Unicystic ameloblastoma: a possible pitfall in periapical diagnosis
Aim: To report a clinical case of unicystic ameloblastoma previously misdiagnosed as radicular cyst.
Summary: A 49-year-old white male was referred to a private practitioner complaining of an asymptomatic bony hard swelling of the left posterior mandible. The patient’s dental history indicated that his left mandibular first molar had been extracted approximately 10 years previously. At that time, preoperative radiographic examination demonstrated a radiolucent area of 1.5 cm diameter with well-defined margins involving the distal root of tooth 36. The lesion was diagnosed as cystic and surgery for its removal was advised, but not performed. At presentation, radiography demonstrated a well-defined 3 cm diameter radiolucency extending from the second premolar to the second molar. The lesion was enucleated and histopathological examination confirmed a diagnosis of unicystic ameloblastoma.
Key learning points:
- Despite a clinical diagnosis of periapical disease of endodontic origin, a nonendodontic lesion may be present.
- Unicystic ameloblastoma located on the periapical area of a tooth can lead to a pulp-periapical misdiagnosis, and should be considered in differential diagnosis.
- All tissue specimens recovered in apical surgery should be submitted to histopathlogical analysis.
Introduction
Ameloblastoma is an aggressive but benign epithelial neoplasm of odontogenic origin (Small & Waldron 1955, Hollows et al. 2000, Ord et al. 2002). Robinson & Martinez (1977) were the first to describe a distinct variant of ameloblastoma that was referred to as unicystic ameloblastoma. On the basis of previous reports, the unicystic ameloblastoma tends to occur at an earlier age than the solid or multicystic forms (Gardner & Corio 1983, Eversole et al. 1984, Ackermann et al. 1988, Philipsen & Reichart 1998). It frequently presents as a unilocular well-defined radiolucency surrounding the crown of an unerupted mandibular third molar, and may also mimic a dentigerous cyst (Eversole et al. 1984, Leider et al. 1985, Ackermann et al. 1988, Ord et al. 2002). Ackermann et al. (1988) described three distinct histological types as (i) cyst lined by variable epithelium with no infiltration into the fibrous cyst wall, (ii) cyst showing intraluminal plexiform epithelial proliferation with no infiltration, and (iii) cyst with invasion of epithelium into the cyst wall in either a follicular or a plexiform pattern. Microscopically, it was demonstrated that in all types there is a basal layer of columnar pre-ameloblasts with hyperchromatic nuclei polarized away from the basement membrane, with a clear basal cytoplasm, and a more superficial loose stellate reticulum-like epithelium (Leider et al. 1985, Philipsen & Reichart 1998, Li et al. 2000, Neville et al. 2002).
Due to its clinical behaviour, this lesion is commonly seen as an incidental finding on radiographs taken for other purposes. In these circumstances, some lesions could remain undiagnosed in the early stages of their development (Eversole et al. 1984).
This paper illustrates a case of a unicystic ameloblastoma that was initially misdiagnosed as a radicular cyst. At that time, no treatment was performed, and after 10 years, a diagnosis of unicystic ameloblastoma was made.
Report
A 49-year-old white male was referred to an oral and maxillofacial surgeon complaining of a ‘little lump’ in his mouth of about 6-month duration. On clinical examination, asymmetry was noted in the mandibular left quadrant. Examination revealed an asymptomatic bony hard swelling in the left posterior mandible extending from the distal of tooth 35 to tooth 37, covered by normal mucosa. The patient complained only of low level discomfort to pressure and palpation. Tooth 37 responded within normal limits to thermal pulp testing (hot and cold), suggesting its vitality. Radiographic examination showed a well-defined 3 cm diameter radiolucency extending from the distal interproximal area of teeth 35–37. Additionally, tooth 37 appeared to have a shortened mesial root (Fig. 1).

The patient’s dental history indicated that his 36 was extracted approximately 10 years previously. At that time, clinical examination revealed a deep occlusal alloy restoration in tooth 36 and with tooth 37 having a low grade of mobility. Periapical radiographs taken at that time (Fig. 2a) demonstrated a nonroot canal treated tooth 36 showing an oval-shaped radiolucent area with well-defined limits of 1.5 cm diameter involving its distal root and extending to the mesial aspect of tooth 37. Aggressive root resorption was noted on the distal root of tooth 36 without displacing the cortical plate of the alveolar process from the apparent connection of the lesion in the root surface. A minor area of root resorption was also noted in the middle third of the mesial root of 37, and the integrity of the associated cortical bone was lost. The patient had no knowledge of the specific procedures conducted in the management of the apical lesion 10 years previously. Three months after the dental extraction, the patient returned to the office complaining of pain. Periapical radiography showed the lesion to be unchanged and the extraction socket in the process of healing (Fig. 2b). At that time, the lesion was clinically diagnosed as cystic and surgery for its removal was advised. According to the patient, the surgery was not done because he felt unsure of the proposed treatment and he wanted to hear an opinion from another professional. He did not seek a second opinion and had no further intervention until his recent presentation.
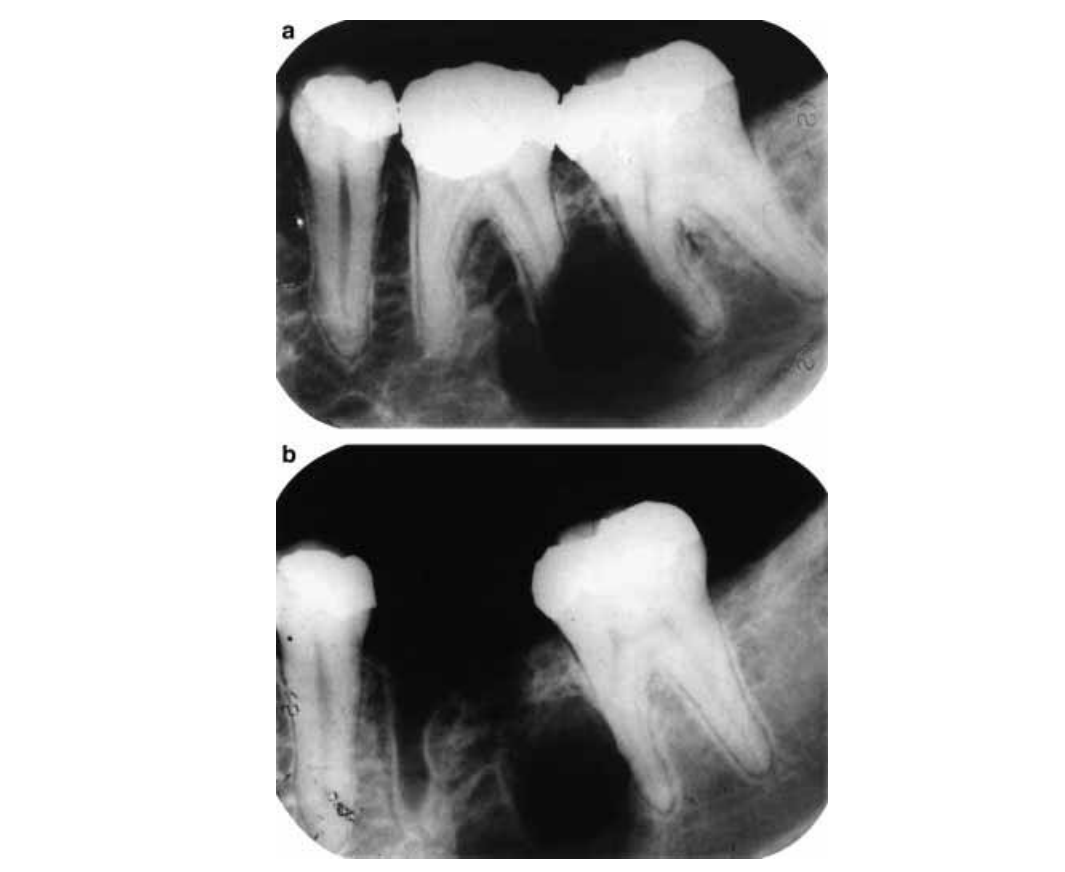
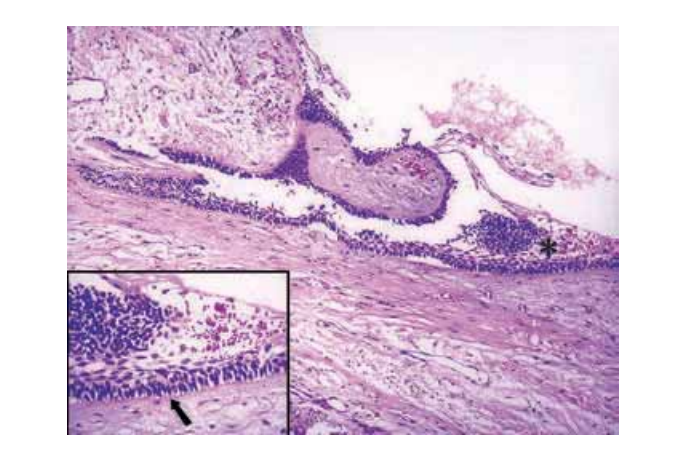
After clinical and radiographic evaluation, needle aspiration was carried out and a serohaemorrhagic fluid was obtained. Cytological examination showed epithelial and haematic cells and a preliminary clinical diagnosis of residual cyst was made. In view of this finding, enucleation of the lesion was again advised, some 10 years after the initial recommendation. After local anaesthesia was administered, a full thickness flap was raised, and an encapsulated lesion located between the perforated lingual and buccal plates was observed. After enlarging the bony access, the lesion was easily enucleated and tissue samples were submitted to histopathological examination that confirmed a diagnosis consistent with a unicystic ameloblastoma (Fig. 3). On the same occasion, tooth 37 was extracted due its mobility and the resorption on the mesial root.
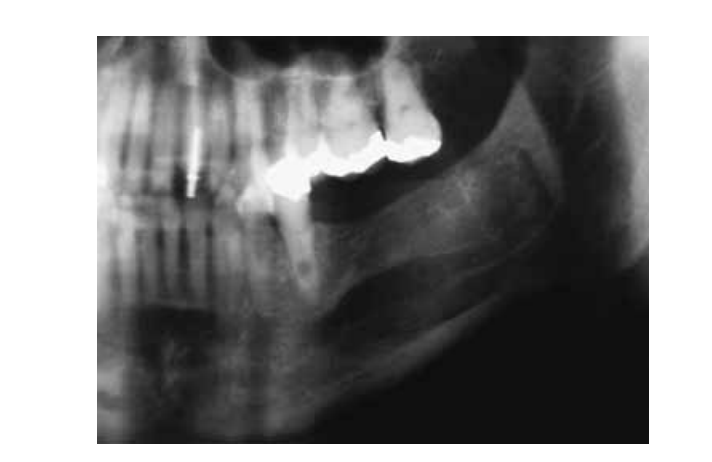
Seven days after the surgical procedure, the patient complained of paraesthesia of the left lower lip and left cheek. An oral examination revealed suppuration through the mesial aspect of the flap, and a drainage procedure was performed with oral administration of cephalexin monohydrate (500 mg every 8 h, for a total dosage of 1.5 g day–1, for 8 days). A 14-day recall showed complete healing of the mucosal wound. At the most recent review (2 years after surgery), a panoramic radiograph demonstrated complete bone formation without clinical symptoms of paraesthesia and signs of recurrence (Fig. 4). However, a balloon-like enlargement in the apical area of 35 pulpal canal could be seen suggesting internal resorption. At the time, the patient was referred to an endodontist for further investigation and treatment.
Discussion
In this paper, a case of unicystic ameloblastoma that was previously diagnosed as an inflammatory periapical lesion has been reported. This misdiagnosis led to the extraction of tooth 36 some 10 years previously, and as a consequence, residual neoplastic epithelium was left in the area. With hindsight, the aggressive appearance of the lesion should have triggered more aggressive intervention and a stronger recommendation for the patient to submit to surgery. At the more recent presentation, the clinical findings and histopathological examination of the lesion removed at surgery confirmed the diagnosis of unicystic ameloblastoma.
Most studies on periradicular lesions focus on radicular cysts and granulomas which are highly prevalent periapical lesions associated with pulpal necrosis and infection (Nair 1997). However, the occurrence of noninflammatory pathoses in this area, including developmental odontogenic cysts, lymphomas, periapical cemento-osseous dysplasias, central giant cell lesions and ameloblastomas (solid and unicystic), among others, have also been described (Wood & Goaz 1985, Dahlkemper et al. 2000, Nary Filho et al. 2004). As these lesions present a different prognosis they should always be considered in differential diagnosis. They are, however, rare. The unicystic ameloblastoma presents a special concern in this respect (Ackermann et al. 1988), being locally aggressive and nonresponsive to root canal treatment or tooth extraction.
Generally, apical inflammatory lesions appear as radiolucent images in intimate contact with the apical root. On radiographic examination, radicular cysts frequently show an oval outline with well-defined limits and uniform, symmetrical concentric growth. Additionally, alveolar cortical bone appears gradually dislocated from the insertion point of the lesion on the dental apex. Although root resorption is frequently associated with chronic periapical lesions, it is not commonly seen in small lesions and is rarely advanced (Wood & Goaz 1985, Dahlkemper et al. 2000, Neville et al. 2002). Moreover, clinical manifestations of cystic lesions include slow asymptomatic growth, buccal cortical bone expansion and needle aspiration usually liberates serous fluid (Shear 1983, Neville et al. 2002).
Some of these clinical and radiographic features are common to unicystic ameloblastoma (Eversole et al. 1984). However, the aspects reported in this case might help the practitioner in the differential diagnosis of apical lesions, and suggest the presence of a noncystic lesion. Radiographic examination showed apparent connection between the lesion and the apical root. However, evidence of a significant area of root resorption associated with a small radiolucent lesion, an asymmetric bony lesion (Fig. 2a) and the absence of alveolar bone dislodgment from the root surface should have discouraged a diagnosis of radicular cyst or granuloma. In this sense, most unicystic ameloblastomas have been associated with an expansive unilocular radiolucency with root resorption but not with cortical erosion and perforation (Li et al. 2000, Ord et al. 2002). Moreover, pulp sensitivity testing is important for the differential diagnosis between apical lesions of endodontic and nonendodontic origin. Unfortunately, it was not possible to obtain precise information about the pulpal health of tooth 36 in the patient’s history. Thus, it must be presumed that, at the first appointment, without a differential diagnosis, the clinician suggested tooth extraction in the belief that the root resorption and the periapical lesion were related to pulpal necrosis in tooth 36.
As pointed out by Ackermann et al. (1988), the most interesting aspect of the unicystic ameloblastoma is its biological behaviour. According to some authors (Robinson & Martinez 1977, Gardner & Corio 1984, Gardner 1984, Leider et al. 1985), this lesion is less aggressive than its solid or multicystic form and even curettage has been performed as the indicated therapeutic approach.
However, it is very important to recall that half of these tumours present mural follicular or plexiform ameloblastic intramural proliferation that cannot be readily identified from biopsy specimens (Leider et al. 1985, Wang 1985, Ackermann et al. 1988). In this situation, unicystic ameloblastoma can have similar behaviour to the solid lesion and a conservative surgical approach is discouraged (Gardner 1984). In the present case, no signs of bony recurrence or paraesthesia after 2 years follow-up were observed. However, an oval radiolucent enlargement of the pulp canal was observed in tooth 35 suggesting internal resorption. At the moment, there is no definitive explanation for this. However, pulpal injuries following crown restoration or from the surgical procedures for ameloblastoma enucleation might be considered. There is no reason to believe this represents tumour recurrence, but the patient will continue under review after endodontic intervention by a specialist.
Enucleated tissue fragments should always be referred to the pathologist to be diagnosed. Histologically, the lesion epithelium demonstrated ameloblastomatous appearance, e.g. tall columnar-basal cells, reverse nuclear polarity, subnuclear vacuoles and a thin layer of oedematous, degenerate-appearing stellate cells on the lumen surface (Philipsen & Reichart 1998, Li et al. 2000). These features are key characteristics of ameloblastomatous transformation (Vickers & Gorlin 1970).
Walton (1996) pointed out that alveolar curettage was an unnecessary procedure after the extraction of teeth with apical periodontitis because, in general, the absence of antigenic stimuli from the extracted tooth results in resolution of the chronic inflammatory lesion. However, pretreatment diagnosis of inflammatory apical diseases is not always accurate, and neither nonsurgical root canal treatment nor tooth extraction is totally effective. So, despite a proper clinical diagnosis of a necrotic pulp and subsequent tooth extraction, a nonendodontic lesion may remain (Dahlkemper et al. 2000). Moreover, in some situations, the follow-up of many patients after tooth extraction may be impractical. As reported in the present case, noninflammatory lesions can mimic radicular cysts or granulomas. As curettage was not performed after tooth extraction, the precise diagnosis of ameloblastoma was impossible to determine. In this way, routine histopathological examination of all lesions removed from alveolar sockets should be completed, avoiding misdiagnosis and inappropriate treatment (Schaffer 1997, Nary Filho et al. 2004).
Conclusions
The unicystic ameloblastoma can be found at or near tooth apices, simulating a radicular cyst or periapical granuloma. When evaluating periapical lesions radiographically, care must be taken in looking for scalloped margins and root resorption as these should be viewed with suspicion. The pupal status of affected teeth is important in the differential diagnosis and the treatment should be directed towards the lesion. Periapical surgical specimens should be submitted routinely for histopathological analysis avoiding misdiagnosis and inappropriate treatment. In the case of ameloblastomas, it is important to emphasize the need for long-term periodic follow-up of the patient.
Authors: E. M. Cunha, A. V. Fernandes, M. A. Versiani, A. M. Loyola
References:
- Ackermann GL, Altini M, Shear M (1988) The unicystic ameloblastoma: a clinicopathological study of 57 cases. Journal of Oral Pathology 17, 541–6.
- Dahlkemper P, Wolcott JF, Pringle GA, Lamar Hicks M (2000) Periapical central giant cell granuloma: a potential endodontic misdiagnosis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 90, 739–45.
- Eversole LR, Leider AS, Hansen LS (1984) Ameloblastomas with pronounced desmoplasia. Journal of Oral and Maxillofacial Surgery 42, 735–40.
- Gardner DG (1984) A pathologist’s approach to the treatment of ameloblastoma. Journal of Oral and Maxillofacial Surgery 42, 161–6.
- Gardner DG, Corio RL (1983) The relationship of plexiform unicystic ameloblastoma to conventional ameloblastoma. Oral Surgery, Oral Medicine, and Oral Pathology 56, 54–60.
- Gardner DG, Corio RL (1984). Plexiform unicystic ameloblastoma: a variant of ameloblastoma with a low recurrence rate after enucleation. Cancer 53, 1730–5.
- Hollows P, Fasanmade A, Hayter JP (2000) Ameloblastoma – a diagnostic problem. British Dental Journal 188, 243–4.
- Leider AS, Eversole LR, Barkin ME (1985) Cystic ameloblastoma: a clinicopathologic analysis. Oral Surgery, Oral Medicine, and Oral Pathology 60, 624–30.
- Li T-J, Wu Y-T, Yu S-F, Yu G-Y (2000) Unicystic Ameloblastoma: a clinicopathologic study of 33 Chinese patients. The American Journal of Surgical Pathology 24, 1385–92.
- Nair PNR (1997) Apical periodontitis: a dynamic encounter between root canal infection and host response. Periodontology 2000 13, 121–48.
- Nary Filho H, Matsumoto MA, Fraga SC, Gonc¸ alves ES, Se´ rvulo F (2004) Periapical radiolucency mimicking an odontogenic cyst. International Endodontic Journal 37, 337–44.
- Neville BW, Damm DA, Allen CM, Bouquot JE (2002) Oral and Maxillofacial Pathology, Chapter 15, 2nd edn. Philadelphia, USA: WB Saunders Company, pp. 616–8.
- Ord RA, Blanchaert RH, Nikitakis NG, Sauk JJ (2002) Ameloblastoma in children. Journal of Oral Maxillofacial Surgery 60, 762–70.
- Philipsen HP, Reichart PA (1998) Unicystic ameloblastoma. A review of 193 cases from the literature. Oral Oncology 34, 317–25.
- Robinson L, Martinez MG (1977) Unicystic ameloblastoma: a prognostically distinct entity. Cancer 40, 2278–85.
- Schaffer AB (1997) Residual cyst? (Letter to Editor). Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 83, 640–1.
- Shear M (1983) Cysts of the Oral Regions, Chapter 11, 2nd edn. Oxford, UK: Wright, pp. 114–41. Small JA, Waldron CA (1955) Ameloblastoma of the jaws. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 8, 281–97.
- Vickers RA, Gorlin RJ (1970) Ameloblastoma: delineation of early histopathologic features of neoplasia. Cancer 26, 699–710.
- Walton RE (1996) The residual radicular cyst: does it exist? (Letter to Editor). Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 82, 471.
- Wang JT (1985) Unicystic ameloblastoma: a clinicopathological appraisal. Taiwan Yi Xue Hui Za Zhi 84, 1363–70.
- Wood NK, Goaz PW (1985) Differential Diagnosis of Oral and Maxillofacial Lesions, Chapter 15, 3rd edn. St Louis, MO, USA: Mosby, pp. 320–56.
