Micro-computed tomographic analysis of the mesial root of mandibular first molars with bifid apex
Abstract
Objectives: The aim of the present study was to evaluate the influence of bifid and non-bifid configurations of the mesial root of mandibular first molars in the canal anatomy using micro-computed tomography (micro-CT).
Design: Thirty mesial roots with bifid apex were pair-matched with 30 non-bifid roots by means of micro-CT. Bifid and non-bifid mesial roots were compared regarding morphological aspects at the apical third, dentin thickness, and the presence of isthmus and middle mesial canal (MMC). Data were analyzed using Chi-square test and Student’s t-test with a significant level of 5 %.
Results: Main foramen of mesiobuccal (MB) and mesiolingual (ML) canals were positioned within 2.5 mm from the anatomic apex, and origin and exit of accessory canals were observed mostly between 1.0 and 2.0 mm from the apex in the groups. Despite no statistical difference was observed between bifid and non-bifid roots regarding the number of canal orifices (p > 0.05), the frequency of accessory canals was significantly higher in bifid roots (p < 0.05). Regarding the dentin thickness, a statistically significant difference was observed between bifid and non-bifid roots at the furcation level (p < 0.05). Only 3 mesial roots with bifid apex (10 %) had isthmuses, mostly at the coronal third, while a high incidence of MMC was observed in this group (n = 10, 33.3 %).
Conclusions: Presence of bifid apex in the mesial root of mandibular first molars might be a predictive factor for a complex canal anatomy at the apical third with an increasing number of accessory canals.
Introduction
Mandibular first molars are the most frequent endodontically treated teeth (Wayman, Patten, & Dazey, 1994). This tooth usually has two roots, one mesial and one distal, widely separated at the apex. The mesial root frequently presents a complex canal configuration, with isthmus communications, ramifications, recesses, and accessory canals (Villas-Bôas et al., 2011). This complexity has been found to compromise the chemomechanical protocols for root canal disinfection by the inability of instruments and antibacterial solutions to reach inaccessible areas of the root canal system (Alves et al., 2016). Consequently, in infected canals, the microbial load in additional anatomies must be properly reduced during preparation procedures once harbour persistent bacteria may jeopardize the treatment outcome (Costa et al., 2019).
In 1981, Turner suggested that multiple root number of the maxillary first premolars could be defined on the basis of root bifurcation and used the term ‘bifid-tipped’ to describe an incipient two-rooted appearance observed in this group of teeth (Turner, 1981). According to him, this new nomenclature had a considerable biological significance considering that more anatomical variations could be observed in the area between the tip and the next third of the tooth than beyond the first third of the tooth from the tip. Later, in an anthropological study of Homo sapiens from Northwest Africa, Kupczik and Hublin (2010) described the presence of bifid-tipped mesial roots in mandibular molars. Authors explained that differences in the external root morphology of this group of teeth have been linked to distinct occlusal loading regimes and that variations in their internal root structure has not yet been precisely quantified (Kupczik & Hublin, 2010). More recently, a micro-computed tomographic (micro-CT) study demonstrated that the apical shape of the mesiobuccal root of maxillary first molars had a significant influence in the complexity of the root canal system (Ordinola-Zapata,Martins, Niemczyk, & Bramante, 2019). Despite the anatomy of the mesial root of mandibular first molars had been deeply investigated in the literature (Keleş & Keskin, 2017a, 2018, Villas-Bôas et al., 2011), to date no study had correlated different morphological aspects of this root with its internal anatomy. Therefore, the aim of this study was to evaluate the influence of bifid and non-bifid apical configurations of the mesial root of mandibular first molars in the canal anatomy, using micro-CT imaging system.
Materials and methods
Specimen selection and imaging
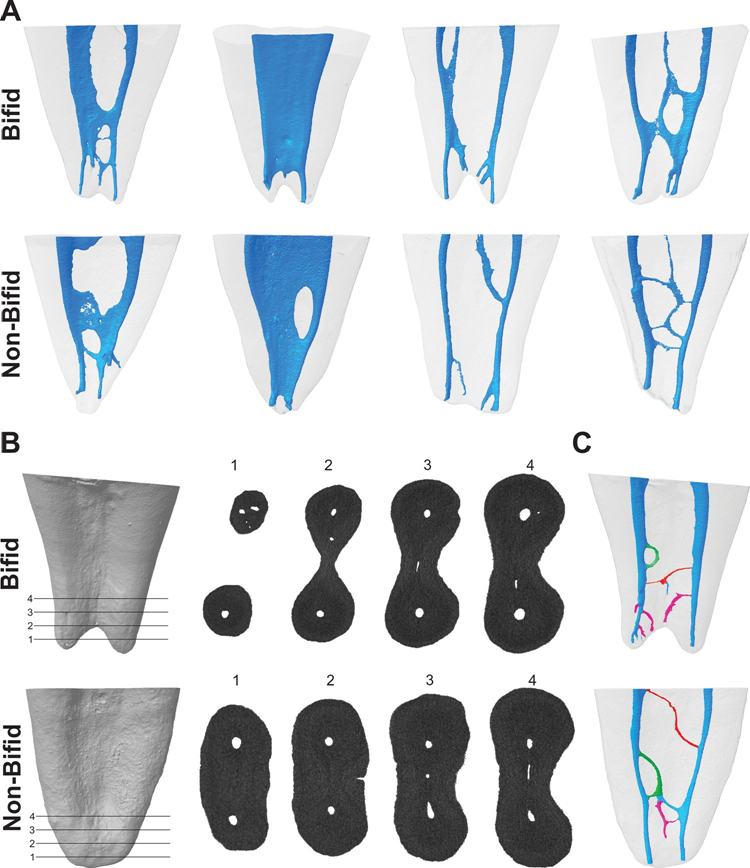
After local ethics committee approval (protocol 2013/145), 250 two-rooted mandibular first molars with no restoration, caries, fracture or immature roots, were collected from Turkish population and imaged (pixel size of 10 μm) using a micro-CT scanner (SkyScan 1172; Bruker-microCT, Kontich, Belgium) at 100 μA, 100 kV, 180° rotation with a step of 0.4°, frame average of 3 and 1400 milliseconds of exposure duration. Data were reconstructed (NRecon v. 1.7.4.2 software; Bruker-microCT) with ring artefact (5), beam-hardening (45 %), and smoothing (2) corrections using an attenuation coefficient ranging from 0 to 0.06. Three-dimensional models of the mesial root canal system were rendered with CTAn v.1.18.8 software (Bruker-microCT) and qualitatively evaluated using CTVol v. 2.3.2.0 software (Bruker-microCT). According to root length and canal configuration type, thirty specimens showing bifid-tipped mesial roots were selected and pair-matched with 30 non-bifid (single-tipped with blunt or conical shape) mesial roots (Fig. 1A). Sample size for this study was estimated following the effect size calculation of the results of a previous study (0.66) (Keleş & Keskin, 2017b). Following the t-test family and difference between two independent means with an alpha-type error by 0.05 and power beta of 0.80 (G*Power 3.1 for Macintosh; Heinrich Heine, Universität Dusseldorf, Dusseldorf, Germany), 29 samples were indicated as the minimum ideal size per group. Then, bifid and non-bifid mesial roots were compared regarding:
Main apical foramen
The apical foramen of the mesiobuccal (MB) and mesiolingual (ML) canals were evaluated regarding their position and vertical distance from the anatomic apex using DataViewer v.1.5.6 software (Bruker- microCT). The vertical distance was determined by measuring the distance from a horizontal plane perpendicular to the root long axis, crossing the anatomic apex, to a second horizontal plane crossing the centre of the foramen, parallel to the long axis of the root.
Foramen-like orifices
The number of foramen-like orifices in the resected root was examined according to Ordinola-Zapata et al. (2019). The orifices were evaluated at 1.0-mm interval for bifid and non-bifid roots from the level of the main foramen up to 4 mm in the coronal direction using Data-Viewer v.1.5.6 software (Bruker-microCT) (Fig. 1B).
Apical foramina and accessory canals
The number of apical foramina (main and accessory foramina) was calculated for each specimen using CTVol v. 2.3.2.0 software (Bruker- microCT). The number and type (patent, loop and anastomoses), as well as, origin and points of exit of the accessory canals, were evaluated according to Ordinola-Zapata et al. (2019), respectively, using CTVol v. 2.3.2.0 (Bruker-microCT) and DataViewer v.1.5.6 (Bruker-microCT) software. Patent accessory canal was defined as any branch that leaves the main canal and communicates with the external surface of the root, the loop accessory canal leaved and rejoined the same canal (recurrent), while the anastomoses connected two different canals (inter-canal branch) (Fig. 1C). The origin position of the accessory canals was determined by measuring the vertical distance, parallel to the long axis of the mesial root, from a horizontal plane perpendicular to the root long axis crossing the anatomic apex, to a second horizontal plane crossing the coronal basis of the accessory canal. The point of exit of accessory canals (i.e. accessory foramen) was determined by measuring the vertical distance, parallel to the long axis of the mesial root, from a horizontal plane perpendicular to the root long axis crossing the anatomic apex, to a second horizontal plane crossing the centre of the foramen. In accessory canals categorized as loops and anastomoses, the merging position was considered as the end point of the canals (Ordinola-Zapata et al., 2019).
Dentine thickness
Three-dimensional map of dentine thickness was obtained with CTAn v.1.18.8 software (Bruker-microCT) (De-Deus et al., 2019 and colour-coded cross-sections were used to identify (CTVox v.3.3.0; Bruker-microCT) and measure (CTAn v.1.18.8, Bruker-microCT) the smallest dentine thickness, in both aspects of the root, related to each root canal at 1.0-mm interval from the furcation level (level 0) up to 7 mm (levels 1–7) to the apex.
Isthmus and middle mesial canals
Isthmus, defined as complete or partial narrow communication between MB and ML canals (AAE, 2020), was classified as present or absent, while the presence and type of middle mesial canal was categorized in accordance to a previous study (Pomeranz, Eidelman, & Goldberg, 1981).
Statistical analysis
Morphological comparisons between bifid and pair-matched non-bifid mesial roots were performed using Chi-square (position of the apical foramen, number of accessory foramina and type of accessory canals) and Student’s t-test (root length and dentine thickness). Bonferroni correction was used when multiple pairwise tests were performed and a statistical difference was found between groups (SPSS v.21.0 software; SPSS Inc., Chicago, IL, USA).
Results
Overall, the incidence of mesial roots with bifid apex in this study was 13.6 % (30 out of 250 mandibular first molar). The degree of the homogeneity between bifid and non-bifid groups was confirmed by comparing root canal configuration and root length of the specimens (p > 0.05).
Main apical foramen
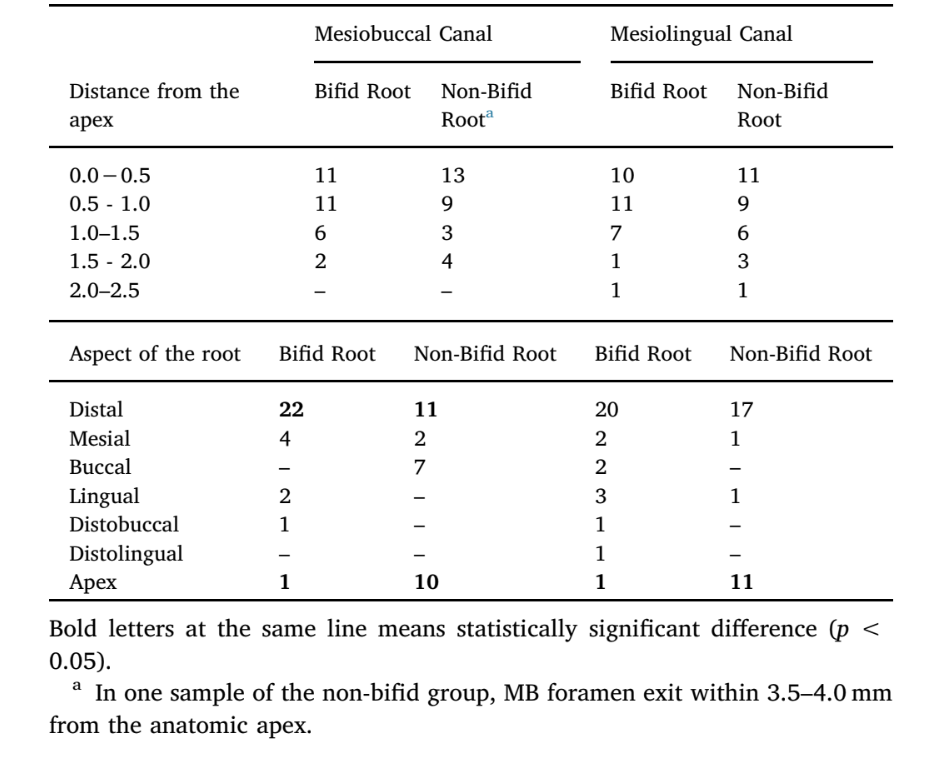
Table 1 displays the position of the main foramen of the MB and ML canals of bifid and non-bifid mesial roots of mandibular first molars. Overall, no statistical difference regarding the distance of the main foramen to the apex was observed between bifid and non-bifid roots for both MB and ML canals (p > 0.05). In MB and ML canals of non-bifid roots, a significant number of foramina exit at the apex (p < 0.05), while in the MB canal of bifid roots, most of the main foramina deviated distally (p < 0.05).
Foramen-like orifices
Table 2 shows the number of canal orifices observed at the apical third of bifid and non-bifid mesial roots and Fig. 1B illustrates the orifices in 4 different levels of 2 representative specimens. Overall, no statistical difference was observed at the apical third of bifid and non- bifid roots regarding the number of orifices in different levels (p > 0.05). Statistical difference was observed only at 2-mm from the anatomic apex (p < 0.05). Cross-section analysis of the apical third showed that most of the specimens had 2–4 orifices at the apical third, but the presence of more than 4 orifices in some specimens was also observed (Table 2).

Apical foramina and accessory canals
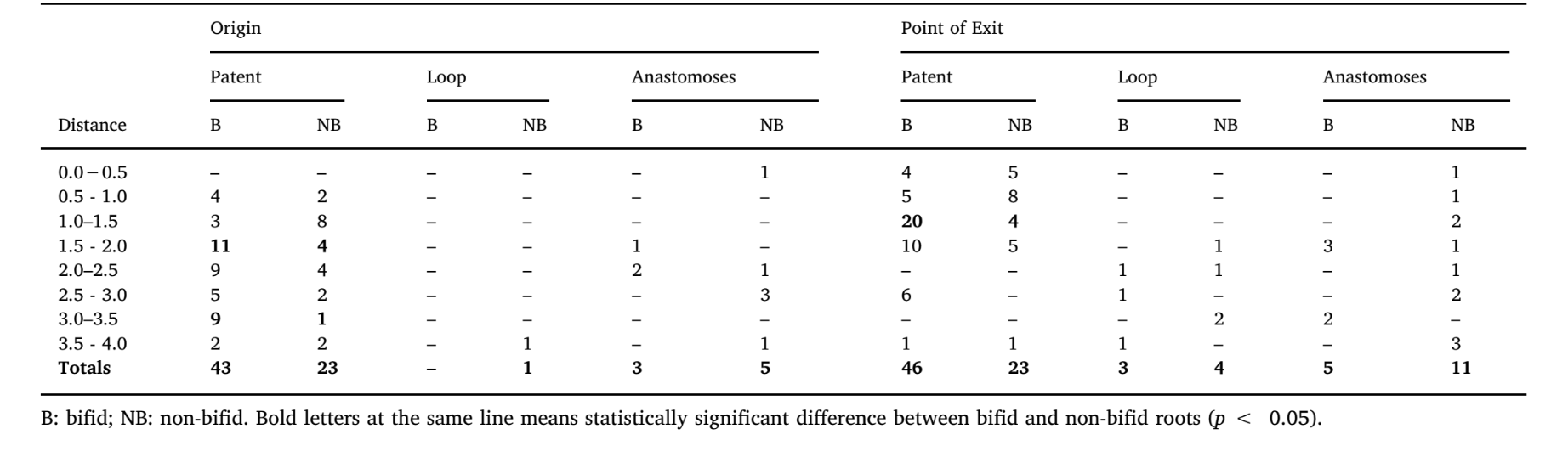
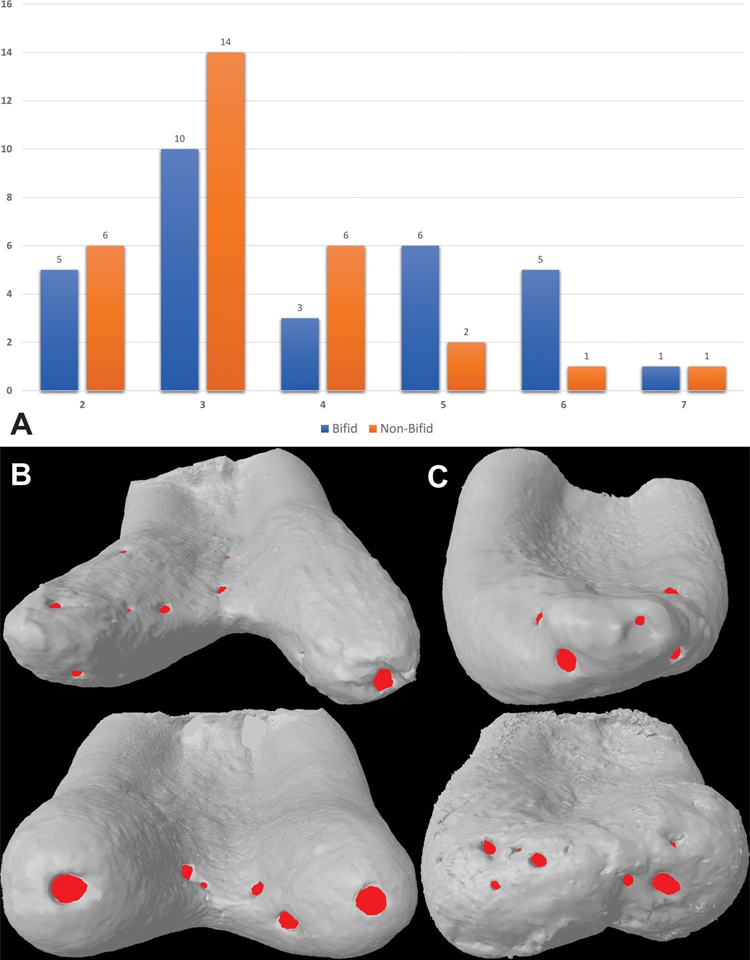
Table 3 shows the distribution of the accessory canal types at the apical third of bifid and non-bifid mesial roots according to the position of their origin and points of exit, while Fig. 1C illustrates the patent, loop and anastomoses accessory canals. Frequency of accessory canals in each level throughout the apical 4 mm of the mesial roots was significantly higher in bifid roots (p < 0.05); however, there was a higher number of patent canals compared with loop and anastomoses accessory types, in all specimens (p < 0.05). Overall, origin and exit of patent canals were observed mostly between 1.0 and 2.0 mm from the apex (p < 0.05). Bifid and non-bifid roots showed a total of 119 and 101 apical foramina, respectively (Fig. 2A). Multiple foramina (> 4) were observed in 41.7 % of the roots (n = 25) (Fig. 2B and C).
Dentine thickness
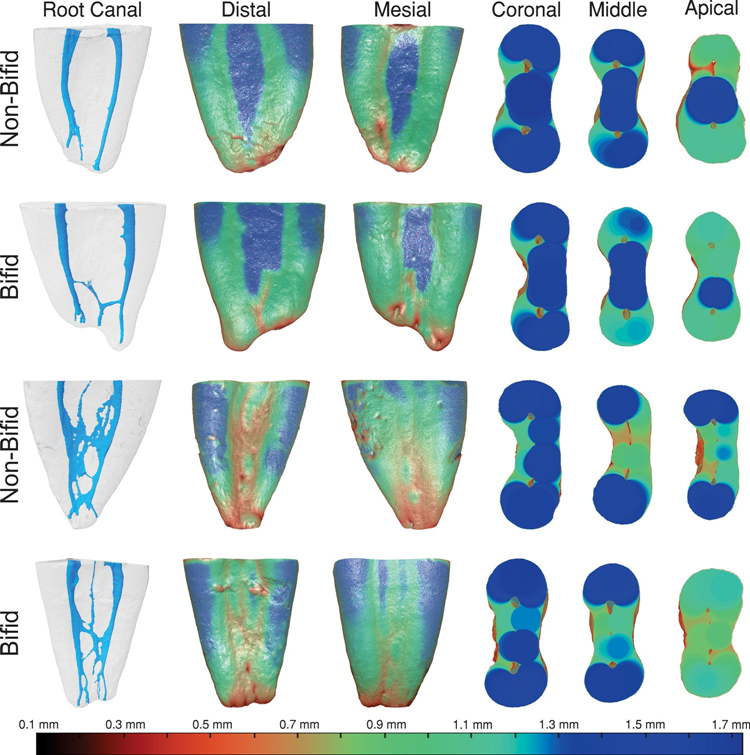
Table 4 and Fig. 3 display the dentine thickness values of the MB and ML canals of bifid and non-bifid mesial roots, in either mesial or distal directions, at 1.0-mm interval from the furcation level up to 7 mm in the apical direction. Fig. 4 shows representative pair-matched colour-coded models of bifid and non-bifid mesial roots showing different canal configurations. While in the mesial direction, dentine thickness varied from 0.49 to 1.88 mm (bifid roots) and from 0.43 to 1.85 mm (non-bifid roots), in the distal direction it varied from 0.32 to 2.14 mm (bifid roots) and from 0.40 to 1.92 mm (non-bifid roots). Significant difference between bifid and non-bifid roots was observed only at levels 0 (furcation) and 1 towards distal of the ML canal (p < 0.05) (Table 4). Despite the mean lowest values were observed to the distal direction of the roots (Table 4), dentine thickness lower than 0.5 mm was detected only at levels 6 and 7 of both canals and directions (Fig. 3). In Fig. 4 it is possible to observe that dentine thickness reduces with the increasing complexity of the root canal system.
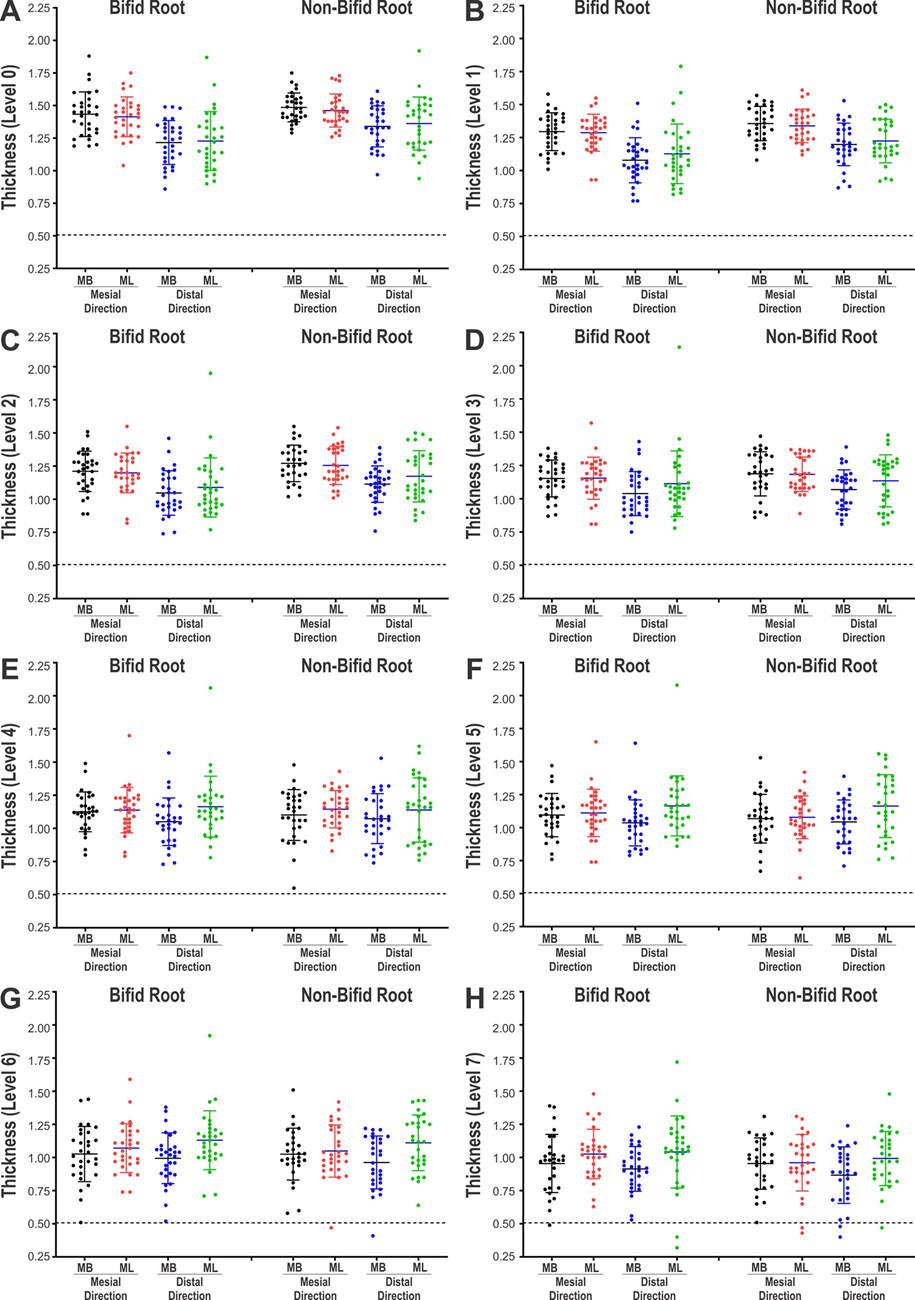
canals of bifid and non-bifid mesial roots from the (A) furcation level (level 0) up to 7 mm (levels 1 to 7) in the apical direction (B-H).
Isthmus and middle mesial canal
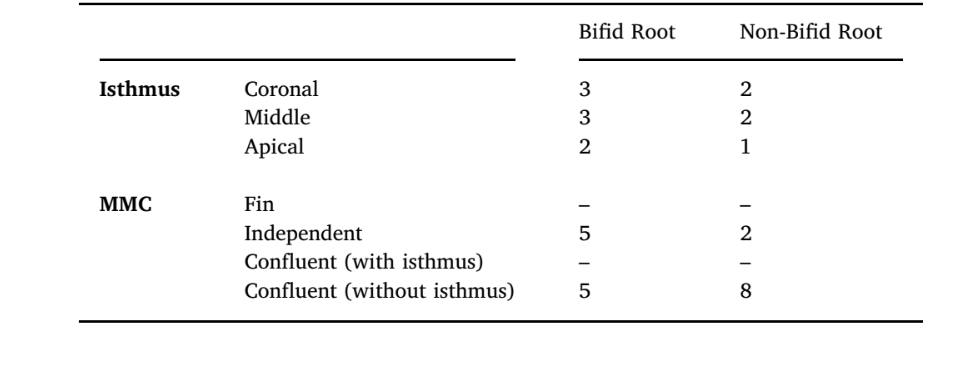
Only 3 mesial roots with bifid apex (10 %) had isthmus, mostly at the coronal third, while a high incidence of MMC was observed (n = 10, 33.3 %). Independent MMC type was observed mostly in bifid roots (n = 5; 16.6 %) and confluent anatomy without isthmus in non-bifid roots (n = 8; 26.6 %) (Table 5). Because of the pair matching methodology used in this study, the same number of specimens with MMC and isthmus was included in the non-bifid group. Therefore, no statistical comparison was performed.
Discussion
In this study, bifid and non-bifid mesial roots of mandibular first molars were compared regarding root morphology and canal anatomy under non-invasive micro-CT technology. It was demonstrated that the incidence of bifid apex in this root is relatively high (13.6 %) and this morphological feature may act as a predictive factor for a complex canal anatomy at the apical third. Accessory canals communicating the root canal system with the external root surface (patent type) were observed mostly between 1.0 and 2.0 mm from the apex and its frequency was significantly higher in bifid (n = 53) than in non-bifid (n = 30) roots (Table 3). On the other hand, loop and anastomoses canal types were not common at the apical area (Table 3), confirming results acquired in previous micro-CT studies (Briseño-Marroquín, Paqué, Maier, Willershausen, & Wolf, 2015; Ordinola-Zapata et al., 2019; Wolf, Paqué, Zeller, Willershausen, & Briseño-Marroquín, 2016). Ex vivo studies using CBCT and micro-CT methods over a large number of teeth reported an average incidence of MMC ranging from 0.26 % (Kim, Kim, Woo, & Kim, 2013) to 18.6 % (Versiani et al., 2016) while, in the present study, a significant high incidence of this extra canal was observed in the bifid roots (n = 10, 33.3 %), half of them (16.6 %) classified as independent type. The presence of an independent MMC may be a problem when its orifice is not detected even after a troughing procedure (Keleş & Keskin, 2017b). If an infected canal is missed, remaining bacteria may maintain or cause disease, compromising the prognosis of the treatment (Costa et al., 2019). Unfortunately, a bifid-tipped mesial root may be difficult to differentiate in clinics using conventional radiography because of the buccolingual superimposition of the root tips, although the presence of a radiolucent line of double periodontal membrane might suggest its presence. Moreover, notwithstanding cone-beam computed tomography (CBCT) is unable to provide information about minor root canal structures at the apical third (Ordinola-Zapata et al., 2017; Sousa et al., 2017), this technology can easily detect the presence of a bifid-tipped root morphology and, consequently, clinicians would be able to assume the existence of anatomical complexities that would demand a proper approach aiming to improve disinfection procedures.
Overall, no statistical difference was observed between bifid and non-bifid roots regarding the position of the MB and ML canal foramina (Table 1), number of foramina (Fig. 2), and number of canal orifices at the apical third (Table 2). These similarities could be explained by the strict criteria used for specimens’ selection using micro-CT data which allowed the formation of balanced pair-matched groups based on root length and canal configuration. In accordance with previous reports (Dummer, McGinn, & Rees, 1984; Vertucci, 2005), the main foramen of MB and ML canals were located within 2.5 mm from the anatomic apex. However, because of the high number of orifices and foramina at the apical third, if surgery becomes necessary, the natural anatomy is altered and additional anatomic features such as isthmus, transverse anastomoses, lateral connections or multiple orifices need to be addressed (Versiani et al., 2016). In the literature, bevelling root apex3mm during surgical procedures has been recommend to remove the vast majority of unprepared and unfilled accessory canals (Degerness & Bowles, 2008; Stropko, Doyon, & Gutmann, 2005) reducing the possibility of failure (Vertucci, 2005). Despite most of the orifices observed in this study were located 3 mm from the apex, as previously reported (Gu, Wei, Ling, & Huang, 2009; Wolf et al., 2016), multiple canal orifices were also observed 4 mm from the apex (Table 2). Thus, in case of apical surgery, it is highly recommended the use of operating microscopy, to benefit from magnification and illumination, together with thin ultrasonic tips for root-end preparation aiming to incorporate and seal all canal orifices (Leoni, Versiani, Pécora, & Sousa-Neto, 2014). Results will be poor if this altered anatomy is not recognized and properly treated. Finally, it is noteworthy to mention that only 3 bifid mesial roots (10 %) had isthmus at the apical third. This is a significant low percentage frequency considering that literature reports the presence of this anatomy in 55 % of the mesial roots of mandibular first molars (De Pablo, Estevez, Péix Sánchez, Heilborn, & Cohenca, 2010), with its greatest incidence at 3–5 mm from the apex (Teixeira et al., 2003).
Evaluation of dentine thickness is important because excessive removal of dentine during mechanical preparation could predispose teeth to root fracture (Kishen, 2006). In general, no significant difference was observed between bifid and non-bifid roots regarding dentine thickness in this study (Table 4, Figs. 3 and 4). As mentioned, this morphological similarity may be explained because of sample distribution into pair-matched groups. In the mesial direction, dentine thickness of MB and ML canals varied from 0.49 to 1.88 mm and from 0.43 to 1.75 mm, respectively, while, in the distal direction, from 0.40 to 1.61 mm and from 0.32 to 2.14 mm, respectively. A recent anatomical study on mesial roots of mandibular molars described dentine thickness values ranging from 0.67 to 1.93 mm (MB canal) and from 0.77 to 1.89 mm (ML canal) (De-Deus et al., 2019). Interestingly, the smallest values were at least twice as the reported in the present study. Besides, in 40 % (n = 9360) of all evaluated cross-sections (n = 23,400), the smallest dentine thickness related to the MB and ML canals was displaced towards the mesial portion of the root, instead of the distal direction (furcation area). These findings were not in line with the present study probably because the cross-sections for measurement were perpendicular to the teeth axis, rather than the axis of the mesial root, and moreover, the mesial root has an inherent curvature toward the distal side; therefore, the cutting line at the mesial aspect is in fact located more apically than the cutting line at the distal aspect of mesial root. Since the mesial root has a taping shape, and the apical level corresponds to a smaller thickness, the thickness at furcation side would be over-estimated.
Conclusions
The present study revealed significant differences in the apical anatomy of bifid and non-bifid mesial roots of mandibular first molars. Results suggest that the presence of a bifid-tipped mesial root in the mandibular first molars may act as a predictive factor for a complex canal anatomy with a high incidence of accessory anatomy at the apical third and an increase percentage frequency of independent middle mesial canals.
Authors: Ali Keleş, Cangül Keskin, Rawan Alqawasmi, Marco Aurelio Versiani
References:
- AAE (2020). Glossary of endodontic terms (10th edn.). Chicago: American Association of Endodontists. https://www.aae.org/specialty/clinical-resources/glossary-endodontic-terms/.
- Alves, F. R., Andrade-Junior, C. V., Marceliano-Alves, M. F., Pérez, A. R., Rôças, I. N., Versiani, M. A., et al. (2016). Adjunctive steps for disinfection of the mandibular molar root canal system: A correlative bacteriologic, micro–computed tomography, and cryopulverization approach. Journal of Endodontics, 42, 1667–1672. https://doi. org/10.1016/j.joen.2016.08.003.
- Briseño-Marroquín, B., Paqué, F., Maier, K., Willershausen, B., & Wolf, T. G. (2015). Root canal morphology and configuration of 179 maxillary first molars by means of micro-computed tomography: An ex vivo study. Journal of Endodontics, 41, 2008–2013. https://doi.org/10.1016/j.joen.2015.09.007.
- Costa, F., Pacheco-Yanes, J., Siqueira, J. F., Jr., Oliveira, A., Gazzaneo, I., Amorim, C. A., et al. (2019). Association between missed canals and apical periodontitis.International Endodontic Journal, 52, 400–406. https://doi.org/10.1111/iej.13022.
- De Pablo, Ó. V., Estevez, R., Péix Sánchez, M., Heilborn, C., & Cohenca, N. (2010). Root anatomy and canal configuration of the permanent mandibular first molar: A systematic review. Journal of Endodontics, 36, 1919–1931. https://doi.org/10.1016/j.joen.2010.08.055.
- De-Deus, G., Rodrigues, E. A., Belladonna, F. G., Simões-Carvalho, M., Cavalcante, D. M., Oliveira, D. S., et al. (2019). Anatomical danger zone reconsidered: A micro-CT study on dentine thickness in mandibular molars. International Endodontic Journal, 52, 1501–1507. https://doi.org/10.1111/iej.13141.
- Degerness, R., & Bowles, W. (2008). Anatomic determination of the mesiobuccal root resection level in maxillary molars. Journal of Endodontics, 34, 1182–1186. https:// doi.org/10.1016/j.joen.2008.07.007.
- Dummer, P. M. H., McGinn, J. H., & Rees, D. G. (1984). The position and topography of the apical canal constriction and apical foramen. International Endodontic Journal, 17, 192–198. https://doi.org/10.1111/j.1365-2591.1984.tb00404.x.
- Gu, L., Wei, X., Ling, J., & Huang, X. (2009). A microcomputed tomographic study of canal ısthmuses in the mesial root of mandibular first molars in a Chinese population.
- Journal of Endodontics, 35, 353–356. https://doi.org/10.1016/j.joen.2008.11.029.
- Keleş, A., & Keskin, C. (2018). A micro-computed tomographic study of band-shaped root canal isthmuses, having their floor in the apical third of mesial roots of mandibular first molars. International Endodontic Journal, 51, 240–246. https://doi.org/10.1111/iej.12842.
- Keleş, A., & Keskin, C. (2017a). Apical root canal morphology of mesial roots of mandibular first molar teeth with vertucci type II configuration by means of micro–computed tomography. Journal of Endodontics, 43, 481–485. https://doi.org/10.1016/j.joen.2016.10.045.
- Keleş, A., & Keskin, C. (2017b). Detectability of middle mesial root canal orifices by troughing technique in mandibular molars: A micro–computed tomographic study. Journal of Endodontics, 43, 1329–1331. https://doi.org/10.1016/j.joen.2017.03.021.
- Kim, S. Y., Kim, B. S., Woo, J., & Kim, Y. (2013). Morphology of mandibular first molars analyzed by cone-beam computed tomography in a Korean population: Variations in the number of roots and canals. Journal of Endodontics, 39, 1516–1521. https://doi. org/10.1016/j.joen.2013.08.015.
- Kishen, A. (2006). Mechanisms and risk factors for fracture predilection in endodontically treated teeth. Endodontic Topics, 13, 57–83. https://doi.org/10.1111/j.1601-1546.2006.00201.x.
- Kupczik, K., & Hublin, J. J. (2010). Mandibular molar root morphology in Neanderthals and Late Pleistocene and recent Homo sapiens. Journal of Human Evolution, 59, 525–541. https://doi.org/10.1016/j.jhevol.2010.05.009.
- Leoni, G. B., Versiani, M. A., Pécora, J. D., & Sousa-Neto, M. D. (2014). Micro-computed tomographic analysis of the root canal morphology of mandibular incisors. Journal of Endodontics, 40, 710–716. https://doi.org/10.1016/j.joen.2013.09.003.
- Ordinola-Zapata, R., Bramante, C. M., Versiani, M. A., Moldauer, B. I., Topham, G., Gutmann, J. L., et al. (2017). Comparative accuracy of the Clearing Technique, CBCT and Micro-CT methods in studying the mesial root canal configuration of mandibular first molars. International Endodontic Journal, 50, 90–96. https://doi.org/10.1111/iej.12593.
- Ordinola-Zapata, R., Martins, J. N. R., Niemczyk, S., & Bramante, C. M. (2019). Apical root canal anatomy in the mesiobuccal root of maxillary first molars: Influence of root apical shape and prevalence of apical foramina – A micro-CT study. International Endodontic Journal, 52, 1218–1227. https://doi.org/10.1111/iej.13109.
- Pomeranz, H. H., Eidelman, D. L., & Goldberg, M. G. (1981). Treatment considerations of the middle mesial canal of mandibular first and second molars. Journal of Endodontics, 7, 565–568. https://doi.org/10.1016/S0099-2399(81)80216-6.
- Sousa, T. O., Haiter-Neto, F., Nascimento, E. H. L., Peroni, L. V., Freitas, D. Q., & Hassan, B. (2017). Diagnostic accuracy of periapical radiography and cone-beam computed tomography in ıdentifying root canal configuration of human premolars. Journal of Endodontics, 43, 1176–1179. https://doi.org/10.1016/j.joen.2017.02.021.
- Stropko, J. J., Doyon, G. E., & Gutmann, J. L. (2005). Root-end management: Resection, cavity preparation, and material placement. Endodontic Topics, 11, 131–151. https://doi.org/10.1111/j.1601-1546.2005.00158.x.
- Teixeira, F. B., Sano, C. L., Gomes, B. P. F. A., Zaia, A. A., Ferraz, C. C. R., & Souza-Filho, F. J. (2003). A preliminary in vitro study of the incidence and position of the root canal isthmus in maxillary and mandibular first molars. International Endodontic Journal, 36, 276–280. https://doi.org/10.1046/j.1365-2591.2003.00638.x.
- Turner, C. G. (1981). Root number determination in maxillary first premolars for modern human populations. American Journal of Physical Anthropology, 54, 59–62. https://doi.org/10.1002/ajpa.1330540108.
- Versiani, M. A., Ordinola-Zapata, R., Keleş, A., Alcin, H., Bramante, C. M., Pécora, J. D., et al. (2016). Middle mesial canals in mandibular first molars: A micro-CT study in different populations. Archives of Oral Biology, 61, 130–137. https://doi.org/10.1016/j.archoralbio.2015.10.020.
- Vertucci, F. J. (2005). Root canal morphology and its relationship to endodontic procedures. Endodontic Topics, 10, 3–29. https://doi.org/10.1111/j.1601-1546.2005.00129.x.
- Villas-Bôas, M. H., Bernardineli, N., Cavenago, B. C., Marciano, M., Del Carpio-Perochena, A., de Moraes, I. G., et al. (2011). Micro-computed tomography study of the internal anatomy of mesial root canals of mandibular molars. Journal of Endodontics, 37, 1682–1686. https://doi.org/10.1016/j.joen.2011.08.001.
- Wayman, B. E., Patten, J. A., & Dazey, S. E. (1994). Relative frequency of teeth needing endodontic treatment in 3350 consecutive endodontic patients. Journal of Endodontics, 20, 399–401. https://doi.org/10.1016/S0099-2399(06)80299-2.
- Wolf, T. G., Paqué, F., Zeller, M., Willershausen, B., & Briseño-Marroquín, B. (2016). Root canal morphology and configuration of 118 mandibular first molars by means of micro-computed tomography: An ex vivo study. Journal of Endodontics, 42, 610–614. https://doi.org/10.1016/j.joen.2016.01.004.
