In vivo comparison of the biocompatibility of two root canal sealers implanted into the subcutaneous connective tissue of rats
Objective. To evaluate the subcutaneous biocompatibility of 2 root canal sealers.
Study design. The subcutaneous implant technique recommended by the Fédération Dentaire International (FDI) was used to test Endométhasone and EndoREZ root canal sealers. These materials were placed in Teflon tubes, 1 mm in diameter and 10 mm in length, and implanted into 2 pockets created in the back of 40 Calomys callosus rodents, 20 for each material. Tissue biopsies were collected and histologically examined 15, 30, 60, and 90 days after the implantation procedure. The overall level of the inflammatory tissue response was graded as none, slight, moderate, or severe on the sealer– connective tissue interface at the opening ends of the tubes. The connective tissue response along the lateral wall outside of each tube served as a negative control.
Results. The tissue reaction to the Endométhasone diminished with time. The EndoREZ sealer was highly toxic during all experimental periods.
Conclusion. Endométhasone root canal sealer presented biocompatibility within the analyzed periods, whereas EndoREZ showed no biocompatible behavior and caused late hypersensitive reaction. (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103:e88-e94)
Successful endodontic therapy depends on correct diagnoses, effective cleaning, elimination of infection, and adequate obturation of root canals. Periapical tissue reaction after root canal treatment may be influenced by various factors depending on the chemical nature of the endodontic sealer. Currently used in clinical practice are resin-, zinc oxide-eugenol-, glass ionomer-, silicon-, and calcium hydroxide-based endodontic sealers. Resin-based sealers are increasingly gaining popularity but studies have demonstrated that some of these sealers present toxicity and mutagenicity. Despite the great variety of sealers available, a root canal sealer that possesses all the desirable physical and biological properties has yet to be found. Biocompatibility is one of the most important properties of root filling materials8 since the release of certain substances by the sealers may generate different reactions in the periapical tissues. Tissue reactions caused by endodontic materials are normally investigated by histological studies following the implantation of the material into animal tissue. Endométhasone (Spécialities Septodont, Saint-Maur-des-Fossés, France) is a zinc oxide-eugenol-based sealer that was previously assessed in various studies regarding its biological properties. Recently, a new methacrylate-based endodontic sealer, EndoREZ (Ultradent Products Inc., South Jordan, UT, USA), has been introduced and its biological properties have been little investigated.
The purpose of this study was to evaluate the reaction of the subcutaneous connective tissue to EndoREZ and Endométhasone root canal sealers following the requirements recommended by the FDI.
Methods
The protocol for this experiment was approved by the Research Ethics Committee of the University of Uberlândia, and the experiment was carried out in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Forty male Calomys callosus (Rodentia, Cricetidae) rodents, weighing 150 to 200 g, were used. The specimens were divided into 2 groups of 20 animals each for Endométhasone and EndoREZ root canal sealers. Table I shows materials, manufacturers, and main components.
All materials were prepared in the manner advised by the manufacturers for their clinical use and loaded into autoclaved Teflon carriers (polytetrafluorethylene, Du-Pont, Habia, Knivsta, Sweden), 10 mm long with a single lumen and an inner diameter of 1 mm, ensuring that air was not entrapped.
The animals were anesthetized intraperitoneally with 0.2 mL ketamine containing acepromazine in a 1:1 proportion. The dorsal skin was shaved, disinfected with 5% tincture of iodine, and small incisions, approximately 15 mm long, were made with a blade, in both sides of the dorsum. Two separate pockets were created by blunt dissection to implant the tubes in the subcutaneous tissue to a depth of ~15 mm. The tubes containing freshly mixed sealers were then placed into the right and left pockets prepared in each animal. Care was taken to prevent spilling of the materials into the tissue. After implantation, the wounds were sutured.
The animals were killed in groups of 5 each after 15, 30, 60, and 90 days by means of carbon dioxide suffocation assuring minimum discomfort and distress. The tubes were excised together with skin and connective tissue around them. The samples were immersed in 10% formalin in 0.1 mol/L phosphate-buffered solution for 24 hours, dehydrated in graded ethanol solutions at room temperature, and embedded in glycol methacrylate (Historesin; Leica Microsystems, Nussloch GmbH, Germany). For cross-sectioning, the blocks were oriented parallel to the long axis of the tube. The sections were cut to a thickness of 3 µm and stained with toluidine blue 1%. The histologic sections were analyzed at different magnifications under a light microscope, noting tissue reactions on the sealer– connective tissue interface at the opening ends of the tubes. The connective tissue response alongside the lateral wall outside of the tubes served as a negative control.
The interface at the opening of the cup between the material and the bone was examined and evaluated for the intensity of the inflammation. The Fédération Dentaire International (FDI) criteria evaluation is exclusively qualitative, and no scoring index was used. Thus, the overall level of the tissue reaction was rated as none, slight, moderate, or severe, depending of the presence or absence of neutrophilic leukocytes, macrophages, lymphocytes, plasma cells, giant foreign body cells, dispersed material, capsule, newly formed healthy bone, necrotic tissue, and resorption. The overall level of the inflammatory tissue response was graded as follow: 1) none/slight: thickness of the reaction zone similar to or only slightly wider than the thickness along the side tube, with no or a few inflammatory cells; 2) moderate: increased reaction zone in which macrophages, plasma cells, or both are present; and 3) severe: increased reaction zone in which macrophages and plasma cells and occasional foci of neutrophil granulocytes, lymphocytes, or both are present.
The interpretations of the results were based on the following FDI criteria: no to slight reaction at both 2 and 12 weeks is acceptable; no to slight reaction at 2 weeks that increases to moderate or severe reaction at 12 weeks is not acceptable; moderate reaction at 2 and 12 weeks is not acceptable; moderate reaction at 2 weeks that diminishes at 12 weeks is acceptable; and a severe reaction at any period is unacceptable.
Results
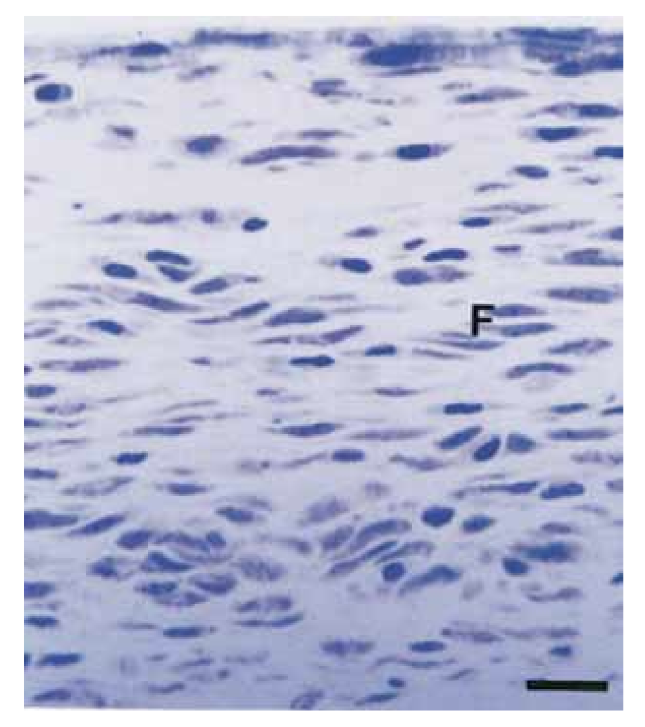
The intensity of the inflammatory response in all experimental periods of both sealers was analyzed. The connective tissue response alongside the lateral wall outside of the Teflon tubes served as a negative control for the technique and showed no inflammatory reaction in all experimentation periods (Fig. 1). The numbers of the samples in each inflammatory category at the different time frames for the 2 types of sealers are presented in Table II.
Endométhasone
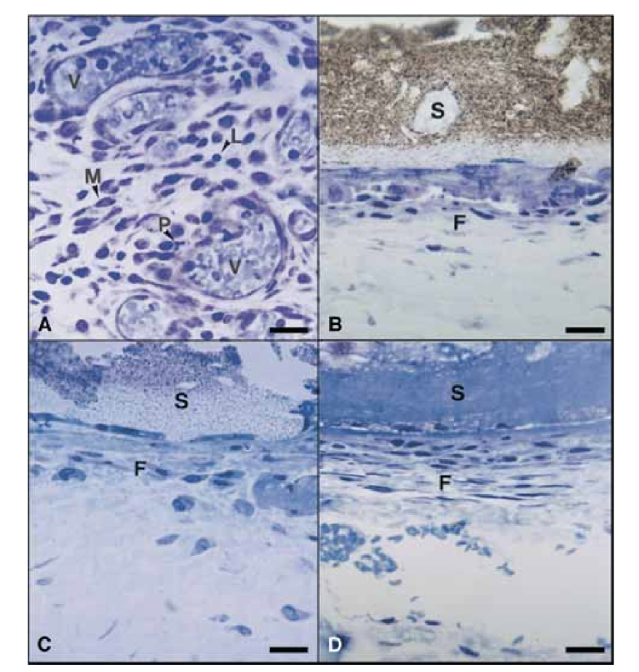
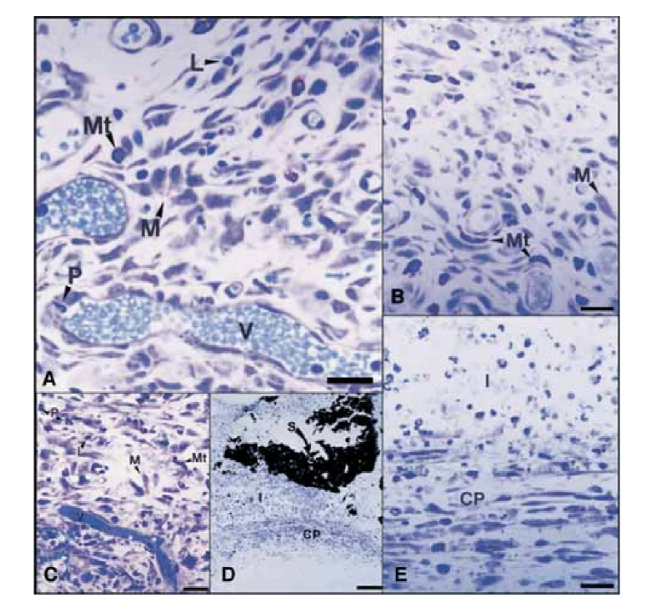
On the 15th day, a moderate to severe inflammatory reaction was observed. The tissue was infiltrated predominantly with macrophages and lymphocytes. The presence of polymorphonuclear leukocytes, hyperemia and a morphologically altered extracellular matrix was also observed (Fig. 2, A). On the 30th, 60th, and 90th days, the connective tissue showed no inflammatory reaction (Fig. 2, B, C, and D).
EndoREZ
On the 15th day, a moderate to severe inflammatory reaction was observed. The tissue was infiltrated predominantly with macrophages, a few polymorphonuclear leukocytes, and lymphocytes. Vessel hyperemia and a reduced and morphologically altered extracellular matrix were also observed (Fig. 3, A). From the 30th day, a severe inflammatory reaction was observed and an inflammatory infiltrate consisting predominantly of polymorphonuclear leukocytes, lymphocytes, and macrophages appeared to be invading the lumen of the Teflon tubes. In addition, at the sealer– connective tissue interface, blood vessels surrounded by mast cells were observed (Fig. 3, B). On the 60th day, a severe inflammatory reaction with macrophages, lymphocytes, and polymorphonuclear leukocytes still persisted. At the opening of the Teflon tubes, the sealer was substituted by an inflammatory infiltrate with macrophages and lymphocytes prevalent. Congested blood vessels surrounded by mast cells were observed (Fig. 3, C). A consistent fibroblastic proliferation surrounding the inflammatory tissue was also detected. On the 90th day, a severe inflammatory infiltrate consisting of macrophages, lymphocytes, polymorphonuclear leukocytes, red cells, and dead cells invaginated into the lumen of the Teflon tubes, substituting the sealer, was observed. At the opening end of the tubes, the granulomatous tissue was walled off by fibrous encapsulation (Fig. 3, D and E).
Discussion
When a new material is introduced into the market, or an existing material is proposed for a different application, its properties should be investigated and the results compared with those other authors. In the United States, the Food and Drug Administration (FDA) has responsibility for assessing and evaluating the biologic effects of all drugs, materials, and devices used in human beings, including most dental products and devices. The FDA also provides for the recognition of standards established by private organizations, such as the ANSI/ADA. The revision of the first published Recommended Standard Practices for Biologic Evaluation of Dental Materials was delayed to incorporate the essentials of the FDA’s recommendations. This new document did not intended to discourage the industrial development of new and improved dental products by demanding overkill with biologic tests, but, in this era of concern for chemical hazards, a toxicity profile on all new and improved materials should be developed to obtain relevant data on safety and efficacy. Although no amount of experimental study can guarantee absolute safety for any substance, toxicologic investigations provide data from which reasonable projections and predictions can be made about the conditions under which the agent can be safely used.
Although many tests, such as cytotoxicity, hemolysis, Ame’s test, Styles cell transformation, subcutaneous and bone implantations, sensitization, and endodontic usage, are listed for various levels of testing, they are not all required for each product. A judgment has to be made as to which tests are relevant. The initial tests are to provide a profile of toxicity in a biologic system, so that on a comparison basis the manufacturer will have a ballpark appreciation and realization of where the product lies. To the FDA, regulated devices fall under 3 classes, however, and the majority of dental devices and appliances would not be subject to standard-setting for premarket clearance. In other words, sometimes even basic safety testing is not required before products can be promoted for clinical use.
The biocompatibility of dental materials is an important requirement because the toxic components present in these materials could produce irritation or even degeneration of the surrounding tissues, especially when accidentally extruded into the periradicular tissues. The subcutaneous connective tissue implantation in animals is one of the most reliable methods of evaluating biocompatibility of dental materials because inflammatory reactions are a characteristic features for all connective tissues. The mouse-like wild rodent Calomys callosus is commonly used to investigate experimental infection and humoral immune response and was used in the present study owing to its reproducibility as an established model widely accepted in the literature.
The samples were embedded in glycol methacrylate owing to its advantages compared with paraffin such as producing less distortion, providing thin sections that offer good cellular definition, permitting the preparation of sections without removal of the tubes, and staining with good quality and few technical artifacts, favoring a more precise evaluation of the inflammatory reaction.
In the present study, the connective tissue alongside the lateral wall served as a negative control and did not present inflammatory reactions (Fig. 1), demonstrating the biocompatibility of Teflon and that the inflammatory reaction at the opening of the tube was related to the material studied.
The biologic properties of Endométhasone, a zinc oxide–eugenol (ZOE)– based root canal sealer, has been previously studied regarding antibacterial activity, cytotoxicity, and tissue biocompatibility. Eugenol (4-allyl-2-methoxyphenol) is an extract of clove oil widely used in dentistry as a therapeutic agent, most commonly as a component of zinc oxide– eugenol cement applied as a base or temporary dressing to dentine or as a root canal sealer. Several studies have been reported on the histopathologic influence of ZOE-based root canal sealers on tissues. Eugenol that leaches out of ZOE-based root canal sealers may participate in the development of periapical inflammation or the continuation of a pre-existing periapical lesion.
In the present study, the subcutaneous tissue inflammatory reactions to Endométhasone decreased with time, similarly to the results obtained by Kaplan et al., probably owing to the neutralization of the eugenol liberated at the start and by the local liberation of corticoids such as dexamethasone and hydrocortisone. Many investigators have suggested that the irritative ability of ZOE– based sealers could be attributed primarily to eugenol and secondarily to zinc ions. Eugenol can inhibit the macrophage function and may influence the inflammatory reactions in the periapical tissues. Paraformaldehyde has been shown to cause allergic reaction and necrosis of the connective tissue. However, despite the high con centration of paraformaldehyde in Endométhasone sealer, in the present study such findings were not observed.
Recently, a new methacrylate-based endodontic sealer was introduced as a root canal sealer. EndoREZ is a hydrophilic, 2-component, chemical-set material containing zinc oxide, barium sulfate, resins, and pigments in a matrix of urethane dimethacrylate resin.
The biologic properties of EndoREZ have been previously investigated regarding its cytotoxicity and tissue biocompatibility. Studies regarding the tissue biocompatibility of resin-based sealers have shown moderate to severe inflammatory reaction. In the present study, EndoREZ caused severe inflammatory reaction at all experimental periods. Because the average life span of a neutrophil outside the blood vessel is 7 days, the presence of polymorphonuclear cells at all experimental periods suggests toxicity behavior of this material. Using cultured cells, it was demonstrated that EndoREZ became more cytotoxic with time of exposure and presented significant cytotoxic risks when freshly mixed. The connective tissue reaction to silicone tubes filled with EndoREZ implanted into the subcutaneous tissue of rats showed a severe reaction that significantly changed its profile after 30 days, with a moderate reaction after 3 months and absence of inflammation after 4 months. Nevertheless, it was also reported that, in some animals, the inflammatory response persisted in all experimental periods. It was considered that after subcutaneous implantation of fresh EndoREZ, components such as zinc and barium were in direct contact with the tissue and caused the severe initial reaction. In contrast to the results of the present research, Zmener et al. demonstrated a satisfactory bone tissue response at the 60-day observation period of EndoREZ implanted in the tibias of rats. The slow breakdown of EndoREZ sealer, illustrated by the dispersed material, and subsequent endocytosis by macrophages, might have been the cause of such persistent chronic inflammation. Besides, root canal therapy performed with laterally condensed gutta-percha cones in conjunction with EndoREZ seems to present a good overall success rate after 14-24 months recall evaluation.
Additionally, in the present study the presence of many mast cells in the connective tissue was observed, mainly in the surrounding area of blood vessels, at the 30th, 60th, and 90th day periods, suggesting a late hypersensitive reaction to the components of EndoREZ. Although the late hypersensitive reaction to endodontic materials is not frequently reported, these adverse systemic reactions, which occur more frequently than reported in the literature, should not be dismissed. According to Bratel et al., endodontic materials can induce a late hypersensitive reaction even in subtoxic concentrations.
Conclusion
According to FDI criteria, the results obtained in the present study allowed the conclusion that Endométhasone root canal sealer presented biocompatibility within the analyzed periods, whereas EndoREZ showed no biocompatible behavior and a suggestion of late hypersensitive reaction.
Authors: Edilson José Zafalon, Marco Aurélio Versiani, Cássio José Alves de Souza, Camila Christian Gomes Moura, Paula Dechichi
References:
- Bernath M, Szabo J. Tissue reaction initiated by different sealers. Int Endod J 2003;36:256-61.
- Bouillaguet S, Wataha JC, Lockwood PE, Galgano C, Golay A, Krejci I. Cytotoxicity and sealing properties of four classes of endodontic sealers evaluated by succinic dehydrogenase activity and confocal laser scanning microscopy. Eur J Oral Sci 2004; 112:182-7.
- Kaplan AE, Ormaechea MF, Picca M, Canzobre MC, Ubios AM. Rheological properties and biocompatibility of endodontic sealers. Int Endod J 2003;36:527-32.
- Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 2. Root-canal-filling materials. Int Endod J 2003;36:147-60.
- Gençoĝlu N, Turkmen C, Ahiskali R. A new silicon-based root canal sealer (Roekoseal-Automix). J Oral Rehabil 2003;30:753-7.
- Zmener O, Banegas G, Pameijer CH. Bone tissue response to a methacrylate-based endodontic sealer: a histological and histometric study. J Endod 2005;31:457-9.
- Huang TH, Yang JJ, Li H, Kao CT. The biocompatibility evaluation of epoxy resin–based root canal sealers in vitro. Biomaterials 2002;23:77-83.
- Ozbas H, Yaltirik M, Bilgic B, Issever H. Reactions of connective tissue to compomers, composite and amalgam root-end filling materials. Int Endod J 2003;36:281-7.
- Ho Y-C, Huang F-M, Chang Y-C. Mechanisms of cytotoxicity of eugenol in human osteoblastic cells in vitro. Int Endod J 2006;39:389-93.
- Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 1. Intracanal drugs and substances. Int Endod J 2003;36:75-85.
- Sousa CJ, Loyola AM, Versiani MA, Biffi JC, Oliveira RP, Pascon EA. A comparative histological evaluation of the biocompatibility of materials used in apical surgery. Int Endod J 2004;37:738-48.
- Tepel J, Darwisch el Sawaf M, Hoppe W. Reaction of inflamed periapical tissue to intracanal medicaments and root canal sealers. Endod Dent Traumatol 1994;10:233-8.
- Gerosa R, Menegazzi G, Borin M, Cavalleri G. Cytotoxicity evaluation of 6 root canal sealers. J Endod 1995;21:446-8.
- Vajrabhaya L, Sithisarn P. Multilayer and monolayer cell cultures in a cytotoxicity assay of root canal sealers. Int Endod J 1997;30:141-4.
- Bratel J, Jontell M, Dahlgren U, Bergenholtz G. Effects of root canal sealers on immunocompetent cells in vitro and in vivo. Int Endod J 1998;31:178-88.
- Serper A, Ucer O, Onur R, Etikan I. Comparative neurotoxic effects of root canal filling materials on rat sciatic nerve. J Endod 1998;24:592-4.
- Ersev H, Schmalz G, Bayirli G, Schweikl H. Cytotoxic and mutagenic potencies of various root canal filling materials in eukaryotic and prokaryotic cells in vitro. J Endod 1999;25:359-63.
- Telli C, Serper A, Dogan AL, Guc D. Evaluation of the cytotoxicity of calcium phosphate root canal sealers by MTT assay. J Endod 1999;25:811-3.
- Huang FM, Tai KW, Chou MY, Chang YC. Cytotoxicity of resin-, zinc oxide-eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int Endod J 2002;35:153-8.
- Schwarze T, Fiedler I, Leyhausen G, Geurtsen W. The cellular compatibility of five endodontic sealers during the setting period. J Endod 2002;28:784-6.
- Schwarze T, Leyhausen G, Geurtsen W. Long-term cytocompatibility of various endodontic sealers using a new root canal model. J Endod 2002;28:749-53.
- Gomes BP, Pedroso JA, Jacinto RC, Vianna ME, Ferraz CC, Zaia, AA, et al. In vitro evaluation of the antimicrobial activity of 5 root canal sealers. Braz Dent J 2004;15:30-5.
- Perassi FT, Filho IB, Berbert FL, Carlos IZ, de Toledo Leonardo R. Secretion of tumor necrosis factor-alpha by mouse peritoneal macrophages in the presence of dental sealers, sealapex and endométhasone. J Endod 2004;30:534-7.
- Pizzo G, Giammanco GM, Cumbo E, Nicolosi G, Gallina G. In vitro antibacterial activity of endodontic sealers. J Dent 2006; 34:35-40.
- Zmener O. Tissue response to a new methacrylate-based root canal sealer: preliminary observations in the subcutaneous connective tissue of rats. J Endod 2004;30:348-51.
- Zmener O, Pameijer CH. Clinical and radiographic evaluation of a resin-based root canal sealer. Am J Dent 2004;17:19-22.
- Louw NP, Pameijer CH, Norval G. Histopathological evaluation of a root canal sealer in subhuman primates [abstract]. J Dent Res 2001;80:654.
- Fédération Dentaire International, Commission of Dental Materials, Instruments, Equipment and Therapeutics. Recommended standard practices for biological evaluation of dental materials. Int Dent J 1980;30:140-88.
- National Institutes of Health. Public Health Service policy on humane care and use of laboratory animals, 2006. Available at: http://grants.nih.gov/grants/olaw/references/phspol.htm.
- National Institutes of Health. Public Health Service policy on humane care and use of laboratory animals clarification regarding use of carbon dioxide for euthanasia of small laboratory animals, 2006. Available at: http://grants.nih.gov/grants/olaw/ Compilation_of_Guidance.doc.
- Stanley HR. Toxicity testing of dental materials. 1st ed. Miami: CRC Press; 1985.
- Sousa CJA, Montes CRM, Pascon EA, Loyola AM, Versiani MA. Comparison of the intraosseous biocompatibility of AH Plus, Endo-REZ, and Epiphany root canal sealers. J Endod 2006;32:656-62.
- Huang FM, Tsai CH, Yang SF, Chang YC. Induction of interleukin-6 and interleukin-8 gene expression by root canal sealers in human osteoblastic cells. J Endod 2005;31:679-83.
- Olsson B, Sliwkowski A, Langeland K. Subcutaneous implantation for the biological evaluation of endodontic materials. J Endod 1981;7:355-67.
- de Oliveira L, Borges MM, Leal RC, Assreuy J, Kloetzel JK. Nitric oxide involvement in experimental Trypanosoma cruzi infection in Calomys callosus and Swiss mice. Parasitol Res 1997;83:762-770.
- Tanisaki M, Ogawa K, Lapa SRC, Da Silva PMC, Watanabe I. Morphometric and high resolution scanning electron microscopy study of Calomys callosus major palatine nerve. Int J Morphol 2005;23:13-8.
- Dost CK, Saraiva J, Zentgraf U, Monesi N, Engels W, Albuquerque S. Is nitric oxide involved in the tolerance of Calomys callosus as a reservoir host towards Trypanosoma cruzi infection? J Infect 2006;52:49-55.
- Taniwaki NN, Andreoli WK, Calabrese KS, da Silva S, Mortara RA. Disruption of myofibrillar proteins in cardiac muscle of Calomys callosus chronically infected with Trypanosoma cruzi and treated with immunosuppressive agent. Parasitol Res 2005;97:323-31.
- Martinez M, Milton FA, de Oliveira SA, de Lima NF, Segatelli TM, Pinheiro PF, et al. Ultrastructural changes on the hard palatine mucosa of Calomys callosus after 120 days of experimental chronic alcoholism. J Submicrosc Cytol Pathol 2005;37:59-65.
- Carroll DS, Mills JN, Montgomery JM, Bausch DG, Blair PJ, Burans JP, et al. Hantavirus pulmonary syndrome in central Bolivia: relationships between reservoir hosts, habitats, and viral genotypes. Am J Trop Med Hyg 2005;72:42-6.
- Gomes-Filho JE, Gomes BP, Zaia AA, Novaes PD, Souza-Filho FJ. Glycol methacrylate: an alternative method for embedding subcutaneous implants. J Endod 2001;27:266-8.
- Pascon EA, Leonardo MR, Safavi K, Langeland K. Tissue reaction to endodontic materials: methods, criteria, assessment, and observations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1991;72:222-37.
- Holland R, Otoboni Filho JA, Bernabé PF, Nery MJ, de Souza V, Berbert A. Effect of root canal status on periodontal healing after surgical injury in dogs. Endod Dent Traumatol 1994;10:77-82.
- Hume WR. Effect of eugenol on respiration and division on human pulp, mouse fibroblasts, and liver cells in vitro. J Dent Res 1984;63:1262-5.
- Gulati N, Chandra S, Aggarwal PK, Jaiswal JN, Singh M. Cytotoxicity of eugenol in sealer containing zinc-oxide. Endod Dent Traumatol 1991;7:181-5.
- Neff T, Layman D, Jeansonne BG. In vitro cytotoxicity evaluation of endodontic sealers exposed to heat before assay. J Endod 2002;28:811-4.
- Ioannis K, Nikolaos E. In vivo comparison of the biocompatibility of 2 root canal sealers implanted into the subcutaneous connective tissue of rats. J Endod 1998;24:82-5.
- Nikolaos E, Ioannis K. Experimental study of the biocompatibility of four root canal sealers and their influence on the zinc and calcium content of several tissues. J Endod 1995;21:122-7.
- Segura JJ, Jimenez-Rúbio A. Effect of eugenol on macrophage adhesion in vivo to plastic surfaces. Endod Dent Traumatol 1998;14:72-4.
- de Oliveira Mendes ST, Ribeiro Sobrinho AP, de Carvalho AT, de Souza Cortes MI, Vieira LQ. In vitro evaluation of the cytotoxicity of two root canal sealers on macrophage activity. J Endod 2003;29:95-9.
- Di Felice R, Lombardi T. Gingival and mandibular bone necrosis caused by a paraformaldehyde containing paste. Endod Dent Traumatol 1998;14:196-198.
- Cotran RS, Kumar V, Robbins SL. Pathologic basis of disease: cellular events. 5th ed. Philadelphia: Saunders; 1994.
- el Sayed F, Seite-Bellezza D, Sans B, Bayle-Lebey P, Marguery MC, Bazex J. Contact urticaria from formaldehyde in a root-canal dental paste. Contact Dermatitis 1995;33:353.
- Ebner H, Kraft D. Formaldehyde-induced anaphylaxis after dental treatment? Contact Dermatitis 1991;24:307-9.
