A comparative study of physicochemical properties of AH PlusTM and EpiphanyTM root canal sealants
Abstract
Aim: To evaluate setting time, solubility and disintegration, flow, film thickness, and dimensional change following setting in a dual-cured resin root canal sealer EpiphanyTM compared with an epoxy-resin-based sealer AH PlusTM.
Methodology: The experiments were performed according to ANSI/ADA Specification 57 which tests the physicochemical properties of endodontic sealing materials. Five samples of each material were tested for each of the properties. In addition, deionized distilled water from the solubility test of EpiphanyTM was submitted for analysis of the cations Fe, Ni, Ca, Mg, Zn, Na, and K in an atomic absorption spectrometer. Three samples were analysed.
Results: There were no statistical differences (P > 0.05) in flow (AH PlusTM: 38.57 mm; EpiphanyTM: 35.74 mm) and film thickness (AH PlusTM: 10.6 μm; EpiphanyTM: 20.1 μm). The solubility (AH PlusTM: 0.21%; Epiphany : 3.41%) and dimensional alterations following setting (AH PlusTM: expansion of 1.3%; EpiphanyTM: expansion of 8.1%) were statistically different (P < 0.05). The setting times of both sealants were in accordance with ANSI/ADA requirements.
Conclusions: Setting time, flow, and film thickness tests for both cements conformed to ANSI/ADA standards. Dimensional alteration test for both cements were greater than values considered acceptable by ANSI/ ADA. EpiphanyTM values regarding solubility were also greater than values considered acceptable by ANSI/ ADA.
Introduction
Many different root canal cements are currently being used in combination with gutta-percha to fill the root canal after biomechanical preparation. Resin-filling materials have steadily gained popularity and are now accepted as a root canal filling (ADA Council on Scientific Affairs 1998).
Epoxy resin sealers have been used because of their reduced solubility (Carvalho-Junior et al. 2003), apical seal (Sousa-Neto et al. 2002) and micro-retention to root dentine (Tagger et al. 2002). Recently, methacrylate resin endodontic sealers have been developed (Kardon et al. 2003, Shipper & Trope 2004). However, the combination of gutta-percha cones and methacrylate resin sealer has shown reduced apical sealing ability compared with gutta-percha cones and conventional epoxy-resin sealer (Kardon et al. 2003, Sevimay & Kalayci 2005). Thus, self-etching primers have been used for bonding to root canal dentine (Economides et al. 2004). As epoxy resin sealers do not co-polymerize with methacrylate resin-based adhesives (Tay et al. 2005), a dual-curable methacrylate resin sealer (EpiphanyTM; Pentron Clinical Technologies, Wallingford, CT, USA), was developed with a self-etching primer, and a new thermoplastic filled polymer (ResilonTM; Resilon Research LLC, Madison, CT, USA), in place of the gutta-percha (Jia & Alpert 2003). This resulted in improvements in the apical seal (Shipper & Trope 2004) and adhesion to root dentine (Gogos et al. 2004).
The purpose of this in vitro study was to assess the setting time, solubility and disintegration, flow, film thickness, and dimensional change following setting of EpiphanyTM dual-cured resin sealer in comparison with a conventional, well-established epoxy-amine resin sealer AH PlusTM, according to the recommendations of ANSI/ADA Specification 57 (ANSI/ADA 2000).
Materials and methods
Setting time, solubility, flow test, film thickness, and dimensional change after setting for AH PlusTM (Dentsply DeTrey, Konstanz, Germany) and EpiphanyTM (Pentron Clinical Technologies) root canal sealants were measured according to the standards of the ANSI/ ADA for dental root canal sealing materials (ANSI/ ADA 2000). As EpiphanyTM is a dual-curable resin, it was mixed and manipulated in a radiographic processing room, where there was no daylight.
Setting time
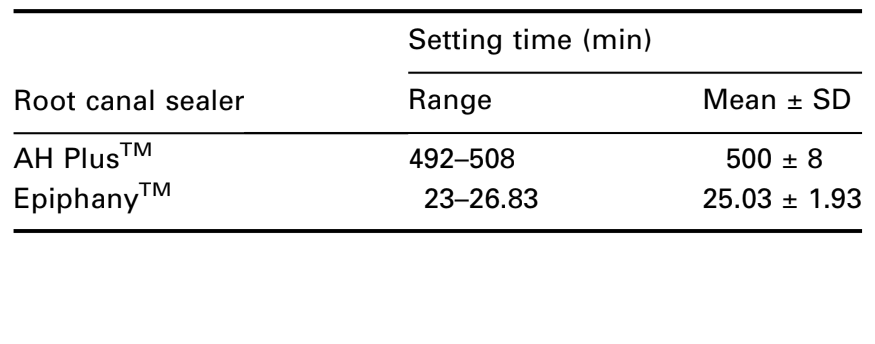
Five plaster of Paris ring moulds, having an internal diameter of 10 mm and a thickness of 2 mm were prepared. The external borders of the moulds were fixed with wax on a 1-mm thick, 25-mm wide and 75-mm long glass plate. The moulds were then filled with the material, mixed according to manufacturer’s directions and transferred to a chamber with 95% relative humidity and a temperature of 37°C. When the setting time stated by the manufacturer approaches, a Gilmore-type needle with a mass of (100 ± 0.5) g having a flat end (2.0 ± 0.1) mm in diameter was carefully lowered vertically onto the horizontal surface of the sealer. The needle tip was cleaned and the movement was repeated until indentations ceased to be visible. The time from the start of mixing to this point was recorded. If the results differed by more than ±5% the test was repeated. The arithmetic mean of five replicates for each sealer was recorded and considered as the setting time.
Solubility
A 1.5-mm-thick cylindrical Teflon® (Polytetrafluroethylene, DuPont, HABIA, Knivsta, Sweden) mould measuring 20 mm in inner diameter was filled with the material, mixed according to the manufacturer’s direc- tions. The mould was supported by a glass plate of larger dimensions than the mould and covered with a cellophane sheet. The mould was filled to a slight excess, an impermeable nylon thread was placed inside the material and another glass plate, also covered with cellophane film, was positioned on the mould and pressed manually in such a way that the plates touched the entire mould in a uniform manner. The assembly was placed in a chamber with 95% relative humidity at 37 °C. The assembly was left to stand for a period corresponding to three times the setting time, and the samples were removed from the mould. Residues and loose particles were removed, the samples were weighed three times on an HM-200 precision scale (A & D Engineering, Inc., Bradford, MA, USA). The mean reading was then recorded.
The samples were suspended by nylon thread and placed inside a plastic vessel with a wide opening, containing 50 mL of distilled and deionized water. Care was taken to avoid any contact between the sample and the inner surface of the container and the liquid. Each sample was placed in a container that was sealed and left for 1 week in an incubator at 37°C and 95% relative humidity. The samples were then removed from the containers, rinsed with distilled and deionized water, and blotted dry with absorbent paper. The samples were placed in a dehumidifier for 24 h and weighed again. The experiment was repeated five times for each sealer. The weight loss of each sample, expressed as percentage of the original mass, was taken as the solubility of the sealer.
There is no information in the literature regarding metals which are released by EpiphanyTM root canal sealer. Therefore, the deionized distilled water from the solubility test of this material was submitted for analysis. A volume of 50 mL of deionized distilled water from the solubility test of EpiphanyTM root canal sealer was used to measure the metal solubility by atomic absorption spectrometry as described in Standard Methods for the Examination of Water and Waste Water of American Public Health Association (APHA), the American Water Works Association (AWWA) and the Water Environment Federation (WEF, formerly Water Pollution Control Federation, WPCF) (APHA, AWWA, WPCF 1989) with Perkin-Elmer (Analyst 700; Shelton, CT, USA). The apparatus was used to measure the levels of Fe, Ni, Ca, Mg, Zn, Na and K.
This spectrophotometer (Perkin-Elmer) is supplied with hollow cathode lamps with different light spectrums exclusively for measuring metallic ions. Eight millilitres of distilled water from each sample, used during the solubility and disintegration test, was poured into a cleaned and dried porcelain crucible. Each crucible was then put into a muffle and burned at 550°C. Ash was dissolved in a concentrated nitric acid using a glass stick. Following this, the samples were put into 50 mL volumetric flasks and the volume made up with ultrapure deionized water (MilliQ, Millipore, Billerica, MA, USA). The solutions thus obtained were then sprayed into the atomic absorption spectrophotometer for measuring.
The arithmetic mean of three replicates for each specimen was recorded and considered as the result of the levels of Fe, Ni, Ca, Mg, Zn, Na and K, expressed as μg mL–1.
Flow test
A volume of 0.5 mL of the cement mixed according to the manufacturer’s recommendations was placed on a glass plate (40 · 40 · 5 mm) using a graduated disposable 3-mL syringe. At (180 ± 5) s after the start of mixing, a load of 100 N plus the top plate with a mass of (20 ± 2) g was placed carefully and centrally on top of the material. Ten min after the commencement of mixing, the load was removed and the average of the major and minor diameters of the compressed discs measured with a digital calliper with a resolution of 0.01 mm (Mitutoyo MTI Corporation, Tokyo, Japan). If both diameters agree to within 1 mm, the results were recorded. If the major and minor diameter discs were not uniformly circular or did not match within 1 mm, the test was repeated. The mean of five such determinations for each sealer, expressed to the nearest millimetre, was taken as the flow of the material.
Film thickness
Two flat glass plates (200 ± 25 mm2) of 5 mm thickness each were placed together and the combined thickness was measured. A volume of 0.5 mL of the material, mixed according to the manufacturer’s recommendations, was deposited on the centre of one glass plate and the second glass plate was placed centrally on top of the sealer. At 180s (±10) after the commencement of mixing, a load of 150 N was carefully applied vertically onto the top glass plate with a loading device (IMI Norgren Inc., Littleton, CO, USA), ensuring that the material filled the entire area between the top and bottom glass plates. Ten minutes after the commencement of mixing, the thickness of two glass plates and the interposed sealer film was measured with an outside micrometer with a resolution of 0.002 mm (Mitutoyo MTI Corporation). The difference in thickness of the two glass plates, with and without sealer, was taken as the film thickness of the material. The mean value of five such determinations for each sealer was taken as the film thickness of the material.
Dimensional alterations
Five Teflon® moulds were prepared for the production of 12-mm high cylindrical test bodies measuring 6 mm in diameter. The mould was placed on a 1-mm thick, 25-mm wide and 75-mm long glass plate wrapped with a fine cellophane sheet. The mould was filled until a slight excess of material, mixed according to the manufacturer’s directions, was observed at its upper end. A microscope slide also wrapped in cellophane was then pressed onto the upper surface of the mould. The assembled group was then kept firmly joined with the aid of a C-shaped clamp. Five minutes after the mixture was first prepared, the assembly was transferred to an incubator set at 95% relative humidity and 37 °C, left to stand for a period corresponding to three times the setting time and then removed.
The next step consisted of grinding flat the ends of the mould containing the sample with a 600 grit wet sandpaper to obtain a regular surface. The sample was removed from the mould, the length measured with a digital calliper with a resolution of 0.01 mm (Mitutoyo MTI Corporation) and stored in a 50-mL vessel containing 30 mL of deionized distilled water at 37°C and 95% relative humidity for 30 days. The sample was then removed from the container, blotted dry on absorbent paper, and measured again for length.
The percentage of the dimensional alterations was calculated using the formula:
((L30 — L)/L) × 100
where L30 is the length of the sample after 30 days of storage under the experimental conditions and L is the initial length of the sample. The arithmetic mean of five replicates for each sealer was recorded as the dimensional alteration of the cement tested.
Statistical analysis
Five specimens from each group were tested, and the mean was calculated. Data were recorded directly onto coding sheets and then stored in a computer. The mean values were compared statistically using the Mann–Whitney U-test between the experimental groups for each procedure. The significance level was set at 5%.
Results
Setting time
The ANSI/ADA (2000) requirements require that a sealer shall be within 10% of that stated by the manufacturer. According to the guidelines for AH PlusTM and EpiphanyTM, the cements have 8 h (480 min) and 25 min of setting time respectively. The mean values of 500 min for AH PlusTM, and 24.75 min for EpiphanyTM (Table 1) showed agreement with the ANSI/ADA standardization.
Solubility
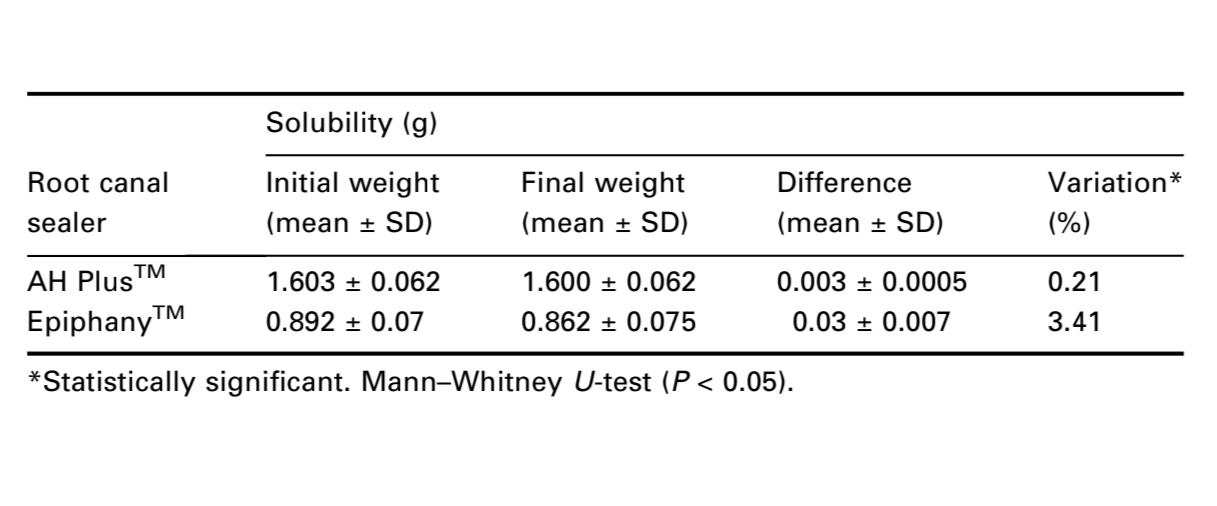
The ANSI/ADA Specification 57 states that a root canal cement should not exceed 3% by mass when the solubility of the set material is tested. In contrast to the AH PlusTM mean result (0.21%), the solubility of EpiphanyTM sealer did not conform to ANSI/ADA standardization (3.41%) (Table 2). The Mann–Whitney U-test showed statistical difference between the cements (P < 0.05).
The deionized distilled water used for the solubility test of the EpiphanyTM root canal sealer was submitted to atomic absorption spectrometry. The resultant levels of seven metals that were analysed were: Fe (0.56 mg L–1), Ni (0.06 mg L–1), Ca (41.46 mg L–1), Mg (0.80 mg L–1), Zn (0.05 mg L–1), Na (4.11 mg L–1) and K (0.50 mg L–1).
Flow test
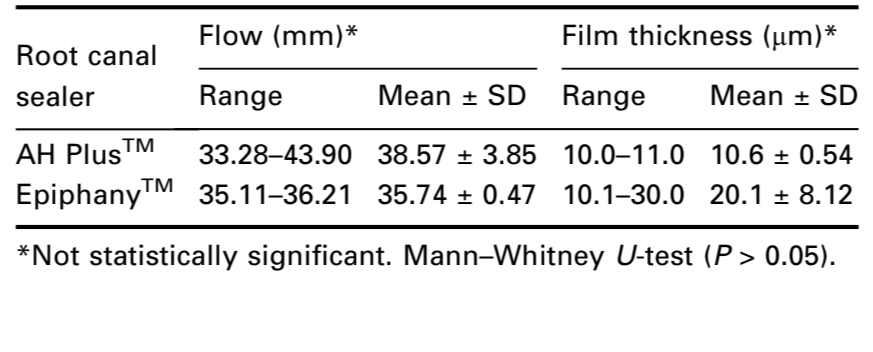
The ANSI/ADA (2000) requires that a sealer shall have a diameter of no less than 20 mm. Both cements conformed to ANSI/ADA standards as the results were 38.57 (±3.85) and 35.74 (±0.47) mm to AH PlusTM and EpiphanyTM respectively (Table 3). Comparing the means pairwise, the Mann–Whitney U-test showed no statistical difference (P > 0.05).
Film thickness
The ANSI/ADA (2000) requires that a sealer shall have a film thickness of no more than 50 μm. Both cements conformed to ANSI/ADA standardization as the results were 10.6 (±0.54) and 20.1 (±8.12)μm to AH PlusTM and EpiphanyTM respectively (Table 3). The Mann– Whitney U-test showed no statistical difference (P > 0.05).
Dimensional alterations
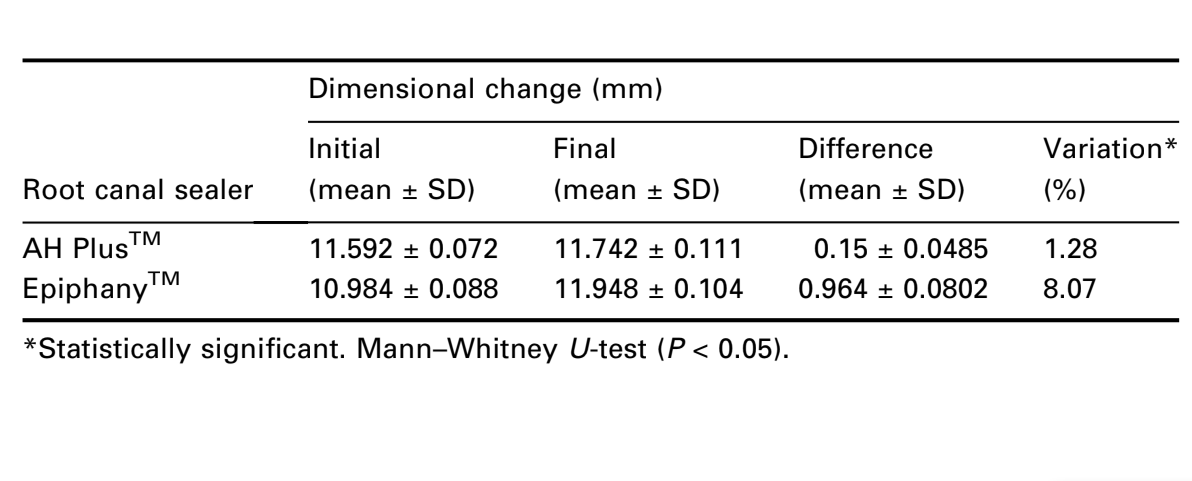
The ANSI/ADA (2000) requirements for this test state that the mean linear shrinkage of the sealer shall not exceed 1% or 0.1% in expansion. Neither cement conformed to the ANSI/ADA standardization. The results showed expansions of 1.3% and 8.1% for AH PlusTM and EpiphanyTM respectively (Table 4). The Mann–Whitney U-test showed statistical difference (P < 0.05).
Discussion
The setting time is the time necessary for the sealer to achieve its definitive properties (Batchelor & Wilson 1969). There is no stipulated standard setting time for sealers, but clinical utility demands that it must be long enough to allow placement and adjustment of root filling if necessary (McMichen et al. 2003). However, it should be as short as possible because of difficulty in maintaining the dryness of the empty prepared canal (Batchelor & Wilson 1969). Once the environment becomes moist, the periapical fluids play an important role in the chemical degradation of the sealer (Ruyter 1995). A better term for the period of time, measured from the start of mixing until the resin endodontic sealer has polymerized, may be ‘polymerization time’. The setting time of sealers is dependent on the constituent components, their particle size, the ambient temperature and relative humidity (Ulrich et al. 1978). Both sealers, EpiphanyTM and AH PlusTM, demonstrated comparable and acceptable setting time as set by ANSI/ADA Specification 57 (ANSI/ADA 2000).
EpiphanyTM sealer is a dual-curable resin composite containing a new redox catalyst (Jin & Jia 2003). The manufacturer states that 40 s of light is required to cure the coronal 2 mm of the canal, whereas the entire filling will self-cure in approximately 15–30 min (Leonard et al. 1996). In this study, EpiphanyTM was mixed and manipulated in a radiographic processing room to ensure that during different experiments the material was not partially cured by light. During the experimental procedure, a thin superficial non-cured layer in EpiphanyTM specimens was always observed after the required setting time. Even when the specimens were exposed to a light cure source, a new batch prepared and left to stand for a period corresponding to five times the setting time period, the non-cured layer still remained.
Oxygen is known to inhibit vinyl polymerization in resins used for restorative dentistry (Franco et al. 2002). Composite materials do not undergo complete polymerization, and about 40–60% of the carbon bonds remain unsaturated (Finger et al. 1996). Therefore, polymerization is compromised by oxygen inhibition. This probably causes the pronounced polymerization inhibition shown by EpiphanyTM. Oxygen may result in a thin film of polymer with a low degree of polymerization, particularly on dentine where oxygen has access to the sealer from the free surface and to some extent through the permeable dentine structure (Rueggeberg & Margeson 1990). This reduced polymerization could compromise the clinical properties.
AH PlusTM, a two-component paste root canal sealer, based on polymerization reaction of epoxy resin amines (Cohen et al. 2000), was tested for comparison. According to the manufacturer’s description, AH PlusTM possesses advantageous properties similar to that of AH26TM, as it preserves the chemistry of the epoxy amines. The material does not release formaldehyde, which interferes negatively with the biocompatibility of AH26TM (Mickel et al. 2003). Therefore, AH PlusTM has been continuously used in comparative studies of physicochemical, biological and antimicrobial properties (Duarte et al. 2004, Gomes et al. 2004, Karadag et al. 2004, Kokkas et al. 2004, Saleh et al. 2004).
Solubility is the ability of a substance to dissolve in another, expressed as the concentration of the saturated solution of the former in the latter (Sousa-Neto et al. 1999). The solubility of the set material, when determined in accordance with ANSI/ADA Specification 57 (ANSI/ADA 2000) shall not exceed 3% by mass. The findings of this study demonstrated that AH PlusTM (0.21%) was within the recommended range, whereas EpiphanyTM (3.41%) showed higher value than the ANSI/ADA (2000) recommendations. A further investigation was conducted to determine the EpiphanyTM components that were released during the test period. The deionized distilled water used for solubility test of EpiphanyTM root canal sealer was submitted to atomic absorption spectrometry and showed an extensive calcium release (41.46 mg L–1). Calcium ion release has been shown to favour a more alkaline pH of the environment leading to biochemical effects that culminate in the acceleration of the repair process (Seux et al. 1991). This high calcium release by EpiphanyTM sealer could explain the reduced apical periodontitis observed clinically (Shipper et al. 2005) and its intraosseous biocompatibility (Souza et al. 2006).
EpiphanyTM is composed of fillers of calcium hydroxide, barium sulphate, barium glass and silica. The total filler content in the sealer is approximately 70% by weight (Leonard et al. 1996). One possible explanation for the higher value verified for solubility test of EpiphanyTM is the erosion of filler particles taking place because of degradation (Soderholm et al. 1984, Gopferich 1996). Water diffusion also leads to erosion of the composite resin material caused by release of unreacted monomers (residual monomers and light-activated monomers) (Gopferich 1996). As a dual curable resin composite sealer, EpiphanyTM resin matrix is a mixture of bisphenol A-glycidyl methacrylate (Bis-GMA), ethoxylated BisGMA, urethane dimetacrylate (UDMA), and hydrophilic difunctional methacrylates (Leonard et al. 1996). It has been shown that residual monomers are the main components released from cured dental composite materials, occurring within the first 7 days from placement (Øysaed et al. 1988, Ruyter 1995). In this study, samples for the solubility test were stored for a period of 7 days and not for 24 h as recommended by ANSI/ADA. These erosion processes will result in mass loss of the dental composite material (Örtengren et al. 2001).
The ability of the sealer to flow and enter uninstrumented accessory root-canal anatomy and between gutta-percha cones is important (McMichen et al. 2003), without increasing the risk of periapical extrusion, which may compromise periapical healing (Sjögren et al. 1990). The results of the flow test showed that both cements were consistent with ANSI/ADA (2000) standards. There was no statistical difference (P > 0.05) in the flow of the two cements. The manufacturer’s instruction for immediately light-curing the coronal root filling to create a coronal seal may also limit flow of resin sealer for stress relief (Davidson & de Gee 1984).
Another important physical property of a sealer is film thickness. A thin film thickness sealer would be expected to wet the surface better than a thick film thickness sealer and thus provide a better seal (De Deus et al. 2003). The results of the film thickness test of both cements conformed to ANSI/ADA (2000) standardization.
As the sealer contributes to the bonding of gutta-percha to the dentinal walls, it should be as stable as possible (Camps et al. 2004). ANSI/ADA Specification 57 (ANSI/ADA 2000) states that the mean linear shrinkage of the sealer shall not exceed 1% or 0.1% in expansion. EpiphanyTM and AH PlusTM presented expansion, although these types of resin sealers promote polymerization shrinkage. AH PlusTM cement presented little expansion (1.3%), when compared with Epiphany (8.1%), which may be due to ADA methodology which recommends that a sample be immersed in water after material setting – in other words, after polymerization. This dimensional alteration could be explained by water sorption suffered by these types of resins after polymerization (Phillips 1991). Water sorption in composite materials is a diffusion-controlled process and occurs mainly in the resin matrix (Braden et al. 1976, Braden & Clarke 1984). Epiphany showed high values of expansion. This could be explained by the presence of hydrophilic difunctional methacrylates. Polymerized materials from mixtures of hydrophilic monomers will show high water sorption (Øysaed & Ruyter 1986). The polar nature of such a polymer matrix and the presence of their linkages are of importance for water sorption and hygroscopic expansion of composite resin materials (Peutzfeldt 1997). Another aspect is the filler content of the composite material which also can affect the water sorption characteristics. EpiphanyTM contains fillers of calcium hydroxide which absorb water (Soderholm et al. 1984, Øysaed & Ruyter 1986).
Conclusion
In conclusion, setting time, flow, and film thickness tests of both cements conformed to American National Standards specifications for endodontic filling materials (ANSI/ADA 2000). However, the solubility and dimensional alteration values of EpiphanyTM sealer, and dimensional alteration values of AH PlusTM were higher than those considered acceptable for the ANSI/ADA specifications (ANSI/ADA 2000).
Authors: M. A. Versiani, J. R. Carvalho-Junior, M. I. A. F. Padilha, S. Lacey, E. A. Pascon, M. D. Sousa-Neto
References
- ADA Council on Scientific Affairs (1998) Statement on posterior resin-based composites. ADA Council on Dental Benefit Programs. Journal of the American Dental Association 129, 1627–8.
- ANSI/ADA (2000) Specification No. 57 Endodontic Sealing Material. Chicago, USA: ANSI/ADA.
- APHA, AWWA, WPCF (1989) Standard Methods for the Examination of Water and Waste Water, 17th edn. Washington: American Public Health Association, American Water Works Association, Water Pollution Control Federation.
- Batchelor RF, Wilson AD (1969) Zinc oxide-eugenol cements. I: The effects of atmospheric conditions on rheological properties. Journal of Dental Research 48, 883–7.
- Braden M, Causton EE, Clarke RL (1976) Diffusion of water in composite filling materials. Journal of Dental Research 55, 730–2.
- Braden M, Clarke RL (1984) Water absorption characteristics of dental microfine composite filling materials. I. Proprietary materials. Biomaterials 5, 369–72.
- Camps J, Pommel L, Bukiet F, About I (2004) Influence of the powder/liquid ratio on the properties of zinc oxide-eugenol based root canal sealer. Dental Materials 20, 915–23.
- Carvalho-Junior JR, Guimarães LF, Correr-Sobrinho L, Pecora JD, Sousa-Neto MD (2003) Evaluation of solubility, disintegration, and dimensional alterations of a glass ionomer root canal sealer. Brazilian Dental Journal 14, 114–8.
- Cohen BI, Pagnillo MK, Musikant BL, Deutsch AS (2000) An in vitro study of the cytotoxicity of two root canal sealers. Journal of Endodontics 26, 228–9.
- Davidson CL, de Gee AJ (1984) Relaxation of polymerization contraction stresses by flow in dentinal composite. Journal of Dental Research 63, 146–8.
- de Deus GA, Martins F, Lima AC, Gurgel-Filho ED, Maniglia CF, Coutinho-Filho T (2003) Analysis of the film thickness of a root canal sealer following three obturation techniques. Brazilian Oral Research 7, 119–25.
- Duarte MAO, Demarchi AC, De Moraes IG (2004) Determination of pH and calcium ion release provided by pure and calcium hydroxide-containing AHPlus. International Endodontic Journal 37, 42–5.
- Economides N, Kokorikos I, Kolokouris I, Panagiotis B, Gogos C (2004) Comparative study of apical sealing ability of a new resin-based root canal sealer. Journal of Endodontics 30, 403–6.
- Finger WJ, Lee KS, Podszun W (1996) Monomers with low oxygen inhibition as enamel/dentin adhesives. Dental Materials 12, 256–61.
- Franco EB, Lopes LG, D’alpino PH, Pereira JC, Mondelli RF, Navarro MF (2002) Evaluation of compatibility between different types of adhesives and dual-cured resin cement. Journal of Adhesive Dentistry 4, 271–5.
- Gogos C, Economides N, Stavrianos C, Kolokouris I, Kokorikos I (2004) Adhesion of a new methacrylate resin-based sealer to human dentin. Journal of Endodontics 30, 238–40.
- Gomes BP, Pedroso JA, Jacinto RC et al. (2004) In vitro evaluation of the antimicrobial activity of five root canal sealers. Brazilian Dental Journal 15, 30–5.
- Gopferich A (1996) Mechanisms of polymer degradation and erosion. Biomaterials 17, 103–14.
- Jia WT, Alpert B (2003) Root canal filling material. United States Patent Application 20030113686, US Patent & Trademark Office, June 19.
- Jin SH, Jia WT (2003) Self-curing system for endodontic sealant applications. United States Patent Application 20030134933, US Patent & Trademark Office, July 17.
- Karadag LS, Bala O, Turkoz E, Mihcioglu T (2004) The effects of water and acetone-based dentin adhesives on apical microleakage. Journal of Contemporary Dental Practice 5, 93– 101.
- Kardon BP, Kuttler S, Hardigan P, Dorn SO (2003) An in vitro evaluation of the sealing ability of a new root-canal-obturation system. Journal of Endodontics 29, 658–61.
- Kokkas AB, Boutsioukis ACH, Vassiliadis LP, Stavrianos CK (2004) The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: an in vitro study. Journal of Endodontics 30, 100–2.
- Leonard JE, Gutmann JL, Guo IY (1996) Apical and coronal seal of roots obturated with a dentine bonding agent and resin. International Endodontic Journal 29, 76–83.
- McMichen FRS, Pearson G, Rahbaran S, Gulabivala K (2003) A comparative study of selected physical properties of five root-canal sealers. International Endodontic Journal 36, 629–35.
- Mickel AK, Nguyen TH, Chogle S (2003) Antimicrobial activity of endodontic sealers on Enterococcus faecalis. Journal of Endodontics 29, 257–8.
- Örtengren U, Wellendorf H, Karlsson S, Ruyter IE (2001) Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. Journal of Oral Rehabilitation 28, 1106–15.
- Øysaed H, Ruyter IE (1986) Water sorption and filler characteristics of composites for use in posterior teeth. Journal of Dental Research 65, 1315–8.
- Øysaed H, Ruyter IE, Sjøvik-Kleven IJ (1988) Release of formaldehyde from dental composites. Journal of Dental Research 67, 1289–94.
- Peutzfeldt A (1997) Resin Composites in dentistry: the monomer systems. European Journal of Oral Science 105, 97–116.
- Phillips RW (1991) Skinner’s Science of Dental Materials, 9th edn. Philadelphia: WB Saunders, pp. 221–32.
- Rueggeberg FA, Margeson DH (1990) The effect of oxygen inhibition on an unfilled/filled composite system. Journal of Dental Research 69, 1652–8.
- Ruyter IE (1995) Physical and chemical aspects related to substances released from polymer materials in an aqueous environment. Advanced Dental Research 9, 344–7.
- Saleh IM, Ruyter IE, Haapasalo M, Ørstavik D (2004) Survival of Enterococcus faecalis in infected dentinal tubules after root canal filling with different root canal sealers in vitro. International Endodontic Journal 37, 193–8.
- Seux D, Couble ML, Hartmann DJ, Gauthier JP, Magloire H (1991) Odontoblast-like cytodifferentiation of human dental pulp ‘in vitro’ in the presence of a calcium hydroxide-containing cement. Archives of Oral Biology 36, 117–28.
- Sevimay S, Kalayci A (2005) Evaluation of apical sealing ability and adaptation to dentine of two resin-based sealers. Journal of Oral Rehabilitation 32, 105–10.
- Shipper G, Trope M (2004) In vitro microbial leakage of endodontically treated teeth using new and standard obturation techniques. Journal of Endodontics 30, 154–8.
- Shipper G, Teixeira FB, Arnold RR, Trope M (2005) Periapical inflammation after coronal microbial inoculation of dog roots filled with gutta-percha or Resilon. Journal of Endodontics 31, 91–6.
- Sjögren U, Hagglund B, Sunfdgvist G, Wing K, (1990) Factors affecting the long-term results of endodontic treatement. Journal of Endodontics 16, 498–504.
- Soderholm KJ, Zigan M, Ragan M, Fischlschweiger W, Bregman M (1984) Hydrolytic degradation of dental composites. Journal of Dental Research 63, 1248–54.
- Sousa CJA, Montes CRM, Pascon EA, Loyola AM, Versiani MA (2006) Comparison of the intraosseous bio compatibility of AH PlusTM, Endo REZTM, and EpiphanyTM root canal sealers. Journal of Endodontics (in press).
- Sousa-Neto MD, Guimarães LF, Saquy PC, Pécora JD (1999) Effect of different grades of gum rosins and hydrogenated resins on the solubility, disintegration, and dimensional alterations of Grossman cement. Journal of Endodontics 25, 477–80.
- Sousa-Neto MD, Passarinho-Neto JG, Carvalho-Junior JR, Cruz-Filho AM, Pecora JD, Saquy PC (2002) Evaluation of the effect of EDTA, EGTA and CDTA on dentin adhesiveness and microleakage with different root canal sealers. Brazilian Dental Journal 13, 123–8.
- Tagger M, Tagger E, Tjan AH, Bakland LK (2002) Measurement of adhesion of endodontic sealers to dentin. Journal of Endodontics 28, 351–4.
- Tay FR, Loushine RJ, Weller RN et al. (2005) Ultrastructural evaluation of the apical seal in roots filled with a polycaprolactone-based root canal filling material. Journal of Endodontics 31, 514–9.
- Ulrich JM, Moser JB, Heuer MA (1978) The rheology of selected root canal sealer cements. Journal of Endodontics 4, 373–9.
