Effects of root canal enlargement on unprepared areas and coronal dentine thickness of three-rooted maxillary first premolars with different root configurations: A stepwise micro-CT study
Abstract
Aim: To evaluate the effects of progressive root canal enlargements on the unprepared surface area and remaining dentine thickness of three-rooted maxillary first premolars with different root configurations.
Methodology: Thirty three-rooted maxillary first premolars with three root configurations (n = 10) were selected and scanned in a micro-CT device. The root canals were sequentially enlarged with rotary instruments sizes 30.02 (step 1), 30.04 (step 2) and 30.06 (step 3). After each step, a new scan was taken. Analysed parameters included morphometric measurements (length, volume and surface area), number of static voxels and minimal dentine thickness. Statistical analyses were performed with one-way ANOVA post hoc Tukey tests and paired sample t-test at a significance level of 5%.
Results: No statistical differences were observed amongst groups regarding the morphometric parameters and static voxels (p > .05). The minimal dentine thickness of the distobuccal root significantly changed depending on the root configuration (p < .05), whilst no differences were observed in the other roots (p > .05). A great variation in the position of the minimal dentine thickness was observed after preparation. Overall, mean percentage reduction in dentine thickness was higher in the buccal roots than in the palatal root (p < .05). In the mesiobuccal and distobuccal root, the number of slices with minimal dentine thickness lower than 0.05 mm increases 2 to 3 times and 3 to 4 times, respectively, from steps 1 to 3.
Conclusions: Instruments sizes 30.02 and 30.04 can be safely and effectively used to enlarge the buccal and palatal canals of three-rooted maxillary first premolars.
Introduction
Different root and root canal configuration types can be found in any group of teeth and a thorough understanding may enhance the chance for a successful treatment outcome. Some factors have been identified as contributors to explain the anatomic variations of teeth including ethnicity (Walker, 1987), age (Peiris et al., 2008), gender (Sert & Bayirli, 2004) and study design (Martins, Marques, Silva, Carames, & Versiani, 2019). This is an important aspect since understanding how demographic factors influence the root canal anatomy may help clinicians in anticipating the presence of complex morphologies in the clinical setting (Martins, Marques, Silva, Carames, & Versiani, 2019). Considering the highly variable internal canal configuration of maxillary first premolars, several studies have investigated their anatomy using different methods (Abella et al., 2015; Ahmad & Alenezi, 2016; Awawdeh et al., 2008; Belizzi & Hartwell, 1981; Bellizzi & Hartwell, 1985; Bürklein et al., 2017; Carns & Skidmore, 1973; Hartmann et al., 2013; Kartal et al., 1998; Marca et al., 2013; Martins, Marques, Silva, Caramês, et al., 2019; Nazeer et al., 2018; Neelakantan et al., 2011; Oi et al., 2004; Ok et al., 2014; Pécora et al., 1992; Saber et al., 2019; Soares & Leonardo, 2003; Tian et al., 2012; Tofangchiha et al., 2018; Vier-Pelisser et al., 2010; Walker, 1987; Willershausen et al., 2006). A recent meta-analysis revealed that the number of roots and root canals in this group of teeth varied according to the geographic region, suggesting that ethnicity may play a role in its external and internal morphologies (Martins, Marques, Silva, Caramês, et al., 2019).
In general, it has been reported that maxillary first premolars have two roots, and Vertucci's Type IV seems to be the most common root canal configuration, whilst the presence of three roots with three root canals (mesiobuccal, distobuccal and palatal) is the most frequently reported anatomic variation, with a prevalence ranging from 0.4% to 9.2% (Ahmad & Alenezi, 2016). Vertucci et al. (1974) classified this variation as Type VIII, defined as three separate and distinct canals from the pulp chamber to the apex. Since then, this classification has been used indistinctly to refer to three-canaled premolars even in cases in which canals are not encased in a single root. This is a misuse of Vertucci's classification, otherwise a three-rooted maxillary molar with three root canals should be also classified as Type VIII, and this is not the case. To provide a more consistent classification of three- rooted maxillary premolars, Belizzi and Hartwell (1981) proposed to categorize them into three types, according to the morphology of the roots: Type 1—fusion of all three roots or only the two buccal ones, and a semi-fused or free palatal root; Type 2—normal separation of the buccal roots at the midroot or apical-third level, with either a semi-fused or free palatal root; and Type 3—normal separation of the buccal roots up to the cervical level, with a free palatal root and the classic tripod appearance. In all of these types, each root usually encase one root canal (Ahmad & Alenezi, 2016), which means that their canal configuration should be classified as Vertucci's Type I.
Although information about diagnosis, morphology and clinical management of three-canaled maxillary premolars has been extensively described in scientific studies and case reports, and some authors have called the attention to their fragility, especially regarded to the mesiobuccal and distobuccal roots (Hartmann et al., 2013; Marca et al., 2013; Vier-Pelisser et al., 2010), no attempt was made so far to investigate the effects of different preparation protocols on the morphology of the roots and root canals. Therefore, the present laboratory investigation aimed to evaluate three-canaled maxillary premolars with different root configurations (Belizzi & Hartwell, 1981) regarding unprepared canal areas and remaining dentine thickness after a progressive enlargement of the root canal space through high-resolution micro-computed tomographic analysis (micro-CT). The null hypotheses tested were that there was no difference in the unprepared canal areas and remaining dentine thickness amongst three-canaled maxillary first premolars with different root morphologies after sequential root canal enlargements.
Material and methods
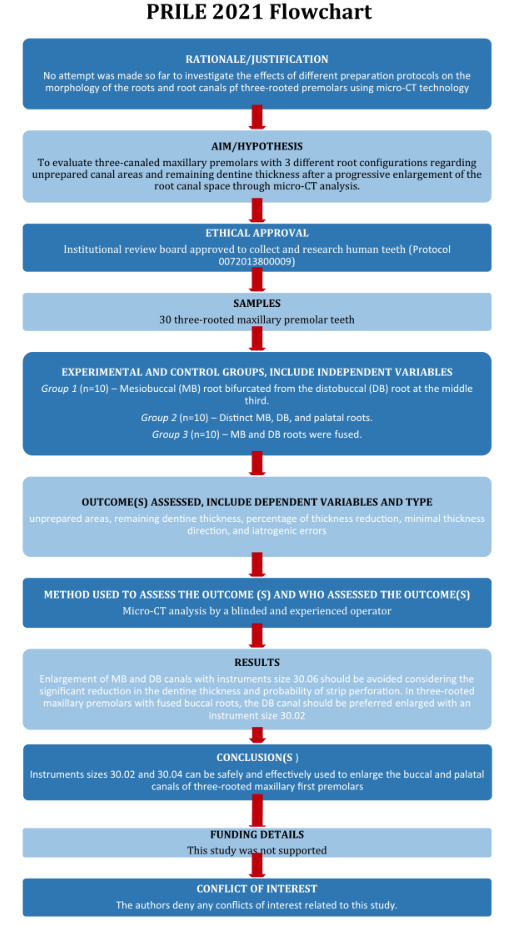
The manuscript of this laboratory study has been written according to Preferred Reporting Items for Laboratory studies in Endodontology (PRILE) 2021 guidelines (Nagendrababu et al., 2021) (Figure 1).
Specimen selection and imaging
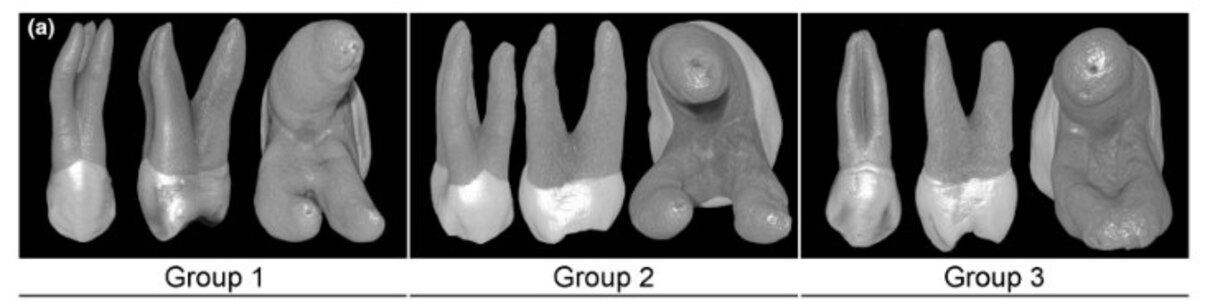
After approval of this study by the local ethics committee (Protocol 0072013800009), one hundred three-rooted maxillary first premolars extracted for reasons not related to this study from a Brazilian subpopulation were scanned in a micro-CT system (SkyScan 1176; Bruker-microCT) at 17 μm (pixel size), 90 kV, 278 μA, 180° rotation with steps of 0.5°, and frame average of 2, filtered by a 0.1-mm-thick copper filter. Demographic data of the donors (age, gender and race) were unknown. After scanning procedures, data sets were reconstructed using NRecon v.1.7.4.2 (Bruker-microCT) with a beam-hardening correction of 15%, smoothing of 3, ring artefact correction of 5 and an attenuation coefficient ranging from 0.0007 to 0.032. CTAn v.1.20.8 (Bruker-microCT) was used to create preoperative 3D models of the external and internal anatomies of the teeth and to measure the length of roots and root canals. Considering that the size of the root trunk was different amongst the selected specimens, the volume and surface area were calculated taken into account the entire root canal system. The canal configuration (DataViewer v.1.5.6.2; Bruker-microCT) and the external morphology of the roots (CTVox v.3.3.1; Bruker-microCT) were then analysed, and 30 maxillary premolars with three independent canals and fully formed apices, but no fillings, caries, fracture or resorptions, were selected and grouped according to their root configuration and canal morphometry (volume and surface area), as follows: Group 1 (n = 10)—mesiobuccal (MB) root bifurcated from the distobuccal (DB) root at the middle third; Group 2 (n = 10)—distinct MB, DB, and palatal roots; Group 3 (n = 10)—MB and DB roots were fused. In all groups, palatal roots were completely separated (Figure 2a).
Root canal preparation
After conventional access cavity preparation, apical patency was achieved with sizes 08 and 10 K-files (Dentsply Sirona), whilst the glide path was created with a size 15 K-file (Dentsply Sirona) up to the working length (WL), established 0.5 mm short of the apical foramen. No coronal flaring was performed, and canal preparation was carried out in three steps. In step 1, all canals were sequentially prepared with a rotary system (RaCe; FKG Dentaire) using instruments sizes 20.02, 15.04, 25.02, 20.04 and 30.02 in a continuous clockwise rotation (X-Smart; Dentsply Maillefer) up to the WL, according to the manufacturer's directions. After three gentle in-and-out motion strokes in an apical direction, the instrument was removed from the canal and cleaned. In each procedure step, irrigation was performed with a total of 10 ml of 2.5% sodium hypochlorite per canal delivered by using a 31-gauge NaviTip needle (Ultradent Products Inc.) adapted to a disposable plastic syringe placed 1 mm short of the WL. A final rinse with 5 ml of 17% EDTA was followed by 5-ml of distilled water. After slightly drying the canals with paper points (Dentsply Sirona), a new scan and reconstruction were performed using the mentioned parameters. Then, canals were further enlarged using rotary instruments sizes 30.04 (step 2) and 30.06 (step 3), respectively. After each enlargement, a new scan was taken. Therefore, four micro-CT scans were performed per specimen. An experienced operator performed all preparation procedures.
Image analysis
Postoperative models of the roots and root canals were rendered with CTAn v.1.20.8 (Bruker-microCT) and coregistered with their respective preoperative data sets using the affine registration algorithm of the 3D Slicer v. 4.5.0 software (available from http://www.slicer.org). The volume of interest (VOI) was selected extending from the cemento-enamel junction at the buccal aspect of the crown up to the apex of the longest root. The surfaces of preoperative root canal models were textured to simulate the pulp tissue, and postoperative models were painted in different colours (Autodesk 3ds Max 2021; Autodesk Inc.) to allow the qualitative comparison of groups after each enlargement step, whilst quantitative evaluation of postoperative morphometric parameters (volume and surface area) was performed using CTAn v.1.20.8 (Bruker-microCT). Unprepared areas were determined by calculating the number of static voxels (voxels present in the same position on the canal surface before and after instrumentation) (CTAn v.1.20.8; Bruker-microCT) expressed as a percentage of the number of static voxel surface (SVn) to the total number of surface voxels (SVt) by the formula: (SVn × 100)/ SVt. CTAn v.1.20.8 software (Bruker-microCT) was also used to create a 3D mapping of the dentine thickness which was saved for structure thickness. Colour-coded cross-sections were used to identify the direction and measure the smallest dentine thickness of each root at 1.0-mm interval from 1 mm below the cemento-enamel junction level at the buccal aspect of teeth (level 1) up to 3 mm towards the apical direction (levels 2 to 4). Qualitative comparisons of the root thicknesses before and after preparation procedures were performed using 3D colour-coded models of the matched roots (CTVox v.3.3.1; Bruker-microCT). Two pre-calibrated examiners (intraclass correlation index around 0.97 for all variables) performed the quantitative measurements and qualitative analyses.
Statistical analysis
Data were normally (Shapiro–Wilk test, p > .05) and homoscedastically (Levene test, p > .05) distributed. One-way ANOVA post hoc Tukey tests were used to compare groups regarding the morphometric parameters of the root and root canals (length, volume, surface area and static voxels), as well as, the minimal dentine thickness at each analysed level of the roots. Paired sample t-test was used to compare the minimal dentine thickness between two sequential canal enlargements in each group. Significance level was set at 5% (SPSS v.21.0 software; SPSS Inc.).
Results
No statistical differences were observed amongst groups regarding morphometric parameters of the roots (length) and root canals (length, volume and surface area) before or after each root canal enlargement (p > .05) (Table 1). No difference was also observed in the percentage of static voxels amongst groups in the different preparation steps (Figure 2b, Table 1).

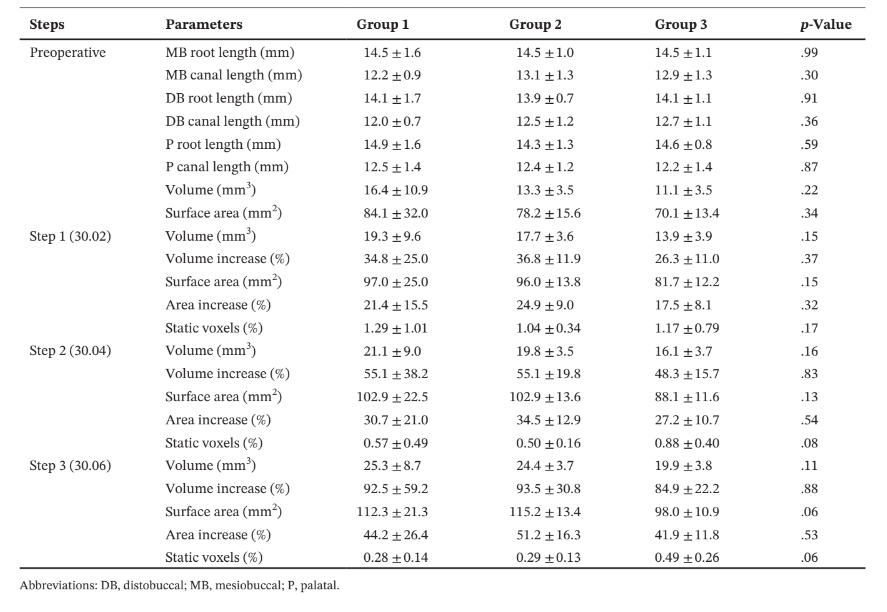
After preparation steps, the minimal dentine thickness of the DB canal in group 3 was significantly lower than the other groups (p < .05), whilst no differences were observed amongst groups in the other root levels (p > .05) (Table 2).
A great variation in the position of the minimal dentine thickness after preparation was observed in each group (Figure 3).
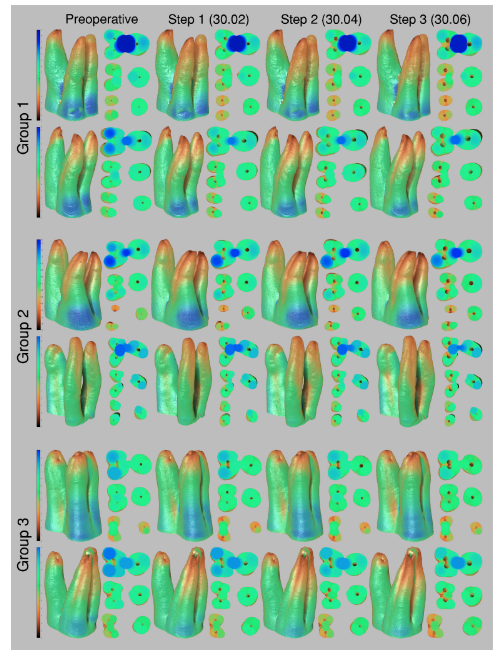
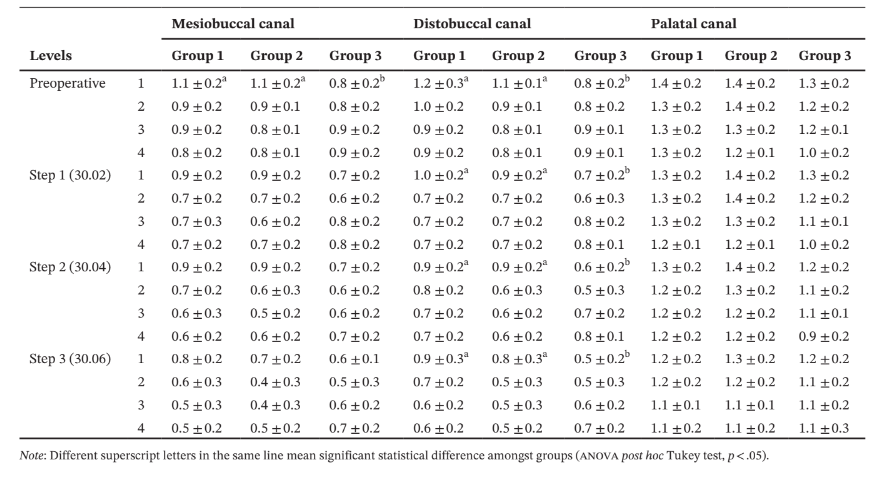
In the MB root of all groups, minimal dentine thickness was mostly observed to the DB direction, whilst in the DB root, it was positioned towards its mesial (Group 2) or MB (Groups 1 and 3) aspects (Table 3).
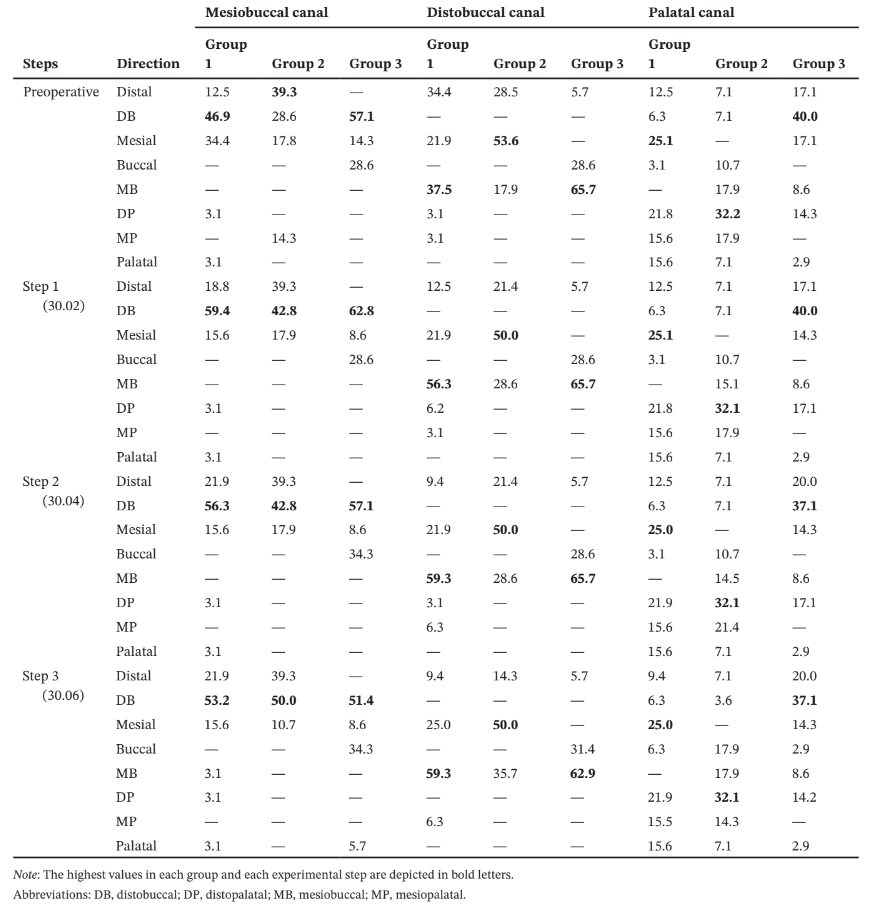
In the palatal root, the minimal dentine thickness was mainly located at its mesial (Group 1), distopalatal (Group 2) and DB (Group 3) aspects. Intragroup comparisons showed a statistically significant decrease in the mean dentine thickness after each canal enlargement (p < .05) (Table 4).
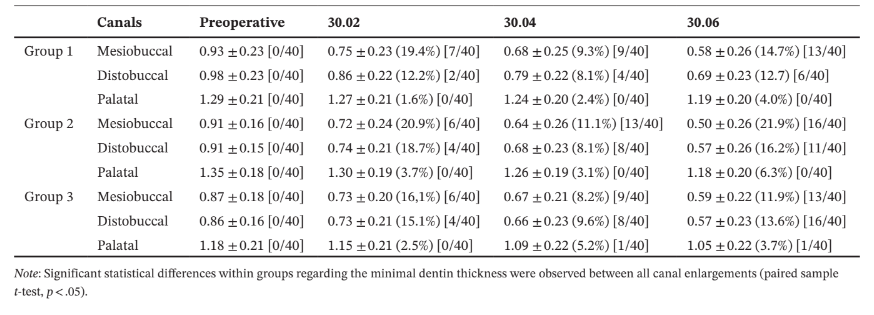
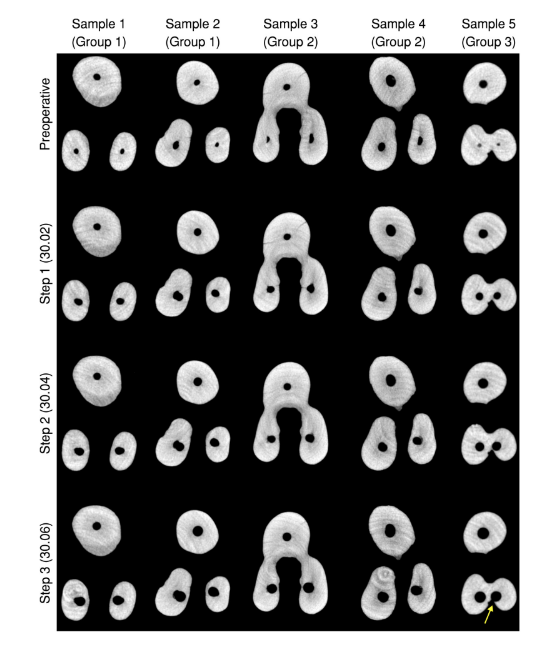
Overall, mean percentage reduction of dentine thickness in the MB and DB canals was higher after enlargement steps 1 (30.02) and 3 (30.06), whilst in the palatal canal, it varied according to the root configurations. In the MB root, the number of slices with minimal dentine thickness lower than 0.05 mm increases 2 (Groups 1 and 3) to 3 (Group 2) times, whilst in the DB root it increases 3 (Groups 1 and 2) to 4 (Group 3) times from the enlargement step 1 (30.02) to 3 (30.06). In the palatal root, most of evaluated slices had minimal dentine thickness larger than 1.0 mm (Table 4). The enlargement steps 1 (30.02) to 2 (30.04) resulted in no slices with less than 0.3 mm; however, after additional enlargement with size 30.06 instrument (step 3), it was observed three specimens from each group with dentine thickness lower than 0.3 mm in the MB and DB roots. Strip perforation was observed at the DB root of only one specimen of Group 3 (Figure 4).
Discussion
In the literature, the most relevant information available about three-rooted maxillary first premolars is their prevalence in different populations (Abella et al., 2015; Ahmad & Alenezi, 2016; Awawdeh et al., 2008; Belizzi & Hartwell, 1981; Bürklein et al., 2017; Kartal et al., 1998; Martins, Marques, Silva, Caramês, et al., 2019; Nazeer et al., 2018; Ok et al., 2014; Pécora et al., 1992; Saber et al., 2019; Sert & Bayirli, 2004; Tian et al., 2012; Tofangchiha et al., 2018; Walker, 1987) and the morphological assessment of their external and internal anatomies (Bellizzi & Hartwell, 1985; Carns & Skidmore, 1973; Hartmann et al., 2013; Marca et al., 2013; Oi et al., 2004; Soares & Leonardo, 2003; Vier-Pelisser et al., 2010). Notwithstanding the relevance of such knowledge, there is still a lack of meaningful scientific data regarding the safe and effective requirements to prepare their challenging root canal system. Therefore, this research used the nondestructive micro-CT technology to add new information into a not yet explored topic by evaluating the root canal shaping of three-rooted maxillary premolars with three different root morphologies after the sequential use of three master apical instruments (30.02, 30.04 and 30.06). The present results highlight the impact of each sequential enlargement steps on relevant aspects of the root canal preparation such as unprepared areas, remaining dentine thickness, percentage of thickness reduction, minimal thickness direction, and iatrogenic errors, throughout a mixed of quantitative and qualitative analyses. Overall, specimens with fused buccal roots (Group 3) showed a significant reduction in the minimal dentine thickness after preparation of the DB canal, leading to the partial rejection of the null hypotheses.
In 1981, Bellizzi and Hartwell classified three-rooted maxillary premolars into three categories according to the configurations of the roots, and called the attention to the importance of distinguishing them preoperatively to avoid missed canals and iatrogenic errors. The present findings suggest that this identification is relevant in a clinical set-up because, depending on the root configuration type, the preparation protocol may have a direct influence on the minimal dentine thickness (Tables 2 and 3). In a radiographic examination, the category with fused buccal roots (as in Group 3) is the most difficult to recognize since the fused roots are usually in line with the palatal root, mimicking a double-rooted configuration (Bellizzi & Hartwell, 1985). In this specific category, root canal preparation is challenging by two main anatomical features: the eccentric position of the buccal canals and the larger mesiodistal width of the fused roots (Belizzi & Hartwell, 1981; Sieraski et al., 1989). The latter aspect results in buccal canals with a common coronal branch that bifurcates more apically (Vier-Pelisser et al., 2010), which may compromise their identification and management without a proper access cavity design, enhanced visualization of the operating field and the use of advanced imaging techniques such as cone-beam computed tomography (Ahmad & Alenezi, 2016). In its turn, the eccentric position of buccal canals is a common anatomic characteristic of all three-rooted maxillary premolars. From a clinical perspective, the eccentricity of the buccal canals usually requires changes in the outline form of the access cavity from the traditional oval shape to a triangular shape with the base on the buccal side, resulting in a cavity with a T-shaped outline (Ahmad & Alenezi, 2016; Sieraski et al., 1989). Considering that buccal orifices are very close to each other, extra care should be taken to avoid excessive removal of dentine at the coronal level during access preparation and instrumentation since dentine thickness at this level is already thin preoperatively (Table 2). Another consequence of the eccentric position of the buccal canals, depicted by the different directions of the minimal dentine thickness before preparation (Table 3), is that root canal preparation may result in the transportation of the MB and DB canals to the distal and mesial aspects of the roots, respectively, even using flexible nickel-titanium instruments (Figure 4). A previous study from Hartmann et al. (2013) on three-rooted maxillary first premolars corroborates the present results regarding the preoperative dentine thickness (Tables 2 and 4) and its position in each root (Table 3). However, although authors did not categorize their sample according to the root configuration, as in the present study, they reported additional information about the minor and major diameters of each root canal at 1.0-mm interval from the apical foramen to the apex. At the last millimetre of the apical third, all canals showed mean diameters smaller than 0.28 mm, supporting the use of instruments with a tip size of 0.30 mm to promote an effective circumferential preparation at this level, as used in this study. In addition, the sequential analysis of the diameters in each section (Hartmann et al., 2013) also allowed to observe that all canals had a tapered shape. In fact, based on the mean reported diameters of the canals at 1 and 10 mm levels, it was possible to calculate the average tapers of the root canals as 2.7% (DB canal), 4.1% (MB canal) and 5.9% (palatal canal). This is an interesting morphological aspect as it suggests that the surface area of the buccal canals, for example, could be effectively prepared using small tapered instruments. This assumption is corroborated by the present results that showed mean percentages of static voxels lesser than 1.29% (Table 1, Figure 2b) after root canal enlargement with a size 30.02 instrument (step 1).
In necrotic cases, it may be necessary to improve the irrigant flow by increasing the canal taper (Boutsioukis et al., 2010) to reduce the intracanal contamination (Mickel et al., 2007). The present results also demonstrated an effective and safe preparation procedures after step 2 (30.04 instrument), considering that whilst static voxels was reduced by half (Table 1), the minimal dentine thickness at the coronal level was kept higher than 0.5 mm in all canals (Tables 2 and 4). On the contrary, as it would be expected (Weiger et al., 2006), the further enlargement of buccal canals to a size 30.06 instrument (step 3) caused a significant percentage reduction in the dentine thickness (Table 4, Figures 3 and 4), strip perforation in one specimen (Figure 4), whilst did not significantly decrease the mean percentage of static voxels (Table 1). Although the enlargement of the palatal canal with a size 30.06 instrument did not affect the integrity of the palatal root, the use of a size 30.04 instrument was sufficient to significantly reduce the percentage of static voxels to less than 1% (Table 1, Figure 2b) and increase the percentage volume of the root canals (Table 1). It is important to point out that, in three-rooted maxillary premolars with fused buccal roots (Group 3), safety preparation of the DB root canal should be done up to an instrument size 30.02 considering that a significant reduction in the minimal dentine thickness to less than 0.3 mm was noticed in several specimens after preparation with larger instruments (Table 2), including a strip perforation (Figure 4).
It may be argued that the main limitation of the present study was the inability to evaluate the impact of other preparation protocols using a large number of specimens.
However, it may be said that this type of sample with different root configurations is extremely difficult to collect. On the contrary, the main strength was the assessment of the progressive enlargements of root canals and their influence on the root and root canals of three-rooted maxilary first premolars using a 3D approach with high internal validity (Aksoy et al., 2021; Hartmann et al., 2013; Marca et al., 2013; Oi et al., 2004; Sousa-Neto et al., 2018), considering that previous literature focussed mostly on reporting prevalence rates in specific geographical locations or in analysing morphometric data obtained from radiographs or axial slices. Additional research is recommended to evaluate the effect of different root canal preparation protocols on the fracture resistance of three-rooted maxillary premolars.
Conclusion
Root canal preparation of three-rooted maxillary first premolars can be effectively and safely achieved with instruments sizes 30.02 and 30.04. Enlargement of MB and DB canals with instruments size 30.06 should be avoided considering the significant reduction in the dentine thickness and probability of strip perforation. In three-rooted maxillary premolars with fused buccal roots, the DB canal should be preferred enlarged with an instrument size 30.02.
Authors: Marco A. Versiani, Kleber K. T. Carvalho, Jorge N. R. Martins, Antonio L. N. Custódio, Maurício A. A. Castro, Emílio Akaki, Yara T. C. S. Silva-Sousa, Manoel D. Sousa-Neto
References:
- Abella, F., Teixido, L.M., Patel, S., Sosa, F., Duran-Sindreu, F. & Roig, M. (2015) Cone-beam computed tomography analysis of the root canal morphology of maxillary first and second premolars in a spanish population. Journal of Endodontics, 41, 1241–1247.
- Ahmad, I.A. & Alenezi, M.A. (2016) Root and root canal morphology of maxillary first premolars: a literature review and clinical considerations. Journal of Endodontics, 42, 861–872.
- Aksoy, U., Kucuk, M., Versiani, M.A. & Orhan, K. (2021) Publication trends in micro-CT endodontic research: a bibliometric analysis over a 25-year period. International Endodontic Journal, 54, 343–353.
- Awawdeh, L., Abdullah, H. & Al-Qudah, A. (2008) Root form and canal morphology of Jordanian maxillary first premolars. Journal of Endodontics, 34, 956–961.
- Belizzi, R. & Hartwell, G. (1981) Evaluating the maxillary premolar with three canals for endodontic therapy. Journal of Endodontics, 7, 521–527.
- Bellizzi, R. & Hartwell, G. (1985) Radiographic evaluation of root canal anatomy of in vivo endodontically treated maxillary premolars. Journal of Endodontics, 11, 37–39.
- Boutsioukis, C., Gogos, C., Verhaagen, B., Versluis, M., Kastrinakis, E. & Van der Sluis, L.W. (2010) The effect of root canal taper on the irrigant flow: evaluation using an unsteady Computational Fluid Dynamics model. International Endodontic Journal, 43, 909–916.
- Bürklein, S., Heck, R. & Schäfer, E. (2017) Evaluation of the root canal anatomy of maxillary and mandibular premolars in a selected german population using cone-beam computed tomographic data. Journal of Endodontics, 43, 1448–1452.
- Carns, E.J. & Skidmore, A.E. (1973) Configurations and deviations of root canals of maxillary first premolars. Oral Surgery, Oral Medicine, and Oral Pathology, 36, 880–886.
- Hartmann, R.C., Baldasso, F.E., Sturmer, C.P., Acauan, M.D., Scarparo, R.K., Morgental, R.D. et al. (2013) Clinically relevant dimensions of 3-rooted maxillary premolars obtained via high-resolution computed tomography. Journal of Endodontics, 39, 1639–1645.
- Kartal, N., Ozcelik, B. & Cimilli, H. (1998) Root canal morphology of maxillary premolars. Journal of Endodontics, 24, 417–419.
- Marca, C., Dummer, P.M., Bryant, S., Vier-Pelisser, F.V., Só, M.V., Fontanella, V. et al. (2013) Three-rooted premolar analyzed by high-resolution and cone beam CT. Clinical Oral Investigations, 17, 1535–1540.
- Martins, J.N.R., Marques, D., Silva, E., Carames, J. & Versiani, M.A. (2019) Prevalence studies on root canal anatomy using cone-beam computed tomographic imaging: a systematic review. Journal of Endodontics, 45, 372–386.e4.
- Martins, J.N.R., Marques, D., Silva, E.J.N.L., Caramês, J., Mata, A. & Versiani, M.A. (2019) Second root and second root canal prevalence in maxillary first and second premolars assessed by cone-beam computed tomography – a systematic review and meta-analysis. Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial, 60, 37–50.
- Mickel, A.K., Chogle, S., Liddle, J., Huffaker, K. & Jones, J.J. (2007) The role of apical size determination and enlargement in the reduction of intracanal bacteria. Journal of Endodontics, 33, 21–23.
- Nagendrababu, V., Murray, P.E., Ordinola-Zapata, R., Peters, O.A., Rôças, I.N., Siqueira, J.F., Jr. et al. (2021) PRILE 2021 guidelines for reporting laboratory studies in Endodontology: explanation and elaboration. International Endodontic Journal, 54, 1491–1515.
- Nazeer, M.R., Khan, F.R. & Ghafoor, R. (2018) Evaluation of root morphology and canal configuration of maxillary premolars in a sample of Pakistani population by using cone beam computed tomography. The Journal of the Pakistan Medical Association, 68, 423–427.
- Neelakantan, P., Subbarao, C., Ahuja, R. & Subbarao, C.V. (2011) Root and canal morphology of Indian maxillary premolars by a modified root canal staining technique. Odontology, 99, 18–21.
- Oi, T., Saka, H. & Ide, Y. (2004) Three-dimensional observation of pulp cavities in the maxillary first premolar tooth using micro-CT. International Endodontic Journal, 37, 46–51.
- Ok, E., Altunsoy, M., Nur, B.G., Aglarci, O.S., Colak, M. & Gungor, E. (2014) A cone-beam computed tomography study of root canal morphology of maxillary and mandibular premolars in a Turkish population. Acta Odontologica Scandinavica, 72, 701–706.
- Pécora, J.D., Saquy, P.C., Sousa Neto, M.D. & Woelfel, J.B. (1992) Root form and canal anatomy of maxillary first premolars. Brazilian Dental Journal, 2, 87–94.
- Peiris, H.R., Pitakotuwage, T.N., Takahashi, M., Sasaki, K. & Kanazawa, E. (2008) Root canal morphology of mandibular permanent molars at different ages. International Endodontic Journal, 41, 828–835.
- Saber, S., Ahmed, M.H.M., Obeid, M. & Ahmed, H.M.A. (2019) Root and canal morphology of maxillary premolar teeth in an Egyptian subpopulation using two classification systems: a cone beam computed tomography study. International Endodontic Journal, 52, 267–278.
- Sert, S. & Bayirli, G.S. (2004) Evaluation of the root canal configurations of the mandibular and maxillary permanent teeth by gender in the Turkish population. Journal of Endodontics, 30, 391–398.
- Sieraski, S.M., Taylor, G.N. & Kohn, R.A. (1989) Identification and endodontic management of three-canalled maxillary premolars. Journal of Endodontics, 15, 29–32.
- Soares, J.A. & Leonardo, R.T. (2003) Root canal treatment of three-rooted maxillary first and second premolars—a case report. International Endodontic Journal, 36, 705–710.
- Sousa-Neto, M.D., Silva-Sousa, Y.C., Mazzi-Chaves, J.F., KKT, C., AFS, B., Versiani, M.A. et al. (2018) Root canal preparation using micro-computed tomography analysis: a literature review. Brazilian Oral Research, 32, e66.
- Tian, Y.Y., Guo, B., Zhang, R., Yu, X., Wang, H., Hu, T. et al. (2012) Root and canal morphology of maxillary first premolars in a Chinese subpopulation evaluated using cone-beam computed tomography. International Endodontic Journal, 45, 996–1003.
- Tofangchiha, M., Bolbolian, M. & Ghasemi, A. (2018) Evaluation of root canal morphology of maxillary first premolars using cone beam computed tomography. Journal of Mashhad Dental School, 42, 31–40.
- Vertucci, F., Seelig, A. & Gillis, R. (1974) Root canal morphology of the human maxillary second premolar. Oral Surgery, Oral Medicine, and Oral Pathology, 38, 456–464.
- Vier-Pelisser, F.V., Dummer, P.M., Bryant, S., Marca, C., So, M.V. & Figueiredo, J.A. (2010) The anatomy of the root canal system of three-rooted maxillary premolars analysed using high-resolution computed tomography. International Endodontic Journal, 43, 1122–1131.
- Walker, R.T. (1987) Root form and canal anatomy of maxillary first premolars in a southern Chinese population. Endodontics and Dental Traumatology, 3, 130–134.
- Weiger, R., Bartha, T., Kalwitzki, M. & Löst, C. (2006) A clinical method to determine the optimal apical preparation size. Part I. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 102, 686–691.
- Willershausen, B., Tekyatan, H., Kasaj, A. & Marroquin, B.B. (2006) Roentgenographic in vitro investigation of frequency and location of curvatures in human maxillary premolars. Journal of Endodontics, 32, 307–311.
