rule was called minimum variability (VM). A correlation of average scores with SD was obtained using the Pearson test. Subsequently, a comparative analysis of the data obtained between men and women was performed using the Mann-Whitney U non-parametric distribution test. The null hypothesis was defined as having no statistically significant difference between the sexes, while the alternative hypothesis was defined as having this said difference.
Results
A total of 50 patients (29 male and 21 female), aged between 24 and 87 years, were included in this study. Female patients had a mean age of 61.1 ± 18.8 (mean ± SD), and males had a mean age of 65.3 ± 11.5 (mean ± SD).
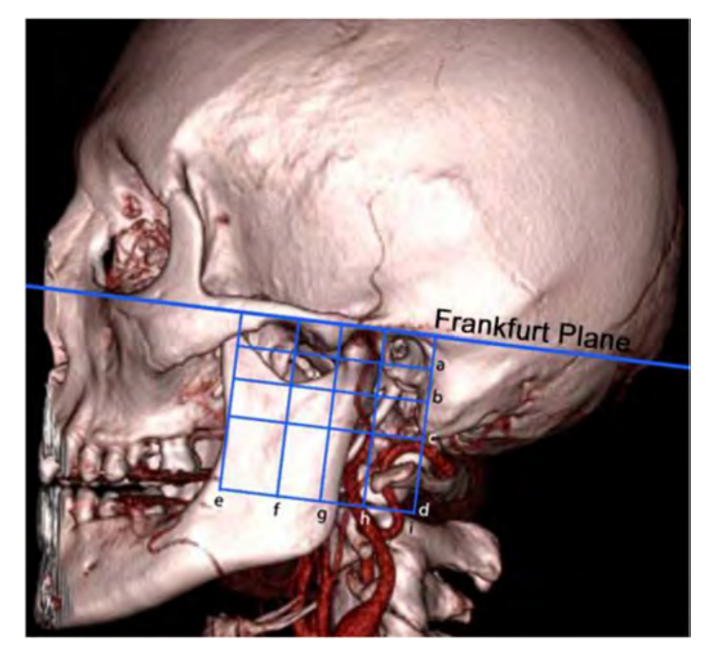
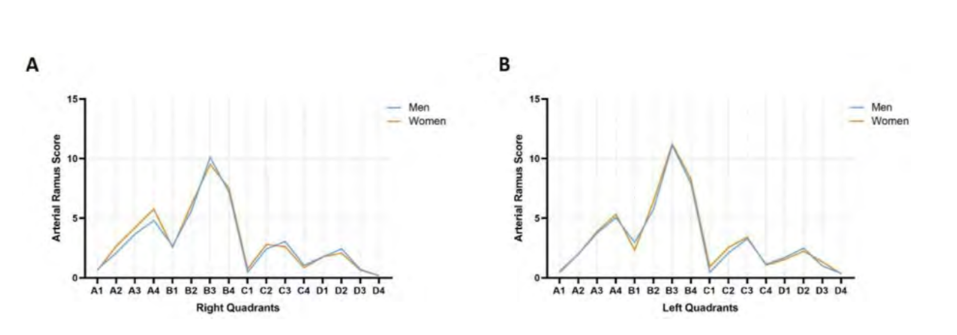
The arterial ramus scores, in accordance with the classification system, obtained in the right and left hemiface are represented in Fig. 2. Bilaterally, the quadrants B3, B4 and B2 had statistically significant greater vascular density (p < 0.0001). Also bilaterally, the quadrant D4 had statistically significant reduced arterial density (p < 0.0001).
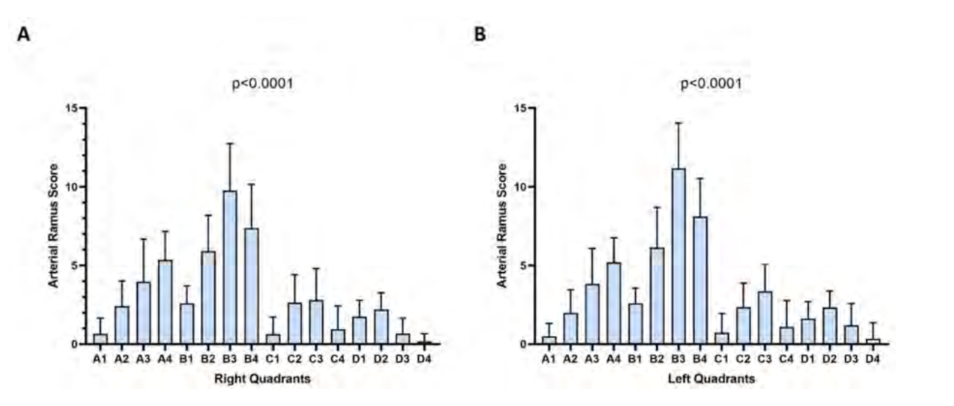
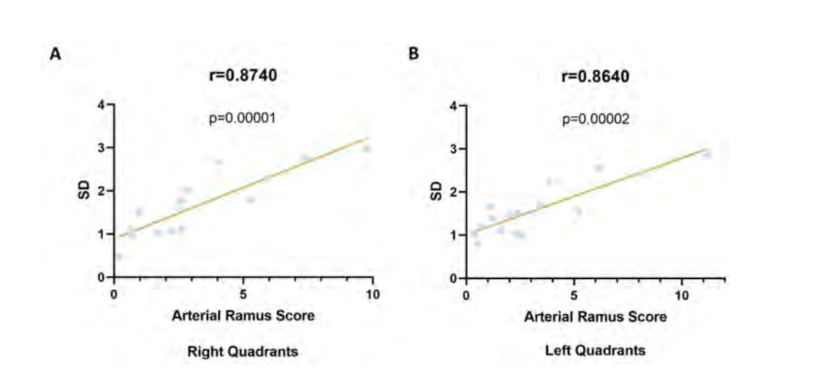
The 95% SD confidence intervals applied the established VM rule (lower limit value of the 95% confidence interval for its SD was greater than 2 mean score). In the right hemiface, quadrants A3 (SD = 2.25 - 3.35), B3 (SD = 2.48 - 3.7), and B4 (SD = 2.33 - 3.47) are the only ones that present significant arterial variability (Fig. 3A). Similarly, in the left hemiface, the quadrants B2 (SD = 2.14 - 3.19), B3 (SD = 2.39 - 3.57), and B4 (SD = 2.03 - 3.02) have significant variability (Fig. 3B). The confidence intervals of the quadrants B2 (SD = 1.89 - 2.82) and A3 (SD = 1.87 - 2.78) on the right and left hemiface, respectively, have considerably high deviations; however, they encompass the value defined in the VM rule, which means their variability cannot be considered significant. Moreover, a Pearson correlation analysis of the SD with the arterial ramus scores allowed us to verify that quadrants with larger SDs correspond to those with higher arterial ramus values in the right (r = 0.87 P = 0.00001, Fig. 4A) and left hemiface (r = 0.87 P = 0.00002, Fig. 4B); this indicates that these quadrants are more vascularized. Similarly, quadrants with smaller SDs have a reduced vascularization (Fig. 4).
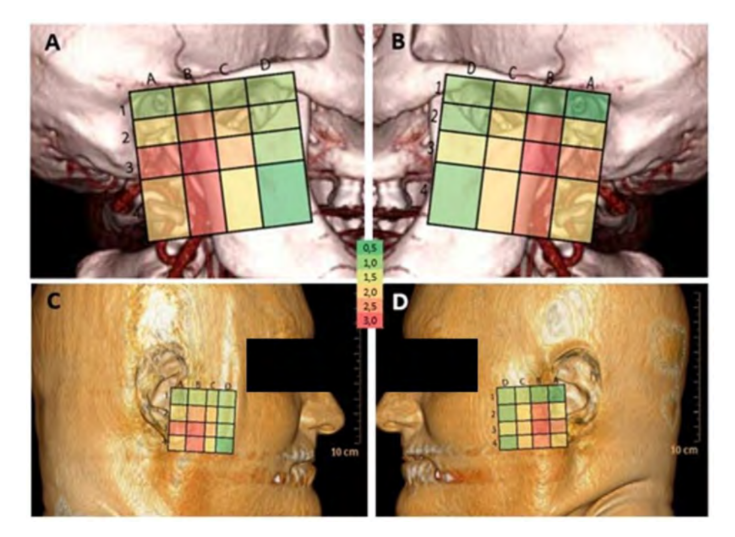
To facilitate the visualization of the variation in blood supply between all quadrants and the anatomical areas to which they correspond, a map was created (Fig. 5) where the warmer colors correspond to a greater variation in arterial anatomy (higher SD value) and colder colors correspond to a lesser variation.
The analysis between the two sexes showed that there are no significant variability differences in each hemiface. The values obtained from the test did not allow rejection of the null hypothesis-meaning the groups are similar for p > 0.05 (Fig. 6).
Discussion
The TMJ is a joint located in a highly vascularized region. Knowledge of vascular variation of this region can contribute to a better understanding of the diseases associated with this joint and their treatments. A 3D volumetric rendering has been used to study the cervicocraniofacial arteries in large (Sparacia et al., 2007) and small caliber (Cuccia et al., 2013) studies. However, analyses of the blood supply distribution in the TMJ area are relatively rare.
The present study showed that 3D volume rendering of CT angiography applying the FP can be effectively used to investigate the variability of the arterial regions surrounding the TMJ region.
The regions delimited by the quadrants (B3, B4), which encompass the posterior aspect of the ascending ramus of the mandible, are the most vascularized and have greater vascular variability (Figs. 2 and 3). Additionally, a correlation between the existence of a greater number of vessels and higher variability and vice versa was found (Fig. 4). This is the result of several factors. The anatomical variations of the mandible itself (Alomar et al., 2007) and the surrounding bone structures can influence the perception of vascular variations by changing the layout of the quadrants created by the authors (Fig. 6). The description of unilateral absences of certain vessels may have been one of the reasons for the bilateral differences found (Ezure et al., 2011). Wasicky and Pretterklieber (2000) described that the origin of ATA varied widely and surprisingly presented laterality. In the same article, 9 cases are also mentioned in which the ATA is depicted in duplicate, as well as 1 case with a triple presentation. Taking this information into account, we can better understand the reason for the greater variability found in these quadrants.
The anatomical territory of the TMJ is also used by several specialties in their surgical methods. Of the different approaches described for TMJ surgeries, the preauricular (Tauro et al., 2020; Vesnaver, 2020; Gerbino et al., 2021; Luo et al., 2021) and endaural (Assef et al., 2019) stand out; both are relatively close to the quadrants addressed in this study. In a recent study of surgical complications in TMJ arthroscopy, the extra-articular bleeding in the posterior puncture was 6.1%, versus 0% in the anterior puncture, supporting the posterior area have an increased risk for bleeding
(Ângelo et al., 2021). Parotid (Kim et al., 2014) and some facial aesthetic surgeries (Giotakis et al., 2020) also involve incisions in the same territory. In all these surgeries, there are associated risks. These include anesthetic, infectious, neurological, otological, and vascular complications, as well as instrumental failures and inflammatory problems (Ishida et al., 2015). Hemorrhages and post-surgical hematomas are common consequences of procedures in this highly vascularized territory (Holmlund et al., 1985). Talebzadeh et al. (1999) reported there are several vascular and nervous structures close to the medial region of the TMJ, which can increase the risk of hemorrhage and neurological damage. The results of this study reinforce for TMJ surgeons that this region presents variability in blood supply and a personalized study for vascular profile could decrease surgical complications.
The main limitations of this study were: 1) the wide range of ages presented by the individuals; 2) the difference in size and area of the same quadrant in different individuals, derived from the anatomical differences in the bone structures used for reference; and 3) the involvement of the “Via Verde do AVC” program in obtaining the images 4) absence of calculation of inter-rater and intra-rater reliability. The “Via Verde do AVC” program, active in Portugal since 2005 (Silva and Gouveia, 2012), is applied to every patient who shows signs of cerebrovascular accident (CVA, stroke) and seeks help from the national health services. This program facilitated the use of CT images for this study; however, it also hindered both selection and evaluation of these images. Additionally, most of these patients have vascular pathology, such as cervical arterial tortuosity or atherosclerotic stenosis of carotid bifurcations, that could result in artifacts during CT angiography acquisition.
Future research can give more robust data to complete the results of this study by increasing the sample size, encompassing a diverse group of individuals, not inquiring about the individual's ethnicity due to privacy reasons in data analysis, and eliminating all the possible vascular changes due to the suspicion of CVA. Since only a small number of similar studies exist, an improvement of any of these factors would contribute to more reliable and representative results in a future study. Usually, the sample selected in studies of the same scope is a set of cadavers, with tests and evaluations being made through the dissection of the anatomical structures (Alomar et al., 2007) and histological analysis of the tissues (Siéssere et al., 2008).
Conclusion
The 3D volume rendering CT angiography has shown great promise to evaluate the arterial variability in TMJ territory. The posterior zone of the condyle and ramus is the most vascularized area, with great variability, representing an increased risk for surgical bleeding. TMJ surgeons among others, must be aware of these considerations, because most of TMJ surgical techniques are performed in this territory.
Authors: David Faustino Ângelo, Jonatas Nogueira, Carolina Pinheiro, Gonçalo Alves, Henrique José Cardoso
References:
- Alomar, X., Medrano, J., Cabratosa, J., Clavero, J.A., Lorente, M., Serra, I., Monill, J.M., Salvador, A., 2007. Anatomy of the temporomandibular joint. Semin. Ultrasound CT MR 28, 170-183.
- Ângelo, D.F., Araújo, R.A.D., Sanz, D., 2021. Surgical complications related to temporomandibular joint arthroscopy: a prospective analysis of 39 single-portal versus 43 double-portal procedures. Int. J. Oral Maxillofac. Surg. 50, 1089-1094.
- Assef, C.A.N., Carvalho, P.H.A., Guerra, R.C., 2019. Arthroscopically-assisted short endaural approach for anchorage of the disc of the temporomandibular joint. Br. J. Oral Maxillofac. Surg. 57, 93-94.
- Cascio, F., Cacciola, A., Portaro, S., Basile, G.A., Rizzo, G., Felippu, A.W.D., Felippu, A.W.D., Bruschetta, A., Anfuso, C., Cascio, F., Milardi, D., Bramanti, A., 2020. Vivo computed tomography direct volume rendering of the anterior ethmoidal artery: a descriptive anatomical study. Int. Arch. Otorhinolaryngol. 24, e38-e46.
- Cillo Jr., J.E., Sinn, D., Truelson, J.M., 2005. Management of middle meningeal and superficial temporal artery hemorrhage from total temporomandibular joint replacement surgery with a gelatin-based hemostatic agent. J. Craniofac. Surg. 16, 309-312.
- Cooney, M., O'Connell, J.E., Vesey, J.A., Van Eeden, S., 2020. Non-surgical management of paediatric and adolescent mandibular condyles: a retrospective review of 49 consecutive cases treated at a tertiary referral centre. J. Cranio-Maxillo- Fac. Surg. 48, 666-671.
- Cuccia, A.M., Caradonna, C., Caradonna, D., Anastasi, G., Milardi, D., Favaloro, A., De Pietro, A., Angileri, T.M., Caradonna, L., Cutroneo, G., 2013. The arterial blood supply of the temporomandibular joint: an anatomical study and clinical im- plications. Imaging Sci Dent 43, 37-44.
- Ezure, H., Mori, R., Ito, J., Otsuka, N., 2011. Case of a completely absent facial artery. Int. J. Acoust. Vib. 4.
- Gerbino, G., Segura-Pallerès, I., Ramieri, G., 2021. Osteochondroma of the mandibular condyle: indications for different surgical methods: a case series of 7 patients. J. Cranio-Maxillo-Fac. Surg. 49, 584-591.
- Giotakis, E.I., Giotakis, A.I., 2020. Modified facelift incision and superficial musculoaponeurotic system flap in parotid malignancy: a retrospective study and review of the literature. World J. Surg. Oncol. 18, 8.
- Godlewski, G., Bossy, J., Giraudon, M., Dussaud, J., Pavart, J.C., Lopez, J.F., 1978. Arterial vascularization of the temporomandibular joint]. Bull. Assoc. Anat. 62, 229-236.
- Holmlund, A., Hellsing, G., 1985. Arthroscopy of the temporomandibular joint. An autopsy study. Int. J. Oral Surg. 14, 169-175.
- Ishida, Y., Chosa, E., Taniguchi, N., 2015. Pseudoaneurysm as a complication of shoulder arthroscopy. Knee Surg. Sports Traumatol. Arthrosc. 23, 1549-1551.
- Kim, D.Y., Park, G.C., Cho, Y.W., Choi, S.H., 2014. Partial superficial parotidectomy via retroauricular hairline incision. Clin Exp Otorhinolaryngol 7, 119-122.
- Luo, X., Bi, R., Jiang, N., Zhu, S., Li, Y., 2021. Clinical outcomes of open treatment of old condylar head fractures in adults. J. Cranio-Maxillo-Fac. Surg. 49, 480-487.
- Mao, Y., Chen, X., Xie, X., Xu, W., Zhang, S., Zhang, S., 2021. Evaluation of an improved anchoring nail in temporomandibular joint disc repositioning surgery: a prospective study of 25 patients. J. Cranio-Maxillo-Fac. Surg. 3, S1010-S5182.
- Shimizu, T., Sakakura, Y., Hattori, T., Yamaguchi, N., Kubo, M., Sakakura, K., 1990. Superselective intraarterial chemotherapy in combination with irradiation: preliminary report. Am. J. Otolaryngol. 11, 131-136.
- Siéssere, S., Vitti, M., Semprini, M., Regalo, S.C., Iyomasa, M.M., Dias, F.J., Issa, J.P., de Sousa, L.G., 2008. Macroscopic and microscopic aspects of the temporomandibular joint related to its clinical implication. Micron 39, 852-858.
- Silva, S., Gouveia, M., 2012. Program “Via verde do AVC”: analysis of the impact on stroke mortality (Portuguese). Revista Portuguesa de Saúde Pública 30, 172-179.
- Sparacia, G., Bencivinni, F., Banco, A., Sarno, C., Bartolotta, T., Lagalla, R., 2007. Im- aging processing for CT angiography of the cervicocranial arteries: evaluation of reformatting technique. La Radiologia medica 112, 224-238.
- Takagi, R., Westesson, P.-L., Ohashi, Y., Togashi, H., 1998. MR angiography of the TMJ in asymptomatic volunteers. Oral Radiol. 14, 69-74.
- Talebzadeh, N., Rosenstein, T.P., Pogrel, M.A., 1999. Anatomy of the structures medial to the temporomandibular joint. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 88, 674-678.
- Taub, P.J., 2007. Cephalometry. J. Craniofac. Surg. 18, 811-817.
- Tauro, D.P., Manay, R.S., 2020. The nuances of temporomandibular joint ankylosis surgery: tips and tricks. J Maxillofac Oral Surg 19, 178-183.
- Toure, G., 2018. Arterial vascularization of the mandibular condyle and fractures of the condyle. Plast. Reconstr. Surg. 141, 718e-725e.
- Vesnaver, A., 2020. Dislocated pediatric condyle fractures - should conservative treatment always be the rule? J. Cranio-Maxillo-Fac. Surg. 48, 933-941.
- Wasicky, R., Pretterklieber, M.L., 2000. The human anterior tympanic artery. A nutrient artery of the middle ear with highly variable origin. Cells Tissues Organs 166, 388-394.
