Adjunctive Steps for the Removal of Hard Tissue Debris from the Anatomic Complexities of the Mesial Root Canal System of Mandibular Molars: A Micro–Computed Tomographic Study
Abstract
Introduction: This in vitro study sought to compare the efficacy of a sonic irrigant activation device with ultrasonic activation and needle irrigation in removing hard tissue debris (HTD) from anatomic complexities of the root canal system.
Methods: Twenty-seven mesial roots of extracted human mandibular molars with 2 canals connected by an isthmus were selected based on micro–computed tomography scans (12-mm voxel size). The mesial canals were mechanically prepared to ProTaper Next X3 (Dentsply Maillefer, Ballaigues, Switzerland) and anatomically distributed into 3 groups (n = 9) according to the final irrigation protocol: sonically activated irrigation (SAI) using the EDDY system (VDW GbmH, Munich, Germany) for 3 X 20 seconds, ultrasonically activated irrigation (UAI) using a size 20 Irrisafe tip (Satelec Acteon, Mérignac, France) for 3 X 20 seconds, and conventional irrigation using a 30-G needle adapted to a syringe. Micro–computed tomographic scans were taken after instrumentation and after supplementary activation of the irrigant. After reconstruction and coregistration, the volume filled with HTD before and after irrigant activation was calculated, and the mean percentage of HTD reduction after final irrigation was compared within and among groups using the paired sample t test and 1-way analysis of variance post hoc Tukey test, respectively (α = 5%).
Results: A significant reduction in the volume filled with HTD after irrigant activation was observed in all groups (P ˂ .05). The percentage reduction of HTD in the UAI group (66.8%) was significantly higher than that in the SAI group (36.4%) (P ˂ .05), whereas the conventional irrigation group result (43.7%) did not differ statistically from the UAI or SAI groups (P ˃ .05).
Conclusions: All tested supplementary irrigation steps significantly reduced the amount of debris created during root canal preparation. Ultrasonic activation resulted in the highest mean debris reduction. (J Endod 2020;46:1508–1514.)
Chemomechanical preparation of the root canal system involves enlargement of the canal space with manual and/or engine-driven instruments in combination with irrigation with antimicrobial solutions. Although mechanical instrumentation is required to allow access of irrigants into the apical area and to enable a proper root filling, it comes with a number of shortcomings. These include the creation of a smear layer on the canal walls, the lack of complete coverage of the canal wall surface, and the production of hard tissue debris (HTD), which may accumulate in secondary anatomy. In infected canals, accumulated HTD may contain bacteria and serve as a nidus for root canal reinfection. In addition, debris packed in the canal system may compromise thorough disinfection and filling. Therefore, irrigating solutions are crucial in order to flush away debris, clean the noninstrumented areas of the root canals, remove the smear layer, and further disinfect the canal space.
The traditional syringe and needle method of irrigation often fails in adequate delivery of irrigant solutions within the complex 3-dimensional (3D) microstructure of the canal system because the fluid penetration beyond the needle tip is limited and canal extensions frequently harbor debris after irrigation. Consequently, different ultrasonic and sonic activation techniques have been proposed to improve the distribution of the irrigant within the root canal space, increasing its effectiveness.
Ultrasonically activated irrigation (UAI) implies the activation of irrigant by an ultrasonically oscillating instrument placed in the center of the canal, usually after its shaping. Although cavitation effects have been observed with UAI, acoustic microstreaming is believed to be the main cleaning action. Numerous
in vitro studies have shown UAI to be more effective than conventional syringe irrigation in cleaning root canal irregularities.
Additionally, significantly cleaner isthmuses have also been demonstrated when UAI is used in comparison with syringe irrigation, both in vitro and in vivo. Drawbacks of UAI include instrument fracture and uncontrolled removal of dentin from the root canal walls.
In comparison with ultrasonically driven instruments, which operate at a frequency above 20 kHz, sonically activated irrigation (SAI) devices operate at lower frequencies (20– 20,000 Hz) and hence produce lower shear stresses. Recently, a new sonic device (EDDY; VDW GbmH, Munich, Germany) driven at a higher frequency (6000 Hz) than other SAI devices was introduced. The EDDY system consists of a smooth tapered polymer tip coupled to a sonic scaler, which is activated within the root canal space after final shaping. A number of in vitro studies showed that EDDY performed better than syringe irrigation in terms of the removal of calcium hydroxide, a biofilm-mimicking hydrogel from the isthmus, canal wall debris and the smear layer, and soft tissue from a root canal wall groove.
When compared with UAI, results are less unequivocal. Although some studies demonstrated better cleaning outcomes with EDDY, others found no difference between them. However, most of these studies used unvalidated models or inadequate surrogate outcomes or resembled very specific situations for canal cleaning; hence, information on standard clinically meaningful outcomes is still missing. Except for 1 study, there is no information on the performance of EDDY in the removal of HTD from large isthmus areas in mesial root canals of mandibular molars. Therefore, the aim of this in vitro study was to compare the efficacy of the EDDY system with ultrasonic activation and syringe irrigation in removing accumulated HTD from anatomic complexities of the mesial root canals of mandibular molars by means of micro–computed tomographic (micro-CT) imaging analysis. The null hypothesis tested was that there were no differences in HTD removal among the 3 supplementary irrigation steps.
Materials and methods
Specimen Selection and Imaging After Ethics Committee approval of Ghent University (protocol EC/2017/1638), Ghent, Belgium, 27 teeth with fully closed apices and 2 canals connected by a large isthmus area in moderately curved mesial roots were selected from a pool of mandibular molar teeth extracted for reasons not related to this study. Sample size calculation indicated that 9 roots per group were required to support analysis with 80% power and a 5% level of significance with a mean difference of 23% debris reduction. The specimens were embedded in self-curing acrylic resin and scanned at a voxel size of 12 mm using the custom-developed HECTOR (High-Energy CT system Optimized for Research) micro-CT scanner (Centre for X- ray Tomography, Ghent, Belgium). The system was set at 120 kV and 138 mA. For each scan,
2001 projections covering a full 360◦ rotation around the vertical axis were acquired. The images were reconstructed into cross- sectional slices with NRecon v.1.6.9 software (Bruker-microCT, Kontich, Belgium) using standardized parameters for beam hardening (15%), ring artifact correction (5%), and similar contrast limits. The volume of interest (the mesial root) was selected from the cementoenamel junction to the apex of the root, and the region of interest in each slice comprised the area of both the root canals and the isthmus. CTAn v.1.14.4 software (Bruker micro-CT) was used to evaluate root canal configuration and to measure the height, width, and length of the isthmus, as well as the length (in mm), volume (in mm3), surface area (in mm2), and the structure model index (SMI) of the mesial root canal system. Three-dimensional models of the mesial canals were also rendered and qualitatively evaluated regarding canal configuration (CTVol v.2.2.1, Bruker-microCT).
Root Canal Preparation and Groups After conventional access cavity preparation, the mesial root canals were sequentially prepared with X1, X2 and X3 (size 30, 0.07 taper) ProTaper Next rotary instruments (Dentsply Maillefer, Ballaigues, Switzerland) up to 0.5 mm from the main apical foramen. The canals were irrigated with 1 mL 2.5% sodium hypochlorite (NaOCl) after each instrument using a 27-G needle (Monoject; Sherwood Medical, Norfolk, NE) adapted to a 3-mL syringe. Then, canals were dried with absorbent paper points, and the roots were submitted to a new scan and reconstruction applying the parameter settings previously mentioned. Postoperative scans were coregistered with their respective preoperative data sets using the rigid registration module of the 3D Slicer 4.3.1 software (available from www.slicer.org), and quantification of HTD was performed using Fiji software (Fiji v.1.47n; Fiji, Madison, WI) as described elsewhere. The presence of material with a density similar to dentin in regions previously occupied by air in the nonprepared root canal space was considered HTD. The total volume of HTD after root canal preparation was measured (in mm3) and expressed as the percentage of the total volume of the canal system (vol% HTD). Aiming to enhance the internal validity of the experiment, the specimens were anatomically matched regarding canal length, canal configuration, isthmus morphology, and vol% HTD and further distributed into 3 groups (n = 9) according to the supplementary irrigation protocol as follows:
- Syringe and needle irrigation (SNI): canals were irrigated with 3 mL 2.5% NaOCl using a 30-G notched needle (Appli-Vac Irrigating Needle Tip; Vista Dental, Racine, WI) adapted to a 3-mL syringe at a mean flow rate of 0.14 mL/s.
- Sonically activated irrigation (SAI): a noncutting polyamide size 25, 0.04 tip (EDDY) operated by an air-driven handpiece (Proxeo; W&H, Bürmoos, Austria) was used in each canal for 3 X 20 seconds at maximum intensity (frequency of 6000 Hz).
- Ultrasonically activated irrigation (UAI): a noncutting, stainless steel size 20 wire (Irrisafe; Satelec Acteon, Mérignac, France) driven by an ultrasonic device (Suprasson Pmax Newtron, Satelec) was used in the root canals for 3 X 20 seconds at 45% of the maximum power (yellow 9). The wire was prebent in order to limit contact with the canal walls.
The tips of all instruments were placed 2 mm from the working length, and irrigation was performed using an up-and-down movement with a 2-mm amplitude. In the SAI and UAI groups, the mesial root canal system was rinsed with 1 mL 2.5% NaOCl in between each 20-second cycle using a 27-G needle adapted to a 3-mL syringe. All procedures were performed by an experienced and previously trained operator.
Imaging Analysis
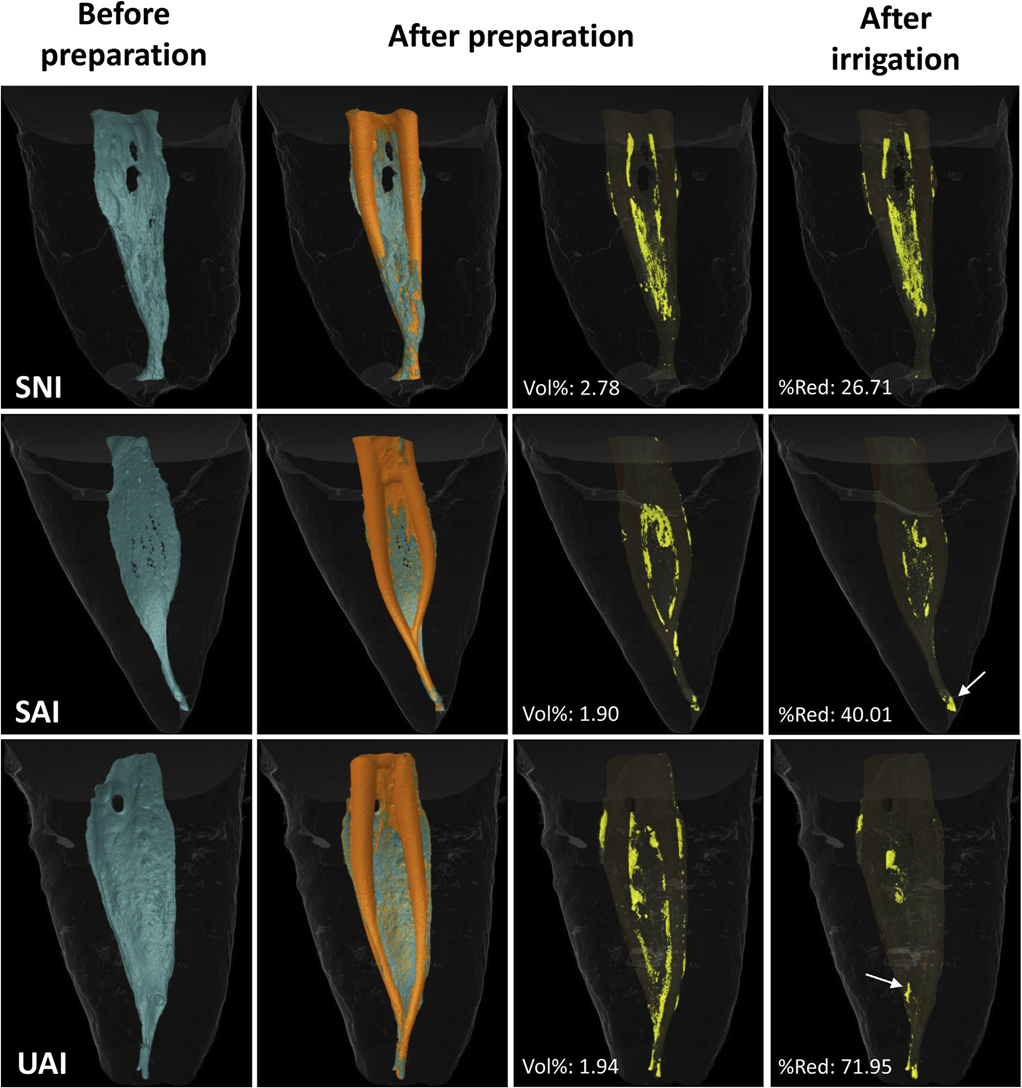
After the supplementary irrigation procedures, each root canal was dried with an absorbent paper point, and a final micro-CT scanning was performed following the same reported parameters. After coregistering the data sets, the vol% HTD was measured and the percentage reduction of HTD calculated according to the following formula: 100 2 ([VAF X 100]/VBF), with VBF and VAF being the volume of HTD before and after the supplementary irrigation protocols, respectively. An examiner blinded to the final irrigation protocol in each specimen performed all of the measurements. Matched color-coded root canal models (green and orange colors indicating pre- and postoperative canal surfaces, respectively) and debris (in yellow color) enabled qualitative comparison of the distribution of the HTD in each portion of the root canals before and after the experimental procedures.
Statistical Analysis
Data were normally distributed (Shapiro-Wilk test, P ˃ .05) and expressed as means with standard deviations. The paired sample t test was used to compare the percentage of HTD values before and after final irrigation in each group. Statistical comparison of the percentage of HTD reduction between groups was performed using 1-way analysis of variance post hoc Tukey tests with the significance level set at 5% (SPSS v25.0; IBM Corp, Armonk, NY).
Results
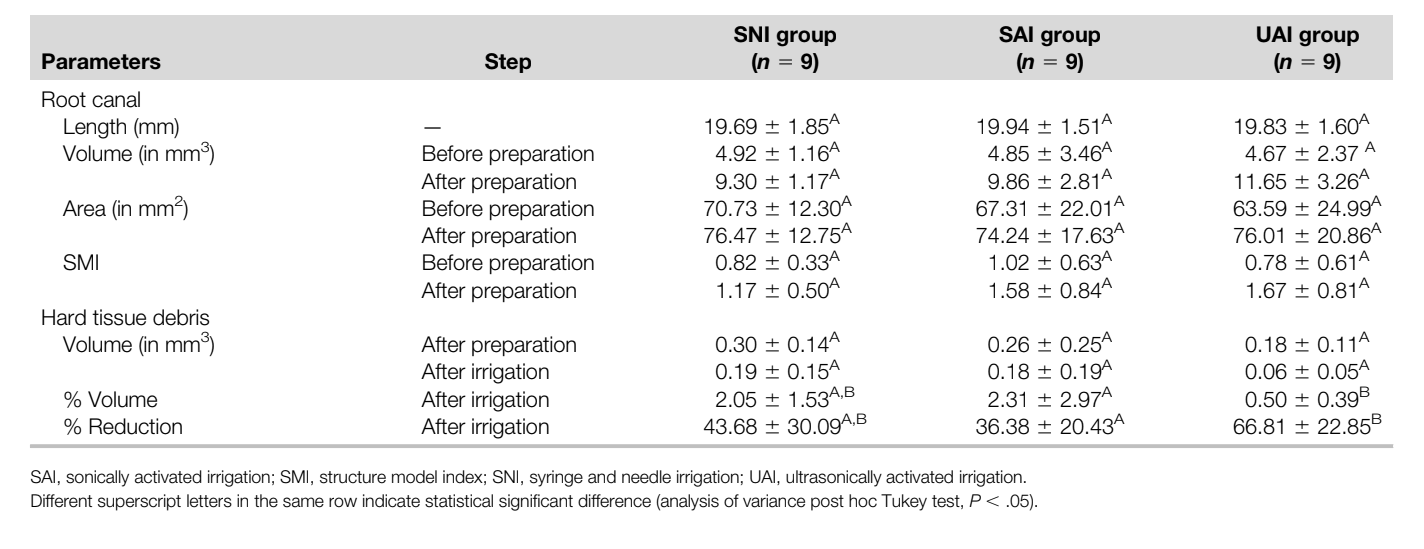
Three-dimensional parameters (length, volume, surface area, and SMI) evaluated before and after preparation and irrigation procedures performed in the mesial root canal system of mandibular molars are detailed in Table 1. No statistically significant differences were noted regarding the analyzed 3D parameters as well as the vol% HTD after preparation (P ˃ .05), demonstrating the degree of homogeneity of the groups pre- and postoperatively. A significant reduction of HTD after the final irrigation protocols was observed in all groups (P ˂ .05). The percentage reduction of HTD in the UAI group (66.8%) was significantly higher than in the SAI group (36.4%) (P ˂ .05; 30.4% difference with 95% confidence interval, 6.3%–54.6%), whereas the percentage of HDT reduction in the SNI group (43.7%) did not differ statistically from the UAI and SAI groups (P ˃ .05). Hence, the null hypothesis was rejected. Representative 3-dimensional models of the mesial root canal system of the SNI, SAI, and UAI groups before and after the experimental procedures are shown in Figure 1. In some specimens, mostly from the SNI and SAI groups, after the irrigation protocol, HTD could be observed in different areas than that observed after preparation, suggesting displacement rather than removal of HTD (Fig. 1).
Discussion
This study sought to evaluate the removal of HTD using different supplementary irrigation protocols after the preparation of complex isthmus-containing mesial roots of mandibular molars. During the mechanical preparation of this challenging canal anatomy, it has been demonstrated that HTD packed in the isthmus area, canal extensions, and apical ramifications may potentially interfere with disinfection by preventing irrigant flow and neutralizing the antibacterial effects of the irrigating solutions. Therefore, in the present study, mesial roots of mandibular molars presenting large isthmus areas were selected instead of less complex anatomies, such as the Vertucci type II configuration used in most of the previous investigations on this topic. Also, aiming to increase the internal validity of the study by reducing the anatomic bias among the specimens, groups were balanced in terms of the 3D morphometric parameters of the root canals (length, volume, surface area, and SMI) obtained after a prescreening of the specimens using micro-CT technology (Table 1).
Overall, our results showed a significant mean percentage reduction of HTD in all groups after the final irrigation protocols (Table 1), which may be clinically translated into improved cleanliness of the root canal system. However, none of the irrigation protocols was able to render the mesial root canal system completely free from dentin particles. This is in accordance with several studies and demonstrates that debris accumulated in areas of anatomic irregularities cannot be removed with the currently available techniques. Apart from this, despite an additional attempt to balance groups regarding the amount of debris created by the mechanical preparation, variations in the percentage of debris reduction among the specimens remained high at the end of the experiment, which may indicate that the distribution of HTD within the root canal space may be unpredictable, irrespective of the preparation and/or irrigation methods. It is also important to highlight that the preparation procedure in this study was aimed to simulate the clinical scenario by using a sequence of rotary instruments and intermittent irrigation steps. As a consequence, it resulted in lower mean vol% HTD (2.86%) after preparation compared with previous micro-CT studies on this topic in which none or only minimal irrigation was performed during instrumentation. Moreover, in some of these studies, canal preparation was performed using reciprocating systems, which has been shown to create larger amounts of dentinal debris than continuous rotary instruments. Finally, despite the fact that irrigation with EDTA after canal shaping has been shown to reduce the levels of HTD, this solution was not used in this study because it would add a confounding variable that would not allow us to observe the isolated effect of the irrigation protocols. Therefore, it remains unclear to what degree the supplementary use of EDTA after different final irrigation protocols would impact the HTD levels.
In the present study, although the statistical significance was borderline (P = .04), UAI resulted in a significantly higher percentage reduction of HTD (66.8%) than activation of the irrigant solution with the EDDY sonic system (SAI = 36.4%). The efficiency of UAI has been explained because of the production of acoustic microwaves, cavitation, and heat generation, which may favor the removal of a greater amount of tissue remnants and dentinal debris compared with sonic activation. Also, although both instruments have equal tip diameters, the IrriSafe file is a nontapered instrument, reducing the likelihood of wall contact and motion dampening during action compared with the tapered EDDY tip. On the other hand, a recent study using micro-CT imaging to evaluate HTD removal from the mesial root canal system with Vertucci type II configuration reported similar results with regard to ultrasonic activation (66.8%) or sonic activation with EDDY (56.9%). Nevertheless, the absence of statistical difference between UAI and SAI in the study of Rödig et al may be explained by the less complex canal anatomy of the specimens (Vertucci type II) and the use of an ultrasonic tip (Irrisafe 25) similar in size with the tip of the master apical instrument (Reciproc R25 [VDW, Munich, Germany]). As has been demonstrated, the efficiency of ultrasonic activation is related to the interaction of the ultrasonic energy and the irrigating solution, which means that the tip of the ultrasonic device should vibrate freely within the root canal space. Therefore, in the present study, the use of a thinner ultrasonic tip (Irrisafe 20) associated with a greater apical enlargement (size 30, 0.07 taper) possibly favored the activation of the irrigant solution and, consequently, increased its efficiency in removing packed HTD.
Despite the lower mean percentage reduction of HTD in the SNI group (43.7%) than in the ultrasonic-activated specimens (66.8%), no statistical difference between these irrigant protocols was observed, which is in disagreement with several publications that reported better root canal debridement after the use of UAI compared with needle irrigation. However, the present results (UAI = 66.8% and SNI = 43.7%) were similar to and corroborate the findings of Rödig et al (UAI = 66.8% and SNI = 44.1%). This result may be explained by the smaller diameter of the irrigation needle during the final irrigation step (30 G) compared with the needle diameter applied during preparation (27 G) and its insertion closest to the working length, which has been proven to significantly enhance the removal of HTD from the mesial root canal system of mandibular molars. In addition, the flow rate in the present study (0.14 mL/s) was at least 40% higher than in most previous reports using flow rates below 0.1 mL/s. Because the irrigant volumes in each group were standardized, the contact time of the irrigant in the UAI and SAI groups was longer than that in the SNI group. However, although the contact time of NaOCl solution may be relevant for antimicrobial action or soft tissue dissolution, considering its chemical properties, it is less important for hard debris removal.
An interesting finding of the present study was the observation that in some specimens, mainly in the SAI and SNI groups, some debris had moved from one place to another and remained within the root canal system instead of being removed by the supplementary irrigation procedures (Fig. 1). It may be assumed that the anatomic complexities of the root canals selected for this study had a noteworthy influence on the efficacy of the tested irrigation protocols, contributing to the present findings. It must also be emphasized that the disruption or detachment of debris does not ensure its removal unless there is a favorable irrigant flow toward the canal orifice, and further studies are required to evaluate other flushing parameters (eg, irrigant volume and flow) with different syringe/needle and sonic activation protocols on the removal not only of dentinal debris but also biofilm from complex canal anatomies. Moreover, although the tested irrigation protocols resulted in a significant reduction in the debris content, its clinical relevance remains unclear, and additional research is needed to evaluate its impact on the success rate of the root canal treatment.
Conclusion
All final adjunctive irrigation steps reduced the amount of debris accumulated after root canal preparation and resulted in improved cleanliness. UAI performed better than the SAI system in terms of the percentage reduction of HTD from the complex mesial root canal system of mandibular molars. None of the tested methods was able to render the root canal systems free of debris.
Authors: Dominique Linden, Matthieu Boone, Mieke De Bruyne, Roeland De Moor, Marco A. Versiani, Maarten Meire
References:
- Haapasalo H, Endal U, Zandi H, et al. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Topics 2005;10:77–102.
- Paqué F, Boessler C, Zehnder M. Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. Int Endod J 2011;44:148–53.
- Gutarts R, Nusstein J, Reader A, et al. In vivo debridement efficacy of ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. J Endod 2005;31:166–70.
- Boutsioukis C, Lambrianidis T, Verhaagen B, et al. The effect of needle-insertion depth on the irrigant flow in the root canal: evaluation using an unsteady computational fluid dynamics model. J Endod 2010;36:1664–8.
- Chan R, Versiani MA, Friedman S, et al. Efficacy of 3 supplementary irrigation protocols in the removal of hard tissue debris from the mesial root canal system of mandibular molars. J Endod 2019;45:923–9.
- Nusstein JM. Sonic and ultrasonic irrigation. In: Bettina B, editor. Endodontic Irrigation: Chemical Disinfection of the Root Canal System. Switzerland: Springer; 2015. p. 173–98.
- van der Sluis LW, Versluis M, Wu MK, et al. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J 2007;40:415–26.
- Macedo R, Verhaagen B, Rivas DF, et al. Cavitation measurement during sonic and ultrasonic activated irrigation. J Endod 2014;40:580–3.
- Jiang LM, Verhaagen B, Versluis M, et al. The influence of the ultrasonic intensity on the cleaning efficacy of passive ultrasonic irrigation. J Endod 2011;37:688–92.
- Lee SJ, Wu MK, Wesselink PR. The effectiveness of syringe irrigation and ultrasonics to remove debris from simulated irregularities within prepared root canal walls. Int Endod J 2004;37:672–8.
- Sabins RA, Johnson JD, Hellstein JW. A comparison of the cleaning efficacy of short-term sonic and ultrasonic passive irrigation after hand instrumentation in molar root canals. J Endod 2003;29:674–8.
- Duque JA, Duarte MA, Canali LC, et al. Comparative effectiveness of new mechanical irrigant agitating devices for debris removal from the canal and isthmus of mesial roots of mandibular molars. J Endod 2017;43:326–31.
- Boutsioukis C, Tzimpoulas N. Uncontrolled removal of dentin during in vitro ultrasonic irrigant activation. J Endod 2016;42:289–93.
- Ahmad M, Pitt Ford TR, Crum LA. Ultrasonic debridement of root canals: an insight into the mechanisms involved. J Endod 1987;13:93–101.
- Donnermeyer D, Wyrsch H, Burklein S, et al. Removal of calcium hydroxide from artificial grooves in straight root canals: sonic activation using EDDY versus passive ultrasonic irrigation and XPendo Finisher. J Endod 2019;45:322–6.
- Swimberghe RC, De Clercq A, De Moor RJ, et al. Efficacy of sonically, ultrasonically and laser- activated irrigation in removing a biofilm-mimicking hydrogel from an isthmus model. Int Endod J 2019;52:515–23.
- Haupt F, Meinel M, Gunawardana A, et al. Effectiveness of different activated irrigation techniques on debris and smear layer removal from curved root canals: a SEM evaluation. Aust Endod J 2020;46:40–6.
- Urban K, Donnermeyer D, Schafer E, et al. Canal cleanliness using different irrigation activation systems: a SEM evaluation. Clin Oral Investig 2017;21:2681–7.
- Conde AJ, Estevez R, Lorono G, et al. Effect of sonic and ultrasonic activation on organic tissue dissolution from simulated grooves in root canals using sodium hypochlorite and EDTA. Int Endod J 2017;50:976–82.
- Marques-da-Silva B, Alberton CS, Tomazinho FS, et al. Effectiveness of five instruments when removing calcium hydroxide paste from simulated internal root resorption cavities in extracted maxillary central incisors. Int Endod J 2020;53:366–75.
- Plotino G, Grande NM, Mercade M, et al. Efficacy of sonic and ultrasonic irrigation devices in the removal of debris from canal irregularities in artificial root canals. J Appl Oral Sci 2019;27:e20180045.
- Rödig T, Koberg C, Baxter S, et al. Micro-CT evaluation of sonically and ultrasonically activated irrigation on the removal of hard-tissue debris from isthmus-containing mesial root canal systems of mandibular molars. Int Endod J 2019;52:1173–81.
- Versiani MA, Alves FR, Andrade-Junior CV, et al. Micro-CT evaluation of the efficacy of hard- tissue removal from the root canal and isthmus area by positive and negative pressure irrigation systems. Int Endod J 2016;49:1079–87.
- Paqué F, Laib A, Gautschi H, et al. Hard-tissue debris accumulation analysis by high-resolution computed tomography scans. J Endod 2009;35:1044–7.
- De-Deus G, Marins J, Silva EJ, et al. Accumulated hard tissue debris produced during reciprocating and rotary nickel-titanium canal preparation. J Endod 2015;41:676–81.
- Freire LG, Iglecias EF, Cunha RS, et al. Micro-computed tomographic evaluation of hard tissue debris removal after different irrigation methods and its influence on the filling of curved canals. J Endod 2015;41:1660–6.
- Keleş A, Alcin H, Sousa-Neto MD, et al. Supplementary steps for removing hard tissue debris from isthmus-containing canal systems. J Endod 2016;42:1677–82.
- Leoni GB, Versiani MA, Silva-Sousa YT, et al. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int Endod J 2017;50:398–406.
- Perez R, Neves AA, Belladonna FG, et al. Impact of the needle insertion depth on the removal of hard-tissue debris. Int Endod J 2017;50:560–8.
- Silva EJ, Carvalho CR, Belladonna FG, et al. Micro-CT evaluation of different final irrigation protocols on the removal of hard-tissue debris from isthmus-containing mesial root of mandibular molars. Clin Oral Investig 2019;23:681–7.
- Yang Q, Liu MW, Zhu LX, et al. Micro-CT study on the removal of accumulated hard-tissue debris from the root canal system of mandibular molars when using a novel laser-activated irrigation approach. Int Endod J 2020;53:529–38.
- Robinson JP, Lumley PJ, Cooper PR, et al. Reciprocating root canal technique induces greater debris accumulation than a continuous rotary technique as assessed by 3-dimensional micro- computed tomography. J Endod 2013;39:1067–70.
- Rödig T, Sedghi M, Konietschke F, et al. Efficacy of syringe irrigation, RinsEndo and passive ultrasonic irrigation in removing debris from irregularities in root canals with different apical sizes. Int Endod J 2010;43:581–9.
- van der Sluis LW, Gambarini G, Wu MK, et al. The influence of volume, type of irrigant and flushing method on removing artificially placed dentine debris from the apical root canal during passive ultrasonic irrigation. Int Endod J 2006;39:472–6.
- Boutsioukis C, Van der Sluis L. Syringe irrigation: blending endodontics and fluid dynamics. In: Bettina B, editor. Endodontic Irrigation: Chemical Disinfection of the Root Canal System. Switzerland: Springer; 2015. p. 45–64.
- Boutsioukis C, Lambrianidis T, Kastrinakis E. Irrigant flow within a prepared root canal using various flow rates: a computational fluid dynamics study. Int Endod J 2009;42:144–55.
