Correlative Bacteriologic and Micro–Computed Tomographic Analysis of Mandibular Molar Mesial Canals Prepared by Self-Adjusting File, Reciproc, and Twisted File Systems
Abstract
Introduction: This ex vivo study evaluated the disinfecting and shaping ability of 3 protocols used in the preparation of mesial root canals of mandibular molars by means of correlative bacteriologic and micro–computed tomographic (mCT) analysis.
Methods: The mesial canals of extracted mandibular molars were contaminated with Enterococcus faecalis for 30 days and assigned to 3 groups based on their anatomic configuration as determined by mCT analysis according to the preparation technique (Self-Adjusting File [ReDent-Nova, Ra’anana, Israel], Reciproc [VDW, Munich, Germany], and Twisted File [SybronEndo, Orange, CA]). In all groups, 2.5% NaOCl was the irrigant. Canal samples were taken before (S1) and after instrumentation (S2), and bacterial quantification was performed using culture. Next, mesial roots were subjected to additional mCT analysis in order to evaluate shaping of the canals.
Results: All instrumentation protocols promoted a highly significant intracanal bacterial reduction (P < .001). Intergroup quantitative and qualitative comparisons disclosed no significant differences between groups (P > .05). As for shaping, no statistical difference was observed between the techniques regarding the mean percentage of volume increase, the surface area increase, the unprepared surface area, and the relative unprepared surface area (P > .05). Correlative analysis showed no statistically significant relationship between bacterial reduction and the mean percentage increase of the analyzed parameters (P > .05).
Conclusions: The 3 instrumentation systems have similar disinfecting and shaping performance in the preparation of mesial canals of mandibular molars. (J Endod 2013;39:1044–1050)
The primary goals of chemomechanical preparation are cleaning and shaping of the root canal. Although distinct, these objectives are achieved simultaneously during preparation through the use of instruments and irrigants. In infected root canals, disinfection is also required, and the best treatment outcome is usually achieved when root canal infection is eradicated or reduced to levels compatible with periradicular healing. Considering that remaining infection is an important risk factor for post-treatment apical periodontitis, chemomechanical preparation assumes a pivotal role in treatment because it acts mechanically and chemically on bacterial communities colonizing the main canal.
Several studies have shown that, regardless of the instrumentation technique and instruments/irrigants used, chemomechanical procedures are unable to promote a thorough cleaning, disinfection, and shaping of the root canal, especially in curved canals or cases with unusual anatomies. Although the main canal and minor anatomic irregularities are generally incorporated into preparation, tissue remnants and bacteria present in other areas such as isthmuses, lateral canals, and apical ramifications usually remain unaffected by preparation because of inherent physical limitations of instruments and the short time of permanence of the irrigants within the root canal. Besides, even areas of the main root canal have been shown to remain untouched by instruments.
In order to circumvent such limitations and make cleaning, disinfection, and shaping more predictable, modifications in instruments and techniques have been devised. The Self-Adjusting File (SAF) (ReDent-Nova, Ra’anana, Israel) is an example of a modified instrument that has been designed to adapt to the main root canal anatomy. Studies have shown that the SAF promotes increased cleaning, disinfection, and shaping when compared with conventional endodontic instruments. The SAF is recommended to be used as a single-instrument technique, and, in this same line, other systems, such as Reciproc (VDW, Munich, Germany), have also been introduced. Unlike the SAF, these instruments have a helical shaft and are not substantially different from conventional rotary instruments but are proposed to be operated in reciprocating motion. Studies have shown that single-file instrumentation techniques using these helical instruments may promote cleaning and shaping effects comparable with instrumentation with a full series of rotary instruments. There are not many studies evaluating the disinfecting ability of single-file systems, but a recent one showed no significant differences in intracanal bacterial reduction induced by Reciproc and a full series of instruments, provided that the volume of irrigants and the time of irrigation were kept similar.
Micro–computed tomographic (mCT) imaging has been widely used to evaluate ex vivo the shaping ability of instruments and techniques. mCT imaging offers a noninvasive reproducible technique for 3-dimensional (3D) assessment of the root canal system before and after preparation, and it can be applied both quantitatively and qualitatively. Using this methodology, it has been shown that a large area of the main root canal walls remains untouched after preparation, regardless of the instrumentation technique or instrument used. Consequently, bacterial biofilms adhered to the walls may remain unaffected in these regions. For evaluation of the disinfecting ability of instruments, techniques, and irrigants, an ex vivo canal disinfection model that used extracted teeth with canals contaminated with Enterococcus faecalis has been widely used. Although very useful for comparisons between different protocols, this experimental model provides information about bacterial persistence, but the location of persisting infection cannot be ascertained. Microscopy could be added for this analysis, but its destructive nature precludes analysis of the root canal before instrumentation. Moreover, because it does not provide a 3D image, the quality of preparation cannot be assessed. The purpose of this ex vivo study was to evaluate the disinfecting and shaping ability of 3 instrumentation protocols used in the preparation of mesial root canals of extracted mandibular molars by means of a correlative bacteriologic and mCT analysis.
Materials and Methods
Specimen Selection and Preparation
This study used straight or slightly curved mesial roots of mandibular molars extracted for reasons not related to this study. Approval for the study protocol was obtained from the Ethics Committee of Estácio de Sá University, Rio de Janeiro, RJ, Brazil. Teeth were initially selected on the basis of radiographs taken in both the buccolingual and mesiodistal directions. Only teeth with 2 mesial canals and no significant calcifications were included. Access cavities were prepared, and hemiresection of the molars was performed using diamond disks. The mesial section (root and crown) was used in the study. Cusps were reduced until all specimens were approximately 13 mm in length. Mesial root canals were explored by using size 08 and 10 K-type files, and only those roots with 2 independent apical foramina were maintained in the study.
All root canals were instrumented 1 mm beyond the apical foramen up to a size 20 hand K-type file in alternating rotary motions under continuous irrigation with water. This was performed to standardize the initial root canal diameter and create room for further contamination of the canals. The smear layer was removed by using 17% EDTA and 2.5% sodium hypochlorite (NaOCl) irrigation. NaOCl was inactivated with 10% sodium thiosulfate.
mCT Analysis of the Root Canals
Morphometric evaluation of the root canal was performed by using mCT imaging. From the initial sample, 18 specimens were randomly selected, dried, mounted on a custom attachment, and scanned using a mCT scanner (SkyScan 1174v2; Bruker-microCT, Kontich, Belgium) at an isotropic resolution of 16.7 mm. Scanning was performed by 180° rotation around the vertical axis with a rotation step of 1.0 using a 0.5-mm-thick aluminum filter. Images of each specimen were reconstructed with a dedicated software (NRecon v.1.6.3, Bruker-microCT) providing axial cross-sections of the inner structure of the samples. CTAn v.1.12 software (Bruker-microCT) was used for 3D evaluation of the root canal regarding volume and surface area. Volume was calculated as the volume of binarized objects within the volume of interest. For the measurement of the surface area, 2 components of the surface were used: (1) the perimeters of the binarized objects on each cross-sectional level and (2) the vertical surfaces exposed by pixel differences between adjacent cross-sections. CTVol v.2.2.1 software (Bruker-microCT) was used for visualization and qualitative evaluation of the root canal system configuration.
The specimens were matched in trios on the basis of the anatomic configuration of the root canal assessed by mCT imaging. One specimen from each threesome was randomly assigned to 1 of the 3 experimental groups. After checking for normality assumption (the Shapiro-Wilk test), the degree of homogeneity (baseline) of the 3 groups, with respect to the aforementioned 3D parameters, was assessed using 1-way analysis of variance with a confidence level set at 5%. Then, a flip of a coin was used to define which group of teeth would be treated with each of the following root canal preparation techniques: SAF, Reciproc, or Twisted File (TF; SybronEndo, Orange, CA) systems.
Bacterial Contamination
The 18 mesial roots selected by mCT screening were used in the correlative bacteriologic/mCT analysis. To strengthen the statistic power of bacteriologic analysis, another 18 specimens, selected on the basis of radiographs, were also included. Both mesial canals were used in the experiment, totaling 36 roots (72 canals) for the bacteriologic analysis (24 canals per experimental group). Five mesial roots were used as controls: 3 for the bacteriologic experiment and 2 for scanning electron microscopy.
The root canals were filled with freshly prepared trypticase soy broth (Difco, Detroit, MI), immersed in the same broth, and ultrasonicated for 1 minute to release entrapped air and allow penetration of the culture medium into root canal irregularities. Then, the tooth specimens were sterilized in an autoclave. E. faecalis strain American Type Culture Collection 29212 was used for root canal contamination for 30 days at 37°C under gentle shaking, with the culture medium being replenished every week. After the contamination period, all teeth had the excess of culture medium dripped off and their external root surface wiped with sterile gauze. Two teeth were fixed in 10% buffered formalin and processed for scanning electron microscopic analysis to confirm bacterial colonization of the root canal as described previously. Six contaminated but uninstrumented mesial canals, which were irrigated with the same substances and volumes as the experimental groups, served as the positive control group.
The apical foramina of each mesial root were sealed with fast-setting epoxy resin in order to prevent apical bacterial leakage and create a closed-end channel that produces the vapor lock effect. Teeth were mounted vertically up to the cervical region in blocks made of a silicone impression material (President Jet; Coltène AG, Cuyahoga Falls, OH). The tooth crown, including the pulp chamber walls, and the silicone surface were disinfected with 2.5% NaOCl followed by inactivation of this substance with 10% sodium thiosulfate. Next, the working length (WL) was determined by introducing a size 10 K-type file in the canal until it reached the apical foramen, and initial bacteriologic samples (S1) were taken from all canals before preparation as follows. The root canal was rinsed with 1 mL sterile 0.85% saline solution to remove unattached cells, and an initial sample was taken by the sequential use of 3 to 5 paper points placed to the WL.
Each paper point remained in the canal for 1 minute. Paper points were transferred to tubes containing 1 mL sterile saline and immediately processed for culture analysis.
Before instrumentation, all root canals received initial irrigation with 2 mL 2.5% NaOCl followed by coronal enlargement using a RaCe file size 25, taper 0.08 mm/mm (BR0; FKG Dentaire, La Chaux-de- Fonds, Switzerland). Next, the root canals were once again irrigated with 2 mL 2.5% NaOCl, and a size 15 K-type file was taken to the WL to confirm patency. The following steps differed according to each experimental group.
SAF Group
Canals were instrumented to the WL using a RaCe file size 25, taper 0.04 mm/mm (FKG Dentaire). After irrigation with 2 mL 2.5% NaOCl, the SAF instrument was inserted in the canal and operated with an in- and-out motion to the WL for a total of 3 minutes. A 1.5-mm diameter SAF was used in a vibrating handpiece (GENTLEpower; KaVo, Bieberach an der Riß, Germany) combined with a RDT3 head (ReDent-Nova) at a frequency of 5000 movements per minute and an amplitude of 0.4 mm. Continuous irrigation with 2.5% NaOCl was applied throughout the procedure at a flow rate of 2 mL/min (total = 6 mL per canal) using a special irrigation apparatus (VATEA, ReDent-Nova).
Reciproc Group
The Reciproc R25 instrument was operated in a reciprocating motion powered by a torque-limited electric motor (VDW Silver) using the preset adjustments. The instrument was introduced into the canal until resistance was felt and then activated. It was moved in the apical direction using an in-and-out pecking motion about 3 mm in amplitude with light apical pressure. After 3 pecking motions, the instrument was removed and cleaned, and the canal was irrigated with 2 mL NaOCl. Patency of the canal was checked by taking a size 15 K-type file to the WL. This protocol was repeated until the WL was reached by the R25 instrument. Irrigation was performed with disposable syringes and 30-G NaviTip needles (Ultradent, South Jordan, UT) taken up to 4 mm short of the WL.
TF Group
In this group, root canals were prepared using the following sequence of TF instruments: 25/0.08, 25/0.06, and 25/0.04 in a crown-down manner, operated at 500 rpm, in continuous clockwise rotation, with the VDW Silver motor. Each instrument was introduced in the canal while rotating until it engaged dentin. Then, it was withdrawn, and the canal was irrigated with 2 mL NaOCl and checked for apical patency with a size 15 K-type file. Irrigation was performed as in the Reciproc group. Preparation was finished by working with the TF 25/0.06 at the WL and the canal irrigated with 2 mL NaOCl.
After preparation was complete, the smear layer was removed by rinsing the canal with 2 mL 17% EDTA per 1 minute followed by 2 mL 2.5% NaOCl. NaOCl was inactivated by rinsing the canal with 1 mL 10% sodium thiosulfate, which was left in the canal for 5 minutes. After another irrigation with 1 mL sodium thiosulfate, a postpreparation bacteriologic sample (S2) was taken as described for S1. In all groups, the total volume of 14 mL of 2.5% NaOCl was used per canal.
Bacterial Quantification
Samples were 10-fold serially diluted in saline (up to 10—5 in S1 and 10—3 in S2). Afterward, aliquots of 100 mL were plated onto Mitis-Salivarius agar plates (Difco) and incubated at 37°C for 48 hours. The colony-forming units grown were counted, and a species-specific polymerase chain reaction was performed to confirm the identification of E. faecalis in all positive samples.
mCT Analysis of Root Canal Preparation
After chemomechanical preparation and S2 taking, the 18 specimens scanned in the first part of this study were autoclaved, dried, and subjected to a new mCT scan applying the same parameter settings. Superimposition of the pre- and postpreparation images of the root canals was performed by means of a previously validated registration software (Mosaic 0.05; Institute of Communication and Computer Systems, Athens, Greece). Color-coded root canal models (green indicates preoperative and red postoperative canal surfaces) enabled qualitative comparison of the matched root canals before and after shaping with CTVol v.2.2.1 software (Bruker-microCT). CTAn v.1.12 (Bruker-microCT) was used for volume and surface area analyses.
The mean percentage increase (%D) of each analyzed parameter was calculated as follows: ([Pa — Pb]/Pb)*100, where Pb and Pa are the total volume or surface area of the root canal before and after preparation, respectively. Spatially registered surface models of the roots, before and after preparation, were compared to evaluate the percentage of remaining unprepared surface area. This variable was calculated by using the distances between the surface of the root canals before and after preparation that had been determined at every surface point.
When an isthmus was present, its relative volume and area were assessed. In this study, the isthmus was defined as the ribbon-shaped or thin structure between the 2 mesial root canals after preparation. Its contribution to the total volume and surface area of the root canal was determined as the relative percentage volume (%RV) and the relative percentage surface area (%RA) of the isthmus. The %RV was calculated as follows: ([Va — RV]/Vb)*100, where Vb and Va are the total volume of the root canal before and after preparation, respectively, and the RV (relative volume) is the volume after preparation without isthmus. The %RA was calculated by the formula (A — RA/A)*100, where A is the total surface area of the root canal after preparation and RA is the surface area of the prepared root canals without isthmus.
Statistical Analysis
The Wilcoxon matched pairs test was used to compare the intragroup reduction in bacterial counts from S1 to S2. In addition, intergroup comparison was accomplished using the Kruskall-Wallis test. For this, the proportion S2/S1 was calculated for each canal and used for comparison between groups. Sample size calculation revealed that 21 specimens per group would suffice to show a 5% difference in S2/S1 proportions, with a power of 90%. Each experimental group was compared with the control group by using the Mann-Whitney U test. For intragroup and intergroup pair-wise analyses of qualitative data (presence/absence), the Fisher exact test was used.
In mCT analysis, because normality could not be verified (Shapiro- Wilk test, P < .05), the mean percentage of volume increase, surface area increase, unprepared surface area, RV, and RA were compared between groups by using the Kruskal-Wallis test and within groups by using the paired-sample t test. Pearson’s correlation analysis was used to verify the relationships between bacterial reduction and the mean percentage increase of the parameters analyzed using mCT imaging. Statistical analyses were performed with STATISTICA version 8 (StatSoft, Tulsa, OK) and SPSS version 17.0 (SPSS Inc, Chicago, IL) with a confidence level set at 5%.
Results
Scanning electron microscopic analysis revealed that E. faecalis colonized the root canal walls, usually forming biofilm-like structures (data not shown). Root canal colonization was further confirmed by culture positive results for S1 samples from all teeth used in the canal disinfection experiment.
Table 1 depicts the mean, median, and range of colony-forming unit counts (quantitative data) observed for the 3 test groups and the control group. Experimental data are shown for the 18 teeth (36 canals) subjected to both bacteriologic and mCT analyses and the overall 36 teeth (72 canals) that were bacteriologically investigated. Of the 18 teeth subjected to correlative analysis, only 7 (39%) showed a negative culture for both mesial canals, regardless of the technique (2 teeth from the Reciproc group, 2 from the SAF group, and 3 from the TF group). Overall, the incidence of positive cultures in S2 was 6 of 24 (25%) for the Reciproc file, 7 of 24 (29%) for the SAF, and 9 of 24 (37.5%) for the TF. Intragroup quantitative analysis evaluating bacterial reduction from S1 to S2 in all groups showed that chemomechanical preparation using the 3 instrumentation systems promoted a highly significant bacterial reduction (P < .001). No significant differences were observed between groups either for quantitative or qualitative analysis (P > .05). All techniques were significantly better than the control group (irrigation with no instrumentation) in reducing the bacterial levels (P < .001).
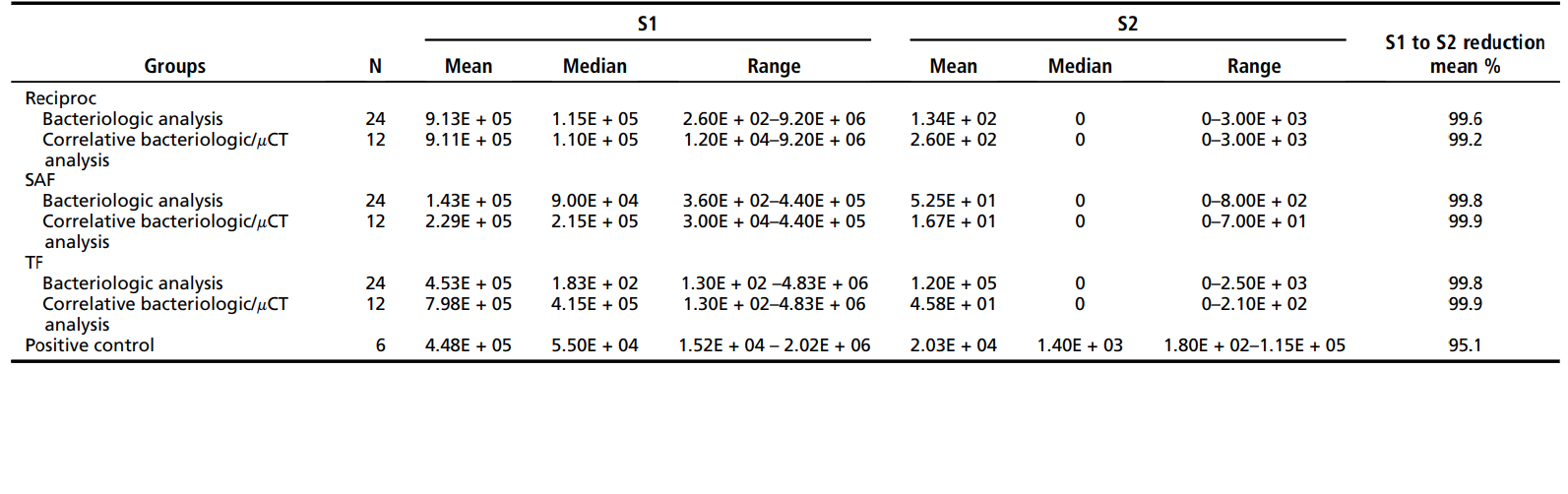
Table 2 displays data from mCT analysis. No statistical difference was observed between Reciproc, SAF, and TF instruments regarding the mean percentage of volume increase, the surface area increase, and the unprepared surface area (P > .05). Intragroup analysis disclosed a statistically significant difference regarding the volume and surface area before and after root canal preparation (P < .05). The %RV and %RA of the isthmus in the Reciproc, SAF, and TF groups were 22.9 ± 15.2 and 23.9 ± 16.2, 16.9 ± 16.51 and 17.5 ± 13.3, and 23.7 ± 16.3 and 23.7 ± 12.8, respectively, with no significant statistical difference between groups (P > .05).
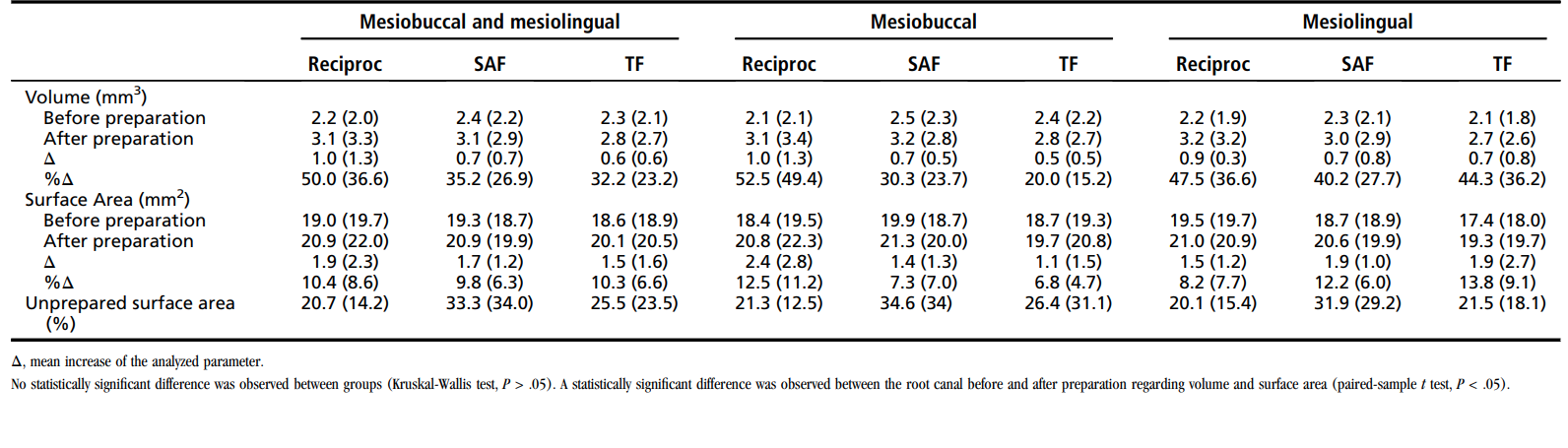
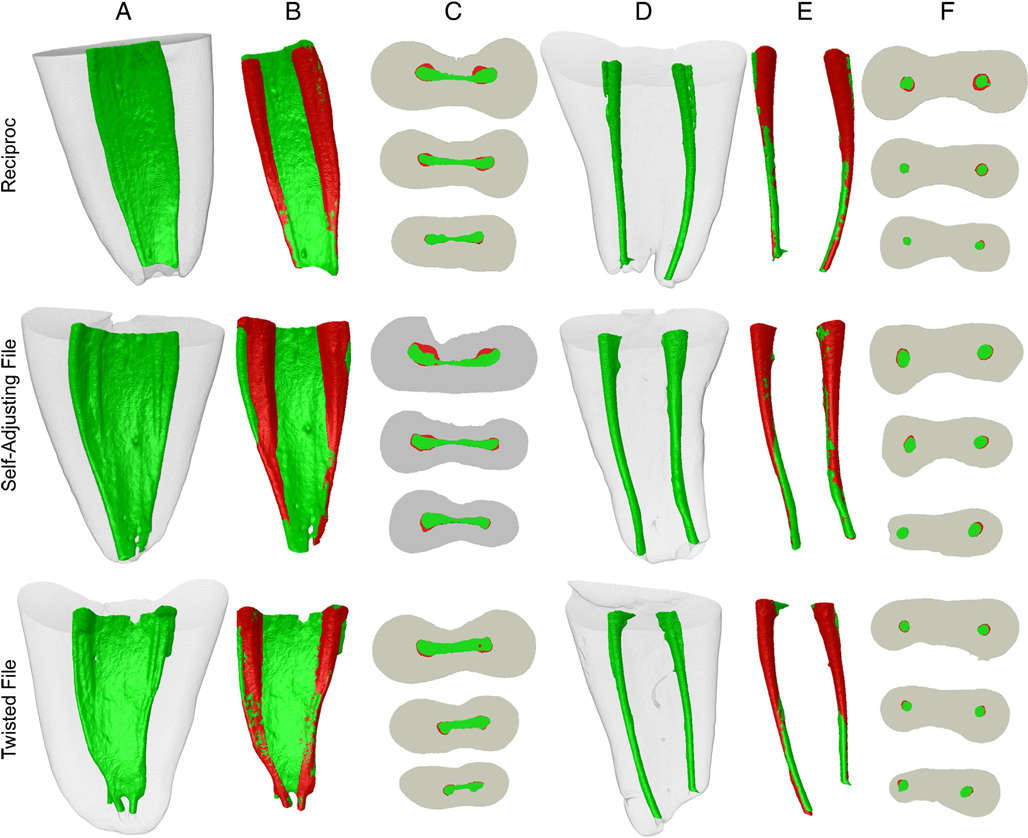
Figure 1A through F shows representative examples of superimposed root canals before and after canal instrumentation in each group. Changes in canal shape are shown as superimpositions of unprepared (green) and prepared (red) areas. Red shows the overlapping areas. All groups showed more untouched areas mainly on the isthmus and apical third of the canal. None of the instrumentation systems was able to prepare all surface areas of the root canal. Correlative analysis showed no statistically significant relationship between bacterial reduction and the percentage mean increase of the analyzed parameters (P > .05).
Discussion
The present study protocol was designed to allow a matched analysis of the ability of 3 instrumentation techniques in shaping and disinfecting mesial root canals of mandibular molars. For this, the shaping ability was evaluated using mCT analysis of the same teeth subjected to bacteriologic analysis.
An innovation of the present study was the use of mCT screening for adequate selection of teeth for the ex vivo canal disinfection assay. Previous studies selected teeth based on the external anatomy and radiographs taken in both the mesiodistal and buccolingual directions. By selecting teeth based on the internal anatomy as revealed by mCT screening, a better distribution of specimens among groups is expected, eliminating potentially significant biases that might interfere with the results. Measurements of the volume and surface area of the root canal were not used in this study for the selection of teeth and distribution among groups and would be interesting additional information for future studies. However, these factors were not found to interfere with the results, as statistically shown.
Bacteriologic analysis showed that chemomechanical preparation with the 3 different systems was statistically equally effective in promoting a highly significant reduction in intracanal bacterial counts. However, many cases still had detectable bacteria after instrumentation. The incidence of positive cultures in S2 samples (qualitative analysis) ranged from 25% (Reciproc group) to 37.5% (TF group), which is in consonance with previous ex vivo and in vivo studies. It is worth pointing out that most previous studies were based on canals of single-rooted teeth, whereas in the present study mesial roots of mandibular molars were used, which are expected to exhibit higher anatomic complexity. The present findings confirm that although chemomechanical preparation can be regarded as the main step in terms of infection control, it may not suffice to predictably disinfect root canals, regardless of the instruments used. Remaining bacteria are conceivably lodged in unprepared areas and recesses of the main canal as well as in isthmuses and other irregularities of the canal system. The main therapeutic challenge is to reach and kill them in those locations.
Single-file techniques have been recently recommended for root canal preparation, and new instruments were launched mostly based on opinion and convenience rather than proven effectiveness. However, it has been shown that the shaping and disinfecting abilities of these systems are comparable with the conventional ones using a full range of instruments. These findings were confirmed in the present study for both disinfection and shaping.
The SAF system is also a single-instrument approach, but the design of the instrument and principle of action are completely different. The instrument is designed to adapt to the root canal morphology in the cross-section. Consequently, the results of the SAF in terms of cleaning, shaping, and disinfection of canals that are curved and/or irregular in the cross-section have been shown to be superior to conventional instruments. Nevertheless, the results of the present study in mesial canals of molars showed no superior results for the SAF either in the shaping ability or antibacterial effectiveness. It should be noted that the SAF was operated for 3 minutes in the canal. Although a previous study reported that the SAF system promoted significant reduction in bacterial populations in oval-shaped canals of incisors and premolars even after only 2 minutes of operation, the most impressive results were obtained after 6 minutes. Whether the SAF effects in mesial canals would be improved after a longer operation time remains to be investigated.
The mesial root of mandibular molars presents a high degree of complexity, making it difficult to achieve optimal results in terms of antibacterial and shaping ability. Many areas of the main root canal remained unprepared regardless of the instrumentation technique used, ranging on average from 20% to 35% (isthmus area excluded). One may reasonably assume that larger apical preparations might have resulted in an increased prepared surface area and improved disinfection, and this is worth further investigation using this correlative approach. Whenever present, isthmuses were not mechanically affected by instrumentation and possibly not even by irrigants. The latter could not be evaluated by mCT analysis, and the limitations of sampling procedures using paper points prevent the bacteriologic analysis from evaluating the permanence of bacteria in isthmuses.
Despite the different designs and tapers of the instruments, efforts were made to standardize the size of preparations as much as possible. Dimensions of the files used for final apical preparation were as follows: TF size 25 with taper 0.06 mm/mm along the helical shaft of the instrument, Reciproc R25 with a 0.08 taper along the last the 3 mm from the tip, and the SAF used for 3 minutes after glide path prepared with a conventional rotary file size 25/0.04. These approaches resulted in preparations with no significant differences in the final volume and prepared surface area as shown by mCT analysis. Because the total volume of irrigants was the same, this lack of a significant difference was also reflected in the antibacterial effectiveness of the 3 instrumentation approaches, which did not differ even with a further increase in sample size.
It was expected that canals showing positive results for bacteria after instrumentation would present a larger unprepared surface area. Nonetheless, even though all specimens showing bacterial presence in S2 had unprepared areas, there were also specimens with large unprepared areas that showed negative cultures. Correlative analysis failed to show statistically significant relationships between bacterial elimination and the percentage mean increase of the parameters analyzed in mCT imaging. It is reasonable to assume that in some specimens the main canal areas untouched by instruments may not have been colonized by bacteria because colonization is not usually uniform along the canal walls. Another possibility is that unprepared areas were successfully disinfected by NaOCl, which has strong antibacterial and antibiofilm activities.
Although this study is innovative in the sense that it combines data from 2 approaches allowing for a correlative analysis, it is not free of limitations. The lack of correlation between the 2 analyses may be related to the factors discussed previously or may have been a result of limitations of sampling procedures using paper points, which may fail to achieve a good representative sample from the root canal system, especially from irregularities, recesses, and areas distant from the main canal. Future studies using either histobacteriologic analysis or cryopulverization of root specimens should circumvent these limitations. However, these approaches also have their own limitations because both are destructive and do not permit specimens to be evaluated before and after instrumentation. Moreover, the histobacteriologic analysis provides 2-dimensional (2D) information of the selected areas and does not give information about viability, whereas cryopulverization does not indicate the location of persisting bacteria. Future studies should consider adding one of these approaches to those used in the present study for a more comprehensive analysis. Further research using this correlative approach may investigate the potential impact of irrigant variables (eg, flow patterns, concentration, exposure time, and temperature) as well as different anatomic configurations and preparation systems on the outcome.
In conclusion, this study showed that the 3 instrumentation techniques have similar disinfecting and shaping performance in the preparation of mesial canals of mandibular molars. Further refinements in the correlative analysis used in this study have the potential to contribute to a method that can comprehensively assess the performance of instruments and instrumentation techniques.
Authors: José F. Siqueira, Jr, Flávio R.F. Alves, Marco A. Versiani, Isabela N. Rôças, Bernardo M. Almeida, Mônica A.S. Neves, Manoel D. Sousa-Neto
References:
- Siqueira JF Jr, Lopes HP. Chemomechanical preparation. In: Siqueira JF Jr, ed. Treatment of Endodontic Infections. London: Quintessence Publishing; 2011:236–84.
- Sjögren U, Figdor D, Persson S, et al. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J 1997;30:297–306.
- Siqueira JF Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod 2008;34:1291–13013.
- Barbizam JV, Fariniuk LF, Marchesan MA, et al. Effectiveness of manual and rotary instrumentation techniques for cleaning flattened root canals. J Endod 2002;28: 365–6.
- Usman N, Baumgartner JC, Marshall JG. Influence of instrument size on root canal debridement. J Endod 2004;30:110–2.
- Siqueira JF Jr, Araujo MC, Garcia PF, et al. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod 1997;23:499–502.
- Peters OA, Schönenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J 2001;34: 221–30.
- Vera J, Siqueira JF Jr, Ricucci D, et al. One-versus two-visit endodontic treatment of teeth with apical periodontitis: a histobacteriologic study. J Endod 2012;38: 1040–52.
- Nair PN, Henry S, Cano V, et al. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after ‘‘one-visit’’ endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99:231–52.
- Metzger Z, Teperovich E, Zary R, et al. The self-adjusting file (SAF). Part 1: respecting the root canal anatomy—a new concept of endodontic files and its implementation. J Endod 2010;36:679–90.
- Metzger Z, Teperovich E, Cohen R, et al. The self-adjusting file (SAF). Part 3: removal of debris and smear layer-A scanning electron microscope study. J Endod 2010;36:697–702.
- De-Deus G, Souza EM, Barino B, et al. The self-adjusting file optimizes debridement quality in oval-shaped root canals. J Endod 2011;37:701–5.
- Ribeiro MVM, Silva-Sousa YT, Versiani MA, et al. Comparison of the cleaning efficacy of self-adjusting file and rotary systems in the apical third of oval-shaped canals. J Endod 2013;39:398–401.
- Siqueira JF Jr, Alves FR, Almeida BM, et al. Ability of chemomechanical preparation with either rotary instruments or self-adjusting file to disinfect oval-shaped root canals. J Endod 2010;36:1860–5.
- Versiani MA, Pecora JD, de Sousa-Neto MD. Flat-oval root canal preparation with self-adjusting file instrument: a micro-computed tomography study. J Endod 2011;37:1002–7.
- Paque F, Peters OA. Micro-computed tomography evaluation of the preparation of long oval root canals in mandibular molars with the self-adjusting file. J Endod 2011;37:517–21.
- De-Deus G, Barino B, Zamolyi RQ, et al. Suboptimal debridement quality produced by the single-file F2 ProTaper technique in oval-shaped canals. J Endod 2010;36: 1897–900.
- Burklein S, Hinschitza K, Dammaschke T, et al. Shaping ability and cleaning effectiveness of two single-file systems in severely curved root canals of extracted teeth: reciproc and WaveOne versus Mtwo and ProTaper. Int Endod J 2012;45: 449–61.
- Berutti E, Chiandussi G, Paolino DS, et al. Canal shaping with WaveOne primary reciprocating files and ProTaper system: a comparative study. J Endod 2012;38: 505–9.
- Dietrich MA, Kirkpatrick TC, Yaccino JM. In vitro canal and isthmus debris removal of the self-adjusting file, K3, and WaveOne files in the mesial root of human mandibular molars. J Endod 2012;38:1140–4.
- Alves FR, Rôças IN, Almeida BM, et al. Quantitative molecular and culture analyses of bacterial elimination in oval-shaped root canals by a single-file instrumentation technique. Int Endod J 2012;45:871–7.
- Peters OA, Paque F. Root canal preparation of maxillary molars with the self-adjusting file: a micro-computed tomography study. J Endod 2011;37:53–7.
- Markvart M, Darvann TA, Larsen P, et al. Micro-CT analyses of apical enlargement and molar root canal complexity. Int Endod J 2012;45:273–81.
- Solomonov M, Paque F, Fan B, et al. The challenge of C-shaped canal systems: a comparative study of the self-adjusting file and ProTaper. J Endod 2012;38: 209–14.
- Paque F, Balmer M, Attin T, et al. Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instruments: a micro-computed tomography study. J Endod 2010;36:703–7.
- Paque F, Ganahl D, Peters OA. Effects of root canal preparation on apical geometry assessed by micro-computed tomography. J Endod 2009;35:1056–9.
- Alves FR, Almeida BM, Neves MA, et al. Time-dependent antibacterial effects of the self-adjusting file used with two sodium hypochlorite concentrations. J Endod 2011; 37:1451–5.
- Siqueira JF Jr, Rôças IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:85–94.
- Tassani S, Matsopoulos GK, Baruffaldi F. 3D identification of trabecular bone fracture zone using an automatic image registration scheme: a validation study. J Biomech 2012;45:2035–40.
- Paiva SS, Siqueira JF Jr, Rôças IN, et al. Supplementing the antimicrobial effects of chemomechanical debridement with either passive ultrasonic irrigation or a final rinse with chlorhexidine: a clinical study. J Endod 2012;38:1202–6.
- Ricucci D, Loghin S, Siqueira JF Jr. Exuberant biofilm infection in a lateral canal as the cause of short-term endodontic treatment failure: report of a case. J Endod 2013;39:712–8.
- Ruckman JE, Whitten B, Sedgley CM, et al. Comparison of the self-adjusting file with rotary and hand instrumentation in long-oval-shaped root canals. J Endod 2013;39: 92–5.
- Siqueira JF Jr, Lima KC, Magalhaes FA, et al. Mechanical reduction of the bacterial population in the root canal by three instrumentation techniques. J Endod 1999;25: 332–5.
- Siqueira JF Jr, Rôças IN, Lopes HP. Patterns of microbial colonization in primary root canal infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 93:174–8.
- Ordinola-Zapata R, Bramante CM, Cavenago B, et al. Antimicrobial effect of endodontic solutions used as final irrigants on a dentine biofilm model. Int Endod J 2012;45:162–8.
- Alves FR, Siqueira JF Jr, Carmo FL, et al. Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. J Endod 2009;35:486–92.
