Comparison of the Cleaning Efficacy of Self-Adjusting File and Rotary Systems in the Apical Third of Oval-shaped Canals
Abstract
Introduction: Cleaning and shaping of root canals are essential steps for the success of endodontic therapy. The purpose of this study was to evaluate the tissue debridement efficacy of the self-adjusting file (SAF) protocol in the apical third of oval-shaped canals of mandibular incisors in comparison with a nickel-titanium rotary system preparation.
Methods: Twenty- six single-rooted human mandibular incisor teeth were selected and assigned to a control (n = 4) and 2 experimental groups (n = 11) according to 1 of 2 instrumentation techniques, SAF and nickel-titanium rotary systems. After root canal preparation, the apical thirds of the specimens were submitted to histologic processing and analyzed by optical microscopy regarding the percentage of debris and uninstrumented root canal walls. The data were statistically compared by using unpaired t test with Welch’s correction, and the level of significance was set at 5%.
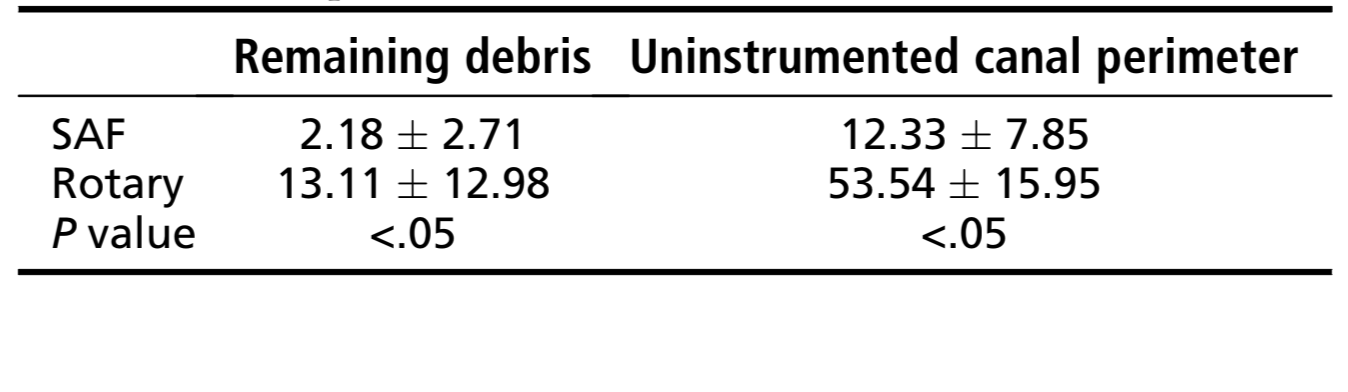
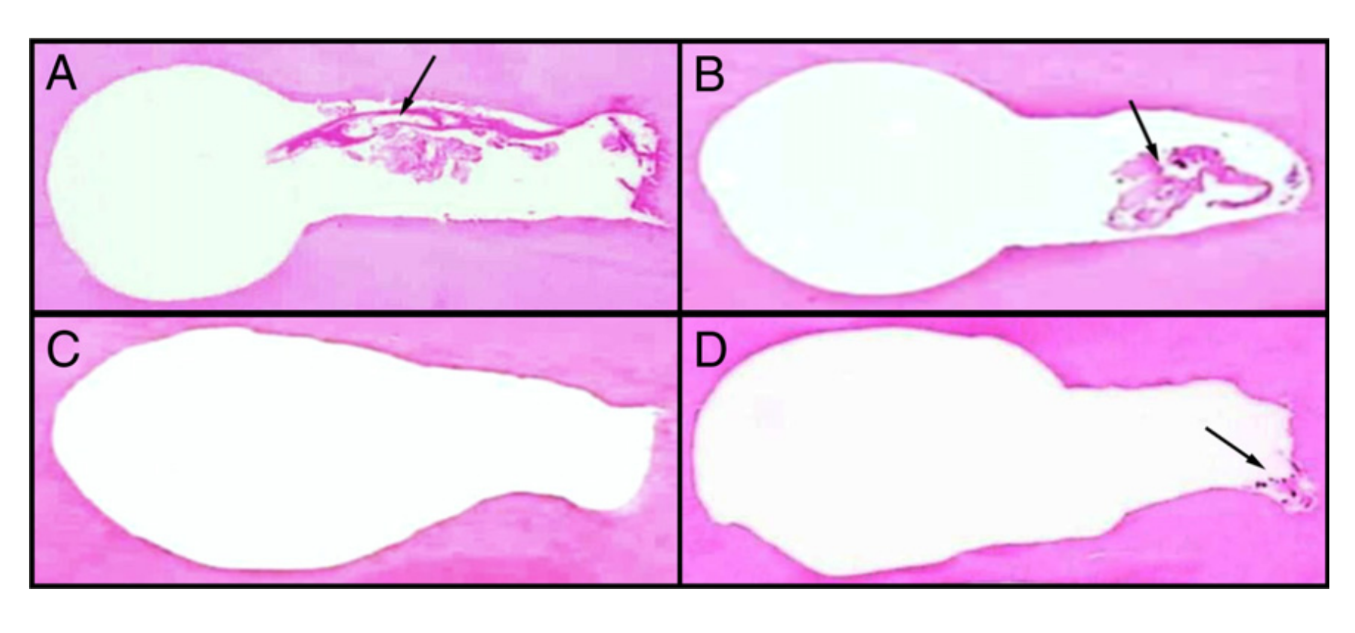
Results: The percentage of remaining debris and uninstrumented canal perimeter was significantly lower in SAF group (2.18 ± 2.71 and 12.33 ± 7.85, respectively) than in rotary group (13.11 ± 12.98 and 53.54 ± 15.95, respectively) (P < .05). In the SAF group most of specimens were completely free of debris, whereas in the rotary group 53% of the canals presented debris.
Conclusions: SAF had significantly more contact to the dentin walls and removed more debris than rotary instrumentation in the apical third of mandibular incisors. (J Endod 2013;■:1–4)
Preparation of the root canal system is recognized as being one of the most important stages in root canal treatment. It includes the removal of vital and necrotic tissues from the root canal system, along with infected root dentin, which gives the canal system a shape that allows easy debridement and predictable placement of locally used medicaments and a permanent root filling of high technical quality. The introduction of nickel-titanium (NiTi) rotary file systems has resulted in significant progress being made in the mechanical preparation of the root canal space. However, the rotary motion of these files tends to prepare the main root canal space into a circular shape, leaving unprepared buccal and lingual extensions, which favors the retention of tissue and bacterial remnants, especially in oval-shaped canals. Thus, although many advances have been made in endodontics in the last decades, canal preparation is still adversely influenced by highly variable root canal anatomy.
The self-adjusting file (SAF) (ReDent-Nova, Ra’anana, Israel) has been devised with the purpose of sidestepping some of the limitations of NiTi rotary instruments. Initial reports of the SAF system in oval-shaped root canals sound promising. During its operation, the file is designed to adapt itself three-dimensionally to the shape of the root canal. Rather than machining a central portion of the root canal into a round cross section, the SAF is claimed to maintain a flat canal as a flat canal with slightly larger dimensions.
Thus, the present study was designed to evaluate the tissue debridement efficacy of SAF protocol in the apical third of oval-shaped canals of mandibular incisors in comparison with a NiTi rotary system preparation.
Materials and Methods
Selection of Teeth
After ethics committee approval (protocol 2009.1.972.58.4, CAAE 0072.0.138.000-09), twenty-six vital single-rooted freshly extracted human mandibular incisor teeth with fully formed apices were selected and stored in 9◦C aqueous 0.1% thymol solution until further use. Each root was radiographed in buccolingual and mesiodistal projections to categorize them and to detect any possible obstruction. When the buccolingual diameter was 4 or more times larger than that of the mesiodistal diameter, the canals were classified as flattened. All teeth presenting isthmus, lateral, accessory, apical curvature, or 2 canals were excluded from the study. After being washed in running water for 48 hours, the root canal was accessed by using high-speed diamond burs. Coronal flaring was accomplished with #2 and #3 Gates Glidden burs (Dentsply Maillefer, Ballaigues, Switzerland) in a low-speed contra-angle handpiece, which was placed to 2–4 mm below the cementoenamel junction by irrigation with 5 mL 2.5% NaOCl delivered in a syringe with a 27-gauge needle (Endo Eze; Ultradent Products Inc, South Jordan, UT). Subsequently, apical patency was determined by inserting a size 10 K-file into the root canal until its tip was visible at the apical foramen, and the working length (WL) was set 0.5 mm short of this measurement. A glide path was confirmed at least to a size #20 K-file. Specimens were then randomly assigned to a control (n = 4) and 2 experimental groups (n = 11) according to the instrumentation technique, SAF and NiTi rotary system. In addition, to achieve a certain degree of uniformity and reduce interoperator variables, all experimental procedures were conducted by the same operator.
Control Group
The negative control group (n = 2) included uninstrumented and unirrigated root canals. In the positive control group (n = 2), root canals had no mechanical preparation; instead, irrigation with distilled water was performed so that the specimens were exposed to the same volume of irrigant (20 mL) for the same length of time (4 minutes) of the experimental groups.
Root Canal Instrumentation with the SAF
A 1.5-mm-diameter SAF was operated for 4 minutes by using a trans-line (in-and-out) vibrating handpiece (Gentle-Power Lux 20LP; KaVo, Biberach, Germany) adapted with a RDT3 head (Re-Dent-Nova) at a frequency of 83.3 Hz (5000 rpm) and amplitude of 0.4 mm. The instrument was used with a manual in-and-out motion to the WL. Continuous irrigation with 2.5% NaOCl was applied throughout the procedure at 5 mL/min by using a special irrigation apparatus (VATEA; ReDent-Nova).
Root Canal Instrumentation by Using the NiTi Rotary System
The coronal and middle thirds were serially enlarged with NiTi rotary instruments sizes 25/.12, 25/.10, and 25/.08 (K3; SybronEndo, West Collins, CA) in a crown-down manner by using gentle in-and-out motion toward the apex. The following sequence was used to the WL at 300 rpm driven by a torque-controlled motor (X-Smart; Dentsply Maillefer): 25/.02, 25/.04, 30/.02, 30/.04, 35/.02, 35/.04, and 40/.02 instruments. To avoid fracture, the instruments were withdrawn when resistance was felt and changed for the next instrument. Also, 2 canals were instrumented with 1 set of instruments. Passive ultrasonic irrigation was performed between each instrument by using a size #20 K-file mounted on a piezoelectric handpiece (JetSonic Four; Gnatus, Ribeirão Preto, SP, Brazil) at a power setting of 3, which was activated for 10 seconds at the WL. Each canal was irrigated with a total of 20 mL 2.5% NaOCl. In all groups after root canal preparation, a final rinse with 3 mL bi-distilled water was performed. Then, teeth were immersed in 10% buffered formalin for 48 hours.
Histologic Preparation and Analysis
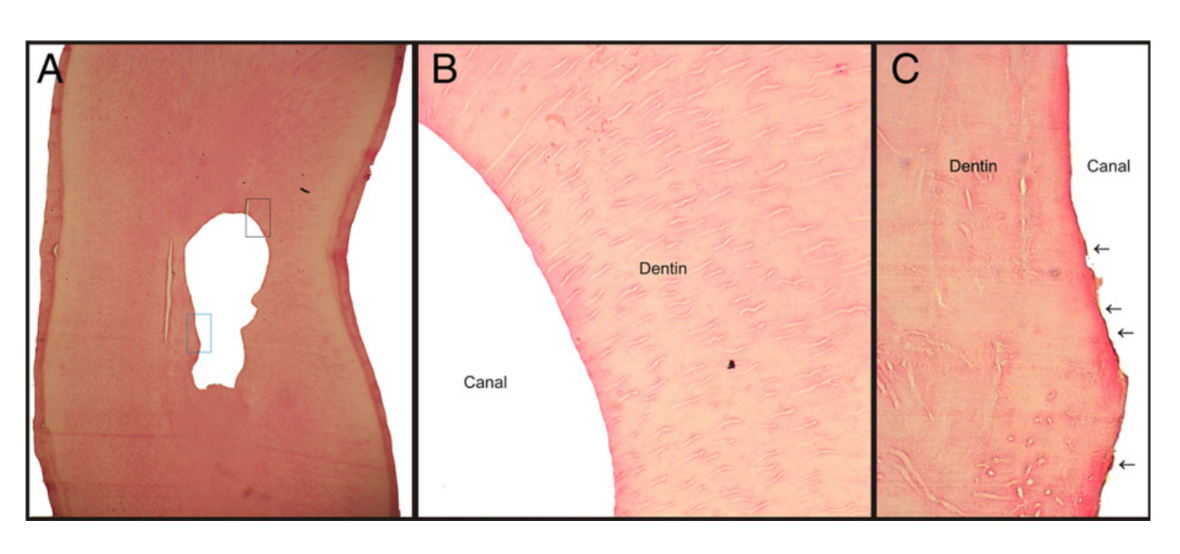
The teeth were washed in running water for 1 hour and decalcified in 10% trichloroacetic acid for 15 days. The apical thirds of the decalcified roots were cut perpendicularly to their long axis with a scalpel at 5 mm from anatomic apex and embedded in paraffin. Care was taken to avoid contamination during the sectioning process. Serial sections (10 semi-serial sections of each specimen), with the microtome set at 6-mm thickness, were stained with hematoxylin–eosin and examined under an optical microscope (Eclipse E 600; Nikon, Shinagawaku, Tokyo, Japan), coupled to a computer, at ×40 magnification. Before viewing the sections, any identification on the slide was masked, and the slides were randomized, which allowed blinded evaluation that was undertaken by 2 trained observers. The percentage of interagreement should be more than 95%; if this percentage was lower than 95%, a consensus should be reached. The images were recorded as a tagged image file format and evaluated for the percentage of debris and uninstrumented root canal walls. The percentage of debris was calculated by placing an integration grid (Image J; National Institutes of Health, Bethesda, MD) over the cross-section images to allow counting the points in the root canal that coincided with either clean areas or areas containing debris. The percentage of uninstrumented root canal walls was determined by calculating the length of the canal outline that was not touched by the instruments in relation to the total length of the canal outline by using Image J software. The action of the instruments on the root canal walls was assessed on the basis of the following criteria: surface regularity, abrupt change on the continuity of root canal wall, and partial or total predentin removal (Fig. 1). The prepared root canal outline was traced in a different color to differentiate it from the uninstrumented canal.
Statistical Analysis
Preliminary tests were performed to determine the sample distribution (Shapiro-Wilk test). The mean percentages of remaining debris and uninstrumented root canal perimeter at the apical third were statistically compared by using unpaired t test with Welch’s correction. Statistical analysis was performed at the 0.05 level of significance by using SPSS software version 17.0 (SPSS Inc, Chicago, IL).
Results
The results of the analysis of the root canal cleanliness are detailed in Table 1. All experimental groups revealed significantly less debris and uninstrumented root canal walls than negative and positive control groups (P < .05). The percentage of remaining debris and uninstrumented canal perimeter was significantly lower in the SAF group (2.18 ± 2.71 and 12.33 ± 7.85, respectively) than in the rotary group (13.11 ± 12.98 and 53.54 ± 15.95, respectively) (P < .05). In the SAF group, most of the specimens were completely free of debris, whereas in the rotary group, 53% of the canals showed some amount of debris. Figure 2 is representative of the root canal condition after preparation with rotary and SAF systems.
Discussion
It has been demonstrated that cleaning of the root canal is not always easily accomplished, especially during the preparation of oval-shaped canals. Because oval canals represent a challenge to any file and/or irrigation system, this type of canal was selected for the present study. Debridement of the apical portion of the root canal is also a big challenge to endodontic treatment, especially because of the complexity of the root canal anatomy and the limitations of instrumentation techniques. Thus, to secure effective apical cleaning, the instruments should be in contact with every part of the canal wall. To deal with this complex problem, several instrumentation techniques and modified instrument designs have been proposed.
In the majority of the studies, postoperative root canal cleanliness has been evaluated in relation to debris and smear layer. Debris may be defined as dentin chips, tissue remnants, and particles loosely attached to the root canal wall, whereas the smear layer is a surface film of debris retained on dentin or other surfaces after instrumentation with either rotary instruments or endodontic files. Overall, no completely cleaned root canals could be found regardless of the instrumentation technique investigated. In this study, optical microscopy was used as a tool to quantify the presence of debris in the root canal as well as the action of the instruments at the dentin walls, according to previous studies. Histologic methods have been considered archaic when compared with micro–computed tomography evaluation; however, they provide valuable information that cannot otherwise be obtained.
The results of this study revealed that preparation of oval-shaped canals of mandibular incisors with the SAF system resulted in a smaller percentage of debris and unprepared root canal walls than the rotary system in the apical third; both of these findings are in accordance with previous studies. Metzger et al demonstrated that the operation of the SAF system with continuous irrigation coupled with alternating sodium hypochlorite and EDTA treatment resulted in a clean and mostly smear layer–free dentinal surface in all parts of the root canal. Siqueira et al showed that the SAF system was significantly more effective than rotary NiTi instrumentation used with syringe/needle irrigation in disinfecting long oval root canals in vitro. De-Deus et al conducted a histologic comparison of the debridement efficacy of the SAF system with the ProTaper system and demonstrated that the SAF system was more efficient in pulpal debridement. By using micro–computed tomography evaluation, Paqué and Peters and Versiani et al showed that shapes generated with the SAF were more complete compared with rotary canal preparation. However, a recent microbiological and scanning electron microscopy evaluation study revealed insufficient apical preparation and inadequate apical irrigation when using the SAF system. This result may be explained by differences in the sample as well as in the evaluation methods.
The present results may be explained by the ability of this instrument to adapt itself to the cross section of the canal and the mechanical debridement efficacy of its continuous irrigation system. The SAF instrument is compressible, thin-walled, and composed of a thin, slightly abrasive NiTi lattice that removes dentin with a back-and-forth grinding motion with vibration. This may explain how it spreads to form closer contact with the canal walls, even in the buccal and lingual recesses that were commonly unaffected by the rotary files. Moreover, a special irrigation device is connected to a silicon tube in the SAF instrument and provides continuous flow of the irrigation solution at a low pressure and at different flow rates. The additional activation of the irrigant by its vibrating motion creates turbulence and allows continuous fresh solution to be present in the root canal at all times, which favors a higher reduction of debris and bacteria than rotary instruments. Taken together, these studies suggested that canal preparation with the SAF does result in homogenous preparation and circumferential removal of a layer of hard tissue in comparison with rotary systems, which explains the better results observed in the present study.
Within the limitations of this ex vivo study, it can be concluded that in the apical third of mandibular incisors, SAF had significantly more contact to the dentin walls and removed more debris than rotary instrumentation. Additional studies combining different methodologies should be performed to compare the cleaning efficacy of the SAF system and rotary files associated with different irrigation devices.
Authors: Marcus Vinícius de Melo Ribeiro, Yara Terezinha Silva-Sousa, Marco Aurélio Versiani, Alessandro Lamira, Dr med dent, Liviu Steier, Jesus Djalma Pécora, Manoel Damião de Sousa-Neto
References:
- Haapasalo M, Endal U, Zandi H, Coil J. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Topics 2005;10:77–102.
- Barbizam JV, Fariniuk LF, Marchesan MA, et al. Effectiveness of manual and rotary instrumentation techniques for cleaning flattened root canals. J Endod 2002;28: 365–6.
- De-Deus G, Souza EM, Barino B, et al. The self-adjusting file optimizes debridement quality in oval-shaped root canals. J Endod 2011;37:701–5.
- Fornari VJ, Silva-Sousa YT, Vanni JR, et al. Histological evaluation of the effectiveness of increased apical enlargement for cleaning the apical third of curved canals. Int Endod J 2010;43:988–94.
- Marchesan MA, Arruda MP, Silva-Sousa YT, et al. Morphometrical analysis of cleaning capacity using nickel-titanium rotary instrumentation associated with irrigating solutions in mesio-distal flattened root canals. J Appl Oral Sci 2003;11:55–9.
- Nadalin MR, Perez DE, Vansan LP, et al. Effectiveness of different final irrigation protocols in removing debris in flattened root canals. Braz Dent J 2009;20:211–4.
- Taha NA, Ozawa T, Messer HH. Comparison of three techniques for preparing oval-shaped root canals. J Endod 2010;36:532–5.
- Peters OA, Laib A, Ruegsegger P, Barbakow F. Three-dimensional analysis of root canal geometry by high-resolution computed tomography. J Dent Res 2000;79: 1405–9.
- Metzger Z, Teperovich E, Cohen R, et al. The self-adjusting file (SAF): part 3—removal of debris and smear layer: a scanning electron microscope study. J Endod 2010;36:697–702.
- Metzger Z, Teperovich E, Zary R, et al. The self-adjusting file (SAF): part 1—respecting the root canal anatomy: a new concept of endodontic files and its implementation. J Endod 2010;36:679–90.
- Siqueira JF Jr, Alves FR, Almeida BM, et al. Ability of chemomechanical preparation with either rotary instruments or self-adjusting file to disinfect oval-shaped root canals. J Endod 2010;36:1860–5.
- Paqué F, Peters OA. Micro-computed tomography evaluation of the preparation of long oval root canals in mandibular molars with the self-adjusting file. J Endod 2011;37:517–21.
- Versiani MA, Pécora JD, de Sousa-Neto MD. Flat-oval root canal preparation with self-adjusting file instrument: a micro-computed tomography study. J Endod 2011;37:1002–7.
- De-Deus G, Barino B, Marins J, et al. Self-adjusting file cleaning-shaping-irrigation system optimizes the filling of oval-shaped canals with thermoplasticized gutta-percha. J Endod 2012;38:846–9.
- Dietrich MA, Kirkpatrick TC, Yaccino JM. In vitro canal and isthmus debris removal of the Self-Adjusting File, K3, and WaveOne files in the mesial root of human mandibular molars. J Endod 2012;38:1140–4.
- Paranjpe A, de Gregorio C, Gonzalez AM, et al. Efficacy of the self-adjusting file system on cleaning and shaping oval canals: a microbiological and microscopic evaluation. J Endod 2012;38:226–31.
- Adigüzel O, Yiğit-Özer S, Kaya S, et al. Effectiveness of ethylenediaminetetraacetic acid (EDTA) and MTAD on debris and smear layer removal using a self-adjusting file. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:803–8.
- Meneghin MP, Nomelini SM, Sousa-Neto MD, et al. Morphologic and morphometric analysis of the root canal apical third cleaning after biomechanical preparation using 3.3% Ricinus communis detergent and 1% NaOCl as irrigating solutions. J Appl Oral Sci 2006;14:178–82.
- Wu MK, van der Sluis LW, Wesselink PR. The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. Int Endod J 2003;36:218–24.
- H€ulsmann M, Peters O, Dummer P. Mechanical preparation of root canals: shaping goals, techniques and means. Endod Topics 2005;10:30–76.
- Sasaki EW, Versiani MA, Perez DE, et al. Ex vivo analysis of the debris remaining in flattened root canals of vital and nonvital teeth after biomechanical preparation with Ni-Ti rotary instruments. Braz Dent J 2006;17:233–6.
- Fariniuk LF, Baratto-Filho F, da Cruz-Filho AM, de Sousa-Neto MD. Histologic analysis of the cleaning capacity of mechanical endodontic instruments activated by the ENDOflash system. J Endod 2003;29:651–3.
- Baratto-Filho F, de Carvalho JR Jr, Fariniuk LF, et al. Morphometric analysis of the effectiveness of different concentrations of sodium hypochlorite associated with rotary instrumentation for root canal cleaning. Braz Dent J 2004;15:36–40.
- American Association of Endodontists. Glossary of Endodontics Terms. 7th ed. Chicago, IL: American Association of Endodontists; 2003.
- Kaya S, Yiğit-Özer S, Adigüzel O. Evaluation of radicular dentin erosion and smear layer removal capacity of Self-Adjusting File using different concentrations of sodium hypochlorite as an initial irrigant. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:524–30.
