Evaluation of physicochemical properties of four root canal sealers
Abstract
Aim: To assess the physicochemical properties and the surface morphology of AH Plus, GuttaFlow, RoekoSeal and Activ GP root canal sealers.
Methodology: Five samples of each material were evaluated for setting time, dimensional alteration, solubility and radiopacity tests, according to ANSI/ ADA Specification 57. A total of 50 mL of deionized distilled water from the solubility tests were used to measure the metal solubility by atomic absorption spectrometry. The morphologies of the external surface and the cross-section of the samples were analysed by means of a scanning electron microscope (SEM). Statistical analysis was performed by using one-way anova and post hoc Tukey–Kramer tests with the null hypothesis set as 5%.
Results: AH Plus had the longest setting time (580.6 ± 3.05 min) (P < 0.05). Activ GP did not have a mean value on the radiopacity and solubility tests (1.31 ± 0.35 mm and 11.8 ± 0.43%, respectively) in accordance with ANSI/ADA, being significantly different from the other materials (P < 0.05), which had mean values for these tests in accordance with the ADA’s requirements. GuttaFlow was the only sealer that conformed to the Specification 57 concerning the dimensional alteration test (0.44 ± 0.16%) (P < 0.05). The spectrometry test revealed significant Ca2+, K+, Zn2+ ion release from Activ GP sealer (32.57 ± 5.0, 1.57 ± 0.22 and 8.20 ± 1.74 μg mL–1, respectively). In SEM analysis, the loss of matrix was evident and the filler particles were more distinguishable in all groups.
Conclusions: The setting time of all sealers was in accordance with ANSI/ADA’s requirements. Activ GP did not fulfill ANSI/ADA’s protocols regarding radiopacity, dimensional alteration and solubility. Gutta-Flow was the only sealer that conformed to the Specification 57 in all tests. SEM analysis revealed that the surfaces of all sealers had micromorphological changes after the solubility test.
Introduction
In root canal treatment, complete sealing of the root canal system after cleaning and shaping is critical to prevent oral pathogens from colonizing and re-infecting the root and periapical tissues. Although gutta-percha is still the most commonly used root canal filling material, a number of new techniques and materials with different physicochemical properties have been developed (Ørstavik 2005, Versiani et al. 2006, Resende et al. 2009).
During canal filling procedures, the endodontic sealer performs several functions including the filling of root canal wall irregularities such as apical ramifications and deltas as well as spaces where gutta-percha was not able to adapt (Kontakiotis et al. 2007). Because the conventional filling materials do not effectively seal the root canal space, new endodontic sealers have been developed (Bouillaguet et al. 2008).
Although AH Plus has adequate long-term dimensional stability, its sealing ability remains controversial partly because AH Plus does not bond to gutta-percha (Ørstavik et al. 2001). RoekoSeal Automix (Coltène Whaledent, Langenau, Germany) is a sealer containing dimethylpolysiloxane with limited data available on its physicochemical properties (Gençoglu et al. 2003). Despite the promising clinical and laboratory data reported for the silicone-based sealer, RoekoSeal has been tested in various studies with contradictory results (De-Deus et al. 2007). GuttaFlow (Coltène Whaledent, Alstätten, Switzerland) is a polyvinylsiloxane-based sealer and was introduced with the intention of reducing the disadvantages of warm gutta-percha techniques (Eldeniz & Ørstavik 2009). It has been claimed that GuttaFlow could improve the seal because of increased flowability and the fact that the material expands slightly on setting (Bouillaguet et al. 2008, Eldeniz & Ørstavik 2009, Roggendorf et al. 2010).
Dental applications of glass ionomer sealers take advantage of their bond to dentine, fluoride release, antimicrobial activity and biocompatibility (Ørstavik 2005). However, some laboratory tests have indicated a propensity for leakage and disintegration (Carvalho-Júnior et al. 2003, Schäfer & Zandbiglari 2003). Recently, Activ GP Precision Obturation System (Brasseler USA, Savannah, GA, USA) was marketed as a root filling monoblock system by using conventional gutta-percha cones that are surface coated with glass ionomer fillers (Roggendorf et al. 2010). The manufacturer claims the product to be superior to previous glass ionomer-based systems in terms of handling characteristics, working time, radiopacity (Fransen et al. 2008), and seal, because of the increased flowability and the fact that the material expands slightly on setting (Kontakiotis et al. 2007).
Although various kinds of endodontic sealers have been proposed as innovative filling materials, the ideal root canal sealer has yet to be found (Ørstavik 1983, 2005, Ørstavik et al. 2001, Versiani et al. 2006, Resende et al. 2009). Therefore, the aim of this laboratory study was to compare the setting time, radiopacity, dimensional change, and solubility of silicone-based sealers (GuttaFlow and RoekoSeal), and an ionomer-based sealer (Activ GP) with a well-established epoxy resin-based sealer (AH Plus), according to ANSI/ADA (2000) standards.
Materials and methods
Setting time, radiopacity, dimensional changes, and solubility, after setting for AH Plus (group I), GuttaFlow (group II), RoekoSeal (group III), and Activ GP (group IV) root canal sealers were measured according to ANSI/ADA (2000) standards for root canal sealing materials. The tested materials (Table 1) were manipulated according to the manufacturer’s instructions. Analyses were performed by a single examiner who was blind to the experimental materials. To standardize and homogenize the amount of material, 0.3 g of sealer was dispensed onto a mixing pad and spatulated for 15 s for each tested sample. For physicochemical tests, the arithmetic mean of five replicates for each sealer was recorded and considered as the result of the test.
Setting time
Five plaster of Paris cast rings, having an internal diameter of 10 mm and a thickness of 2 mm were prepared. The external borders of the moulds were fixed with wax on a glass plate (75 x 25 x 1 mm). The moulds were then filled with the material and transferred to a chamber with 95% relative humidity (RH) and a temperature of 37 °C. After 150 ± 10 s from the start of mixing the sealer, a Gilmore-type needle with a mass of 100 ± 0.5 g having a flat end of 2.0 ± 0.1 mm in diameter was carefully lowered vertically onto the horizontal surface of each sample. The needle tip was cleaned and the probing was repeated until indentations ceased to be visible. If the results differed by more than ±5%, the test was repeated.
Radiopacity test
Five acrylic plates (2.2 cm x 4.5 cm x 1 mm), containing four wells measuring 1 mm in depth and 5 mm in diameter, were prepared and placed over a glass plate covered by a cellophane sheet. Each well was filled with one of the sealers, following a sequence according to the setting time of the material, from the longest to the shortest, so that the samples would be ready for radiographic evaluation after the final setting of all materials. In order to avoid the formation of bubbles, the freshly mixed sealer was introduced into the wells using a syringe. Another glass plate covered with cellophane was placed on top until complete setting and any excess sealer removed. Each plate was kept in an incubator (37°C, 95% RH) for a period corresponding to three times the setting time.
Each one of the acrylic plates containing the sealers was positioned, at the time of the radiographic exposure, alongside to another acrylic plate (1.3 cm x 4.5 cm x 1 mm), containing an aluminium stepwedge, made of 1100 alloy, with the thickness varying from 1 to 10 mm, in uniform steps of 1 mm each (Margraf Dental MFG Inc., Jenkintown, PA, USA). This set of acrylic plates was placed in front of this phosphor plate, next to the aluminium step wedge, and a digital radiograph was taken (Digora™ system; Soredex Orion Corporation, Helsinki, Finland). Radiographic images were obtained using the Spectro 70X X-ray machine (Dabi Atlante, Ribeirão Preto, SP, Brazil), at 70 kVp and 8 mA. The object-to-focus distance was 30 cm and the exposure time was 0.2 s. Exposed imaging plates of the test samples were immediately scanned after exposure (Digora™ Scanner) and analysed using Digora™ for Windows 5.1 software.
Dimensional change
Five Teflon® (Polytetrafluroethylene, DuPont, HABIA, Knivsta, Sweden) moulds, prepared for the production of 3.58-mm high cylindrical test bodies measuring 3 mm in diameter, were placed on a glass plate wrapped with a fine cellophane sheet. The moulds were filled with a slight excess of freshly mixed sealers and a microscope slide, also wrapped in cellophane, was pressed onto the upper surface of the mould. The assembled group was kept firmly joined with a C-shaped clamp and transferred to an incubator (37 °C, 95% RH) left to stand for a period corresponding to three times the setting time. After this period, the flat ends of the moulds, containing the samples, were ground with 600 grit wet sandpaper. The samples were removed from the mould, measured with a digital calliper, stored in a 50-mL vessel containing 2.24 mL of deionized distilled water, and kept in an incubator (37 °C, 95% RH) for 30 days. The sample was then removed from the container, blotted dry on absorbent paper, and measured again for length. The percentage of the dimensional alterations was calculated using the formula:
((L30 — L )/ L) x 100
where L30 is the length of the sample after 30 days of storage and L is the initial length of the sample.
Solubility
A 1.5-mm-thick cylindrical Teflon® mould measuring 7.75 mm in inner diameter was filled with freshly mixed sealer. The mould was supported by a larger glass plate and covered with a cellophane sheet. An impermeable nylon thread was placed inside the material and another glass plate, also covered with cellophane film, was positioned on the mould and pressed manually in such a way that the plates touched the entire mould in a uniform manner. The assembly was placed in an incubator (37 °C, 95% RH) and left to stand for a period corresponding to three times the setting time. As soon as the samples were removed from the mould, they were weighed three times each (HM-200, A & D Engineering, Inc., Bradford, MA, USA), and the mean reading recorded. The samples were suspended by nylon thread and placed two-by- two inside a plastic vessel with a wide opening containing 7.5 mL of deionized distilled water, taking care to avoid any contact between them and the inner surface of the container. The containers were sealed and left for 7 days in an incubator (37 °C, 95% RH). After this period, the samples were removed from the containers, rinsed with deionized distilled water, blotted dry with absorbent paper, and placed in a dehumidifier for 24 h. Afterwards, they were weighed again. The weight loss of each sample (initial mass minus final mass), expressed as a percentage of the original mass (m% = mi – mf), was taken as the solubility of the sealer.
A volume of 7.5 mL of distilled water from each sample was poured into a cleaned and dried porcelain crucible. Each crucible was put into a muffle and burned at 550 °C. Ash was dissolved in 10 mL of a concentrated nitric acid (Merck KGaA, Darmstadt, Germany) using a glass stick. Following this, the samples were put into 50 mL volumetric flasks and the volume made up with ultrapure deionized water (MilliQ, Millipore, Billerica, MA, USA). The attained solutions were sprayed into the atomic absorption spectrophotometer (Perkin Elmer, Überlingen, Germany) to verify the presence of Ca2+, K+, Zn2+ and Ag+ ions. The arithmetic mean of three replicates for each specimen was recorded and considered as the result, expressed as μg mL–1.
Scanning electron microscopy examination
For scanning electron microscopy (SEM) examination, cylindrical Teflon® moulds (3 x 4 mm) were filled with freshly mixed sealers. The moulds were supported by a glass plate covered with a cellophane sheet and placed in a chamber (37 °C, 95% RH) for a period corresponding to three times the setting time. After that, the samples were sectioned with a number 15 disposable surgical scalpel blade, fixed on a metallic stub (10 x 5 mm), and sputter-coated with gold–palladium (Bal-Tec AG, Balzers, Germany) at 20 mA. The morphologies of the external surface and the cross-section of the samples were qualitatively analysed under a field emission SEM (Jeol JSM 5410; Jeol Technic Co., Tokyo, Japan) at an accelerating voltage of 15 kV, a working distance from 6 to 10 mm, and at different magnifications.
Statistical analysis
Five specimens from each group were tested and the means were statistically compared. The Kolmogorov– Smirnov showed that the results were consistent with a normal distribution curve thus, parametric statistical analysis was possible (one-way anova and post hoc Tukey–Kramer test), and the null hypothesis was set as 5% (spss 17.0 for Windows; SPSS Inc., Chicago, IL, USA).
Results
Setting time
The ANSI/ADA specification (2000) requires that the setting time of a sealer shall be within 10% of that stated by the manufacturers. According to them, the setting times of AH Plus, RSA RoekoSeal, and Gutta-Flow are 480, 50, and 30 min, respectively. Conversely, the Activ GP manufacturer does not provide this information and only states that it has been formulated with an extended 15-min working time.
Statistical significant difference was observed between AH Plus (580.6 ± 3.05 min), GuttaFlow (24.0 ± 2.0 min), RoekoSeal (40.0 ± 1.58 min), and Activ GP (15.2 ± 1.30 min) groups (P < 0.05). However, mean values showed agreement with the ANSI/ ADA standardization (Table 2).
Radiopacity test
AH Plus (6.0 ± 0.12 mmAl), GuttaFlow (3.0 ± 0.04 mmAl), and RoekoSeal (4.17 ± 0.45 mmAl) had radiopacity above the 3-mm of aluminium as recommended by ANSI/ADA Specification 57, while Activ GP (1.31 ± 0.35 mmAl) did not meet this requirement. Statistical analysis demonstrated difference between the experimental groups (P < 0.05) (Table 2).
Dimensional change
ANSI/ADA standardization (2000) states that the mean linear shrinkage of the sealer shall not exceed 1% or 0.1% in expansion. Except for GuttaFlow (0.44 ± 0.16%), neither sealer conformed to the Specification 57 (ANSI/ADA 2000). All groups exhibited post-setting expansion except for RoekoSeal that showed shrinkage on setting (-1.33 ± 0.12%). Statistical analysis revealed significant difference between the experimental groups (P < 0.05) (Table 2).
Solubility
ANSI/ADA Specification 57 (2000) states that a root canal sealer should not exceed 3% by mass when the solubility of the set material is tested. Except for Activ GP (11.8 ± 0.43%) all sealers conformed to ANSI/ ADA standardization (P < 0.05) (Table 2). The deionized distilled water used for the solubility test and submitted to atomic absorption spectrometry showed a significant level of Ca2+, K+ and Zn2+ ions release in the Activ GP group compared with AH Plus, Gutta-Flow, and RoekoSeal (P < 0.05). Significant levels of Ag+ release were not observed in any group (Table 3).

SEM examination
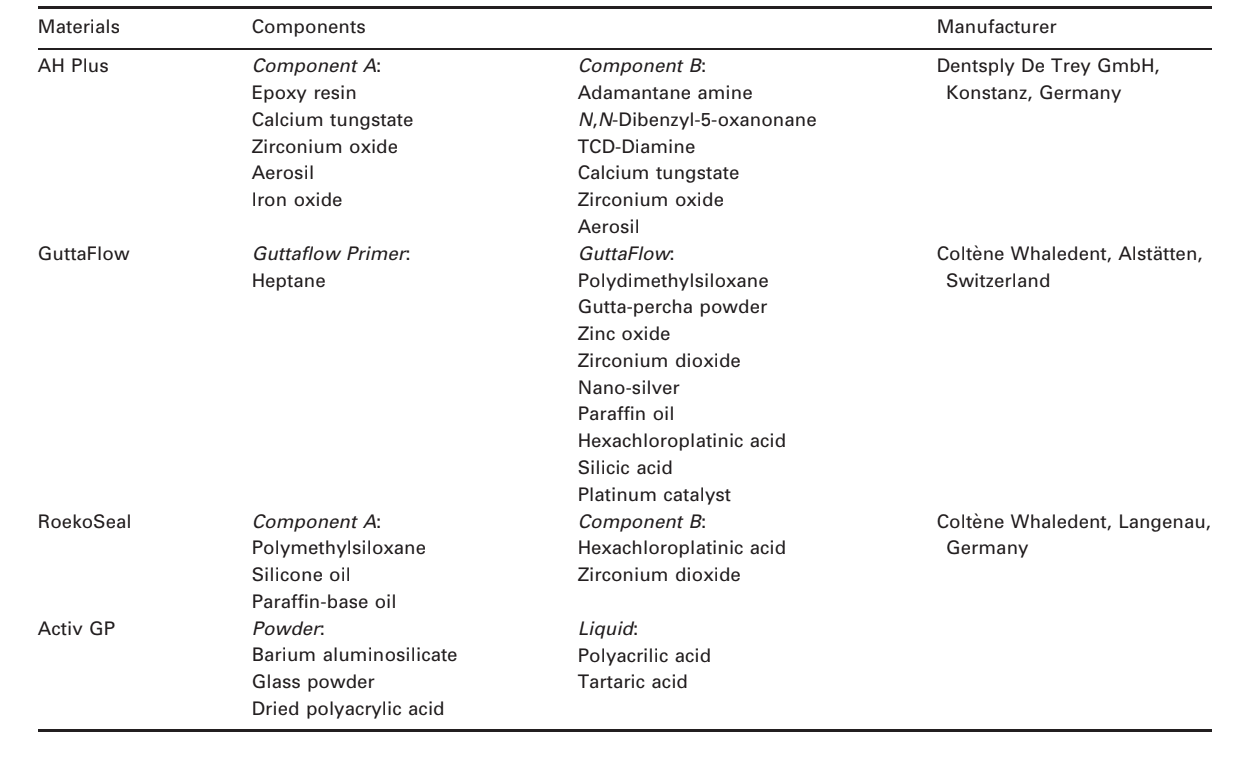
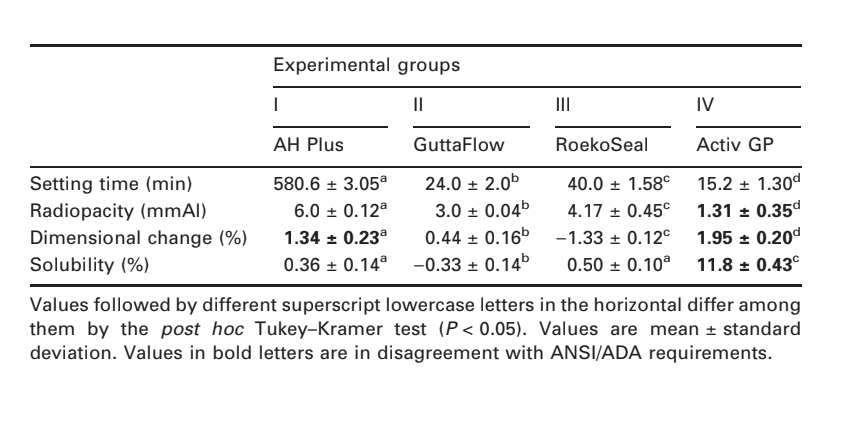
Selected photomicrographs of the polymerized samples obtained before and after water storage for 7 days are presented in Figs 1–4. Overall, it was noted that all surfaces had micromorphological changes after the solubility test. The loss of matrix was evident and the filler particles were more distinguishable. AH Plus (Fig. 1a,b), GuttaFlow (Fig. 2a,b), and RoekoSeal (Fig. 3a,b) groups had an external surface that appeared to be mostly homogeneous rough surfaces that decrease in roughness after the solubility test with a subsequent roughening of the surface. Cracking was not observed in the specimens except for the Activ GP group. The surface of Activ GP samples was relatively rougher when compared with the other groups and the surface material appeared to be more damaged after the solubility test (Fig. 4a,b).
The scanning electron microscopy of the cross-section surface of the specimens revealed the presence of sphere-shaped polymers of different sizes that were nonhomogeneously dispersed. After the solubility test, the inner surface of AH Plus (Fig. 1c,d), GuttaFlow (Fig. 2c,d), and Activ GP (Fig. 4c,d) samples appeared to be much more irregular and rough. Several micro-cracks were seen at the filler-polymeric matrix interfaces in the Activ GP group before and after the solubility test (Fig. 4c,d). On the other hand, RoekoSeal group had a more uniform and compact layer comprised of sphere-shaped polymers with a higher amount of resin matrix (Fig. 3c,d).
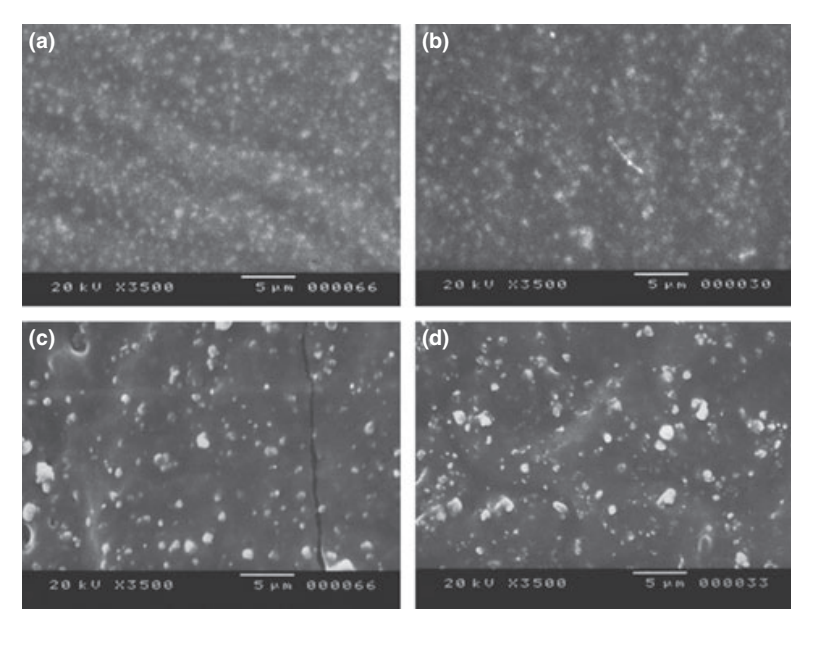
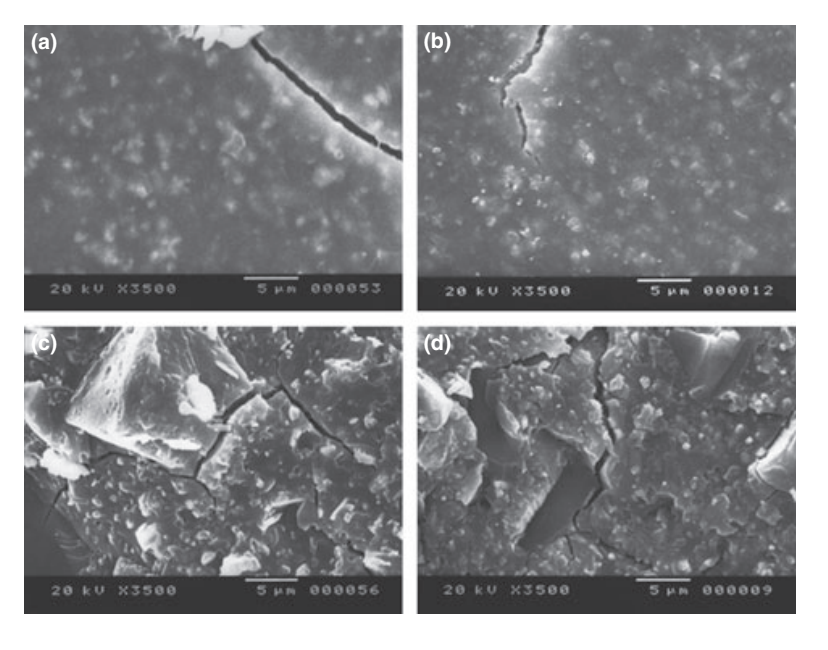
Discussion
Setting time is primarily a control test on the stable behaviour of a product and is dependent on the constituent components, their particle size, the ambient temperature, and relative humidity (Ørstavik 1983, 2005, Ørstavik et al. 2001). In the present study, all results were in accordance with ANSI/ADA specification. The longest setting time of AH Plus might be explained by this sealer being a two-component paste material based on a slow polymerization reaction of epoxy resin amines with high molecular weight (Bisphenol A and Bisphenol F), where the conversion of monomers into polymers occurs gradually (Lin-Gibson et al. 2006, Resende et al. 2009). These results were in agreement with previous research (Versiani et al. 2006, Resende et al. 2009), however, Garrido et al. (2010) reported a setting time of 13 h to AH Plus, which is above the 10% variation permitted by the ADA’s Specification Number 57.
The ANSI/ADA (2000) standards for the radiodensity of root canal sealing materials have been determined using conventional dental films where the radiographic images are obtained by the chemical processing (Taşdemir et al. 2008). However, digital radiography is becoming increasingly more common in the study of endodontic sealers (Baksi Akdeniz et al. 2007, Carvalho-Júnior et al. 2007a, Rasimick et al. 2007, Taşdemir et al. 2008). Thus, in the present study digital images of root filling materials and steps of an aluminium stepwedge were acquired using a phosphor plate system and a scanning, capturing and reading digital system.
An analysis of the composition of the tested materials revealed they all have radiopacifier agents (Table 1), except for Activ GP sealer. According to Tanomaru-Filho et al. (2008), the presence of barium aluminosilicate glass powder in its composition does not provide satisfactory radiopacity. AH Plus contains zirconium oxide, iron oxide and calcium tugstate as radiopacifiers (Tanomaru-Filho et al. 2007) and had the highest mean radiopacity value (6.0 ± 0.12 mmAl), as reported previously (Tagger & Katz 2003, Carvalho-Júnior et al. 2007a, Tanomaru-Filho et al. 2007, Taşdemir et al. 2008). RoekoSeal sealer contains zirconium dioxide as the radiopacifier (Baksi Akdeniz et al. 2007) and the present result (4.17 ± 0.45 mmAl) was in agreement with previous findings that showed its radiopacity ranging from 4.0 to 5.7 (Tagger & Katz 2003, Gambarini et al. 2006, Tanomaru-Filho et al. 2007, Rasimick et al. 2007). The lower radiopacity of GuttaFlow (3.0 ± 0.04 mmAl) in comparison to RoekoSeal was also observed in previous studies (Tagger & Katz 2003, Gambarini et al. 2006, Rasimick et al. 2007, Tanomaru-Filho et al. 2007). As the manufacturer does not provide the chemical type and concentration of nano-silver in GuttaFlow, it is apparent that these particles did not exert a radiopaque characteristic. High solubility of root canal sealers is undesirable because dissolution may cause the release of materials that could irritate periapical tissues and may also permit gaps to form between root canals and filling materials that is likely to increase bacterial leakage over time (Donnelly et al. 2007). Despite ANSI/ADA recommendations, in the present study a modification previously proposed for both tests was used, which achieved similar results with a decrease in the material volume necessary for the production of the test samples (Carvalho-Júnior et al. 2007b). In the present study, AH Plus expanded in the dimensional change test. The slow gain in mass over time was mostly due to the interaction energy between water and the adsorption sites for the system (i.e. a hydroxyl group, an amino group, the polymer chain end, etc.), filling the gaps between the polymer chains (McDermott 1993). Possibly, this expansion has compensated the shrinkage suffered by the resin-based sealer after polymerization (Phillips & Skinner 1991, Carvalho-Júnior et al. 2007b, Resende et al. 2009, Garrido et al. 2010).
The polymerization of the AH Plus is achieved through the polyamines monomers in its composition (1-adamantane amine, N,N’-dibenzyl-5-oxa-nonandi- amine-1,9, TCD-Diamine). When the diepoxide compounds and polyamines paste are mixed together, the amine groups react with the epoxide groups to form a covalent bond. The utilization of amine groups aliphatic cyclic promotes modifications in the curing rate, cross-link density and morphology of epoxy resins. Each NH group can react with an epoxide group, so that the resulting polymer is heavily cross-linked, and is thus rigid and strong (McDermott 1993, Case et al. 2005). It may explain the homogeneous and compact rough surface revealed in SEM analysis and the low solubility results of AH Plus in the present study. Schäfer & Zandbiglari (2003) have also measured the solubility of epoxy resins (AH Plus, AH 26), silicone-based (RoekoSeal), calcium hydroxide (Apexit, Sealapex), zinc-oxide-eugenol (Aptal-Harz) and glass ionomer-based (Ketac Endo) sealers in water and artificial saliva. According to these authors, AH Plus had the least weight loss of all sealers tested, independent of the solubility medium used.
Previous findings have shown that polydimethylsiloxane-based sealers expand slightly during setting and exhibited minimal water sorption and solubility (Donnelly et al. 2007, Monticelli et al. 2007). In agreement with this statement, in the present study GuttaFlow and RoekoSeal sealers had low solubility with minimum metal ion release. Considering the dimensional change test, GuttaFlow was the only sealer that conformed to the Specification 57 (ANSI/ADA 2000), underwent an expansion of approximately 0.44%. The same was not observed with its predecessor, RoekoSeal that demonstrated shrinkage (1.33%). Conversely, this result was not in agreement with Ørstavik et al. (2001) who reported that Roeko-Seal had a small expansion of some 0.2% within 4 weeks, and was stable thereafter.
The presence of gutta-percha and nano-silver particles on GuttaFlow sealer gives better stability to the polymer matrix due to the attraction force between atoms of silver and molecules of gutta-percha into the polymer cross-link (Malynych et al. 2001). These interactions may explain the low silver ion release of GuttaFlow observed in atomic absorption spectroscopy. Besides, the presence of gutta-percha in the polymer cross-link of GuttaFlow resulted in higher water adsorption than RoekoSeal (Gong et al. 2008) explaining the differences on the results of the dimensional change test and the more uniform and compact layer observed on RoekoSeal surface under SEM.
The highest results of solubility and dimensional change of Activ GP sealer may be related to ANSI/ ADA’s methodology that recommends that a sample be immersed in water after a period of three times the setting time of the material. According to Wilson & McLean (1988), the setting time of the ionomer cement is completed only 24 h after mixing. During the maturation time of glass–ionomer cements, the presence of humidity may cause dissolution of anions and cations that form the matrix for areas surrounding the cement (Monticelli et al. 2007). This ion dissolution does not allow water, which is part of cement composition, to hydrate the matrix (Ca2+ and Al3+ form polysalts with groups of COO-of polyacrylic acid), rendering it unstable (Carvalho-Júnior et al. 2003, Schäfer & Zandbiglari 2003, Donnelly et al. 2007). It could explain the rougher surface observed in SEM and the significant level of calcium, potassium and zinc ions release of Activ GP in the present study. As glass ionomer cements’ structures are likely to be influenced by the presence or absence of water, they are sensitive to dehydration early in the setting process (Wilson & McLean 1988). For SEM evaluation, dehydration of the sample is an essential process, which may explain the microcracks observed in the surface of Activ GP samples. Therefore, the results obtained with Activ GP in the present study should be interpreted with caution.
Conclusions
Setting time of all sealers was in accordance with ANSI/ ADA’s requirements. Activ GP did not fulfill ANSI/ADA’s protocols regarding radiopacity, dimensional alteration and solubility. GuttaFlow was the only sealer that conformed to the Specification 57 in all tests. SEM analysis revealed that all sealers’ surfaces presented micromorphological changes after the solubility test.
Authors: D. S. H. Flores, F. J. A. Rached-Júnior, M. A. Versiani, D. F. C. Guedes, M. D. Sousa-Neto, J. D. Pécora
References:
- ANSI/ADA (2000) Specification n° 57 Endodontic Sealing Material. Chicago, USA: ADA Publishing.
- Baksi Akdeniz BG, Eyuboglu TF, Sen BH, Erdilek N (2007) The effect of three different sealers on the radiopacity of root fillings in simulated canals. Oral Surgery Oral Medicine Oral Patholology Oral Radiology and Endodontics 103, 138–41.
- Bouillaguet S, Shaw L, Barthelemy J, Krejci I, Wataha JC (2008) Long-term sealing ability of Pulp Canal Sealer, AH-Plus, GuttaFlow and Epiphany. International Endodontic Journal 41, 219–26.
- Carvalho-Júnior JR, Guimarães LF, Correr-Sobrinho L, Pécora JD, Sousa-Neto MD (2003) Evaluation of solubility, disintegration, and dimensional alterations of a glass ionomer root canal sealer. Brazilian Dental Journal 14, 114–8.
- Carvalho-Júnior JR, Correr-Sobrinho L, Correr AB, Sinhoreti MA, Consani S, Sousa-Neto MD (2007a) Radiopacity of root filling materials using digital radiography. International Endodontic Journal 40, 514–20.
- Carvalho-Júnior JR, Correr-Sobrinho L, Correr AB, Sinhoreti MA, Consani S, Sousa-Neto MD (2007b) Solubility and dimensional change after setting of root canal sealers: a proposal for smaller dimensions of test samples. Journal of Endodontics 33, 1110–6.
- Case SL, O’Brien EP, Ward TC (2005) Cure profiles, crosslink density, residual stresses, and adhesion in a model epoxy. Polymer 46, 10831–40.
- De-Deus G, Brandão MC, Fidel RA, Fidel SR (2007) The sealing ability of GuttaFlow in oval-shaped canals: an ex vivo study using a polymicrobial leakage model. International Endodontic Journal 40, 794–9.
- Donnelly A, Sword J, Nishitani Y et al. (2007) Water sorption and solubility of methacrylate resin-based root canal sealers. Journal of Endodontics 33, 990–4.
- Eldeniz AU, Ørstavik D (2009) A laboratory assessment of coronal bacterial leakage in root canals filled with new and conventional sealers. International Endodontic Journal 42, 303–12.
- Fransen JN, He J, Glickman GN, Rios A, Shulman JD, Honeyman A (2008) Comparative assessment of ActiV GP/Glass Ionomer sealer, Resilon/Epiphany, and Gutta- Percha/AH Plus obturation: a bacterial leakage study. Journal of Endodontics 34, 725–7.
- Gambarini G, Testarelli L, Pongione G, Gerosa R, Gagliani M (2006) Radiographic and rheological properties of a new endodontic sealer. Australian Endodontic Journal 32, 31–4.
- Garrido ADB, Lia RCC, França EC, da Silva JF, Astolfi-FIlho S, Sousa-Neto MD (2010) Laboratory evaluation of the physicochemical properties of a new root canal sealer based on Copaifera multijuga oil-resin. International Endodontic Journal 43, 283–91.
- Gençoglu N, Türkmen C, Ahiskali R (2003) A new silicone-based root canal sealer (Roekoseal-Automix). Journal of Oral Rehabilitation 30, 753–7.
- Gong W, Zeng K, Wang L, Zheng S (2008) Poly(hydroxyether of bisphenol A)-block-polydimethylsiloxane alternating block copolymer and its nanostructured blends with epoxy resin. Polymer 49, 3318–26.
- Kontakiotis EG, Tzanetakis GN, Loizides AL (2007) A comparative study of contact angles of four different root canal sealers. Journal of Endodontics 33, 299–302.
- Lin-Gibson S, Landis FA, Drzal PL (2006) Combinatorial investigation of the structure-properties characterization of photopolymerized dimethacrylate networks. Biomaterials 27, 1711–7.
- Malynych S, Robuck H, Chumanov G (2001) Fabrication of two-dimensional assemblies of Ag nanoparticles and nano-cavities in poly(dimethylsiloxane) resin. Nano Letters 1, 647–9.
- McDermott J (1993) The Structure of the Advanced Composites Industry: Advance Composites Bluebook. Cleveland: Advanstar Communications.
- Monticelli F, Sword J, Martin RL et al. (2007) Sealing properties of two contemporary single-cone obturation systems. International Endodontic Journal 40, 374–85.
- Ørstavik D (1983) Physical properties of root canal sealers: measurement of flow, working time, and compressive strength. International Endodontic Journal 16, 99–107.
- Ørstavik D (2005) Materials used for root canal obturation: technical, biological and clinical testing. Endodontic Topics 12, 25–38.
- Ørstavik D, Nordahl I, Tibballs JE (2001) Dimensional change following setting of root canal sealer materials. Dental Materials 17, 512–9.
- Phillips RW, Skinner EW (1991) Skinner’s Science of Dental Materials, 9th edn. Philadelphia: Saunders.
- Rasimick BJ, Shah RP, Musikant BL, Deutsch AS (2007) Radiopacity of endodontic materials on film and a digital sensor. Journal of Endodontics 33, 1098–101.
- Resende LM, Rached-Junior FJ, Versiani MA et al. (2009) A comparative study of physicochemical properties of AH Plus, Epiphany, and Epiphany SE root canal sealers. International Endodontic Journal 42, 785–93.
- Roggendorf MJ, Legner M, Ebert J, Fillery E, Frankenberger R, Friedman S (2010) Micro-CT evaluation of residual material in canals filled with Activ GP or GuttaFlow following removal with NiTi instruments. International Endodontic Journal 43, 200–9.
- Schäfer E, Zandbiglari T (2003) Solubility of root-canal sealers in water and artificial saliva. International Endodontic Journal 36, 660–9.
- Tagger M, Katz A (2003) Radiopacity of endodontic sealers: development of a new method for direct measurement. Journal of Endodontics 29, 751–5.
- Tanomaru-Filho M, Jorge EG, Guerreiro Tanomaru JM, Goncalves M (2007) Radiopacity evaluation of new root canal filling materials by digitalization of images. Journal of Endodontics 33, 249–51.
- Tanomaru-Filho M, Jorge EG, Tanomaru JM, Goncalves M (2008) Evaluation of the radiopacity of calcium hydroxide- and glass-ionomer-based root canal sealers. International Endodontic Journal 41, 50–3.
- Tas¸demir T, Yesilyurt C, Yildirim T, Er K (2008) Evaluation of the radiopacity of new root canal paste/sealers by digital radiography. Journal of Endodontics 34, 1388–90.
- Versiani MA, Carvalho-Junior JR, Padilha MI, Lacey S, Pascon EA, Sousa-Neto MD (2006) A comparative study of physicochemical properties of AH Plus and Epiphany root canal sealants. International Endodontic Journal 39, 464–71.
- Wilson AD, McLean JW (1988) Glass-Ionomer Cement. Chicago: Quintessence Pub. Co.

/public-service/media/default/145/GbhGY_65311921a3b65.jpg)
/public-service/media/default/148/ix2WY_6531196adc6ec.jpg)
/public-service/media/default/460/aU9ju_671a20a2e53f3.png)
/public-service/media/default/158/GMj69_65311b2333f75.jpg)